Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 11
Color stability of Lucirin-photo-activated resin composite after immersion in different staining solutions: a spectrophotometric study
Authors AlSheikh R
Received 16 May 2019
Accepted for publication 13 August 2019
Published 5 September 2019 Volume 2019:11 Pages 297—311
DOI https://doi.org/10.2147/CCIDE.S216011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Rasha AlSheikh
Restorative Dental Sciences Department, College of Dentistry, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia
Correspondence: Rasha AlSheikh
Restorative Dental Sciences Department, College of Dentistry, Imam Abdulrahman Bin Faisal University, Po Box 30018 AlKhobar, Dammam 31952, Saudi Arabia
Email [email protected]
Introduction: Exceptional optical properties characterize teeth. As such, an esthetic restorative material should enable the dental professional to imitate the optical properties of natural teeth accurately. IPS Empress Direct was introduced to the market with the claim that it can mimic optically dental tissues with superior clinical performance.
Aim: To evaluate the ability of IPS Empress Direct to mimic tooth appearance and color and resist staining.
Materials and methods: Three disc specimens were prepared for each shade of enamel and dentin of Lucirin-based composite, IPS Empress Direct (Ivoclar Vivadent) (A1, A2, A3, B1, and B2) (total of 60). The specimens were submitted to colorimetric evaluation in comparison after immersion in 10 different solutions (coffee, coffee with sugar, coffee with milk, red tea, red tea with sugar, red tea with milk, tomato juice, pomegranate juice, coke, and distilled water as a control group) for 4 weeks using spectrophotometric analysis.
Results: After plotting the data and conducting linear regression analyses, IPS Empress showed high potential in mimicking the optical properties of natural tooth color according to the CIE color space. Three solutions showed a change in color higher than 3, coffee, coffee with sugar, and red tea. All other solution showed changes in color that are acceptable clinically.
Conclusion: IPS Empress Direct can satisfactory mimic teeth appearance and color while manifesting high stability of color resisting staining upon clinical aging.
Keywords: composite color, composite shade, restoration staining, IPS Empress
Introduction
The desire to have a more pleasant smile has become an essential esthetic need among today’s patients. Multiple components contribute to a patient’s smile, with anterior teeth playing a significant role in smile aesthetics. Teeth are characterized by exceptional optical properties, which far exceed qualities associated with color; translucency and opalescence are two significant factors affecting tooth appearance and color perception.1–4 Tooth color is determined by a combination of complex optical properties of both enamel and dentin, as well as the surrounding soft tissues.5–9 In the oral cavity, teeth are prone to decay, discoloration, trauma, or fracture. Restoring anterior teeth has always been a challenge, requiring the restoration of contour, texture, optical characteristics, and color stability. Teeth have a complex, layered structure, and their colors are the net result of a combination of colors from each layer.10–12 For example, dentin is far more opaque and intensely colored than is enamel.13,14 As such, the color-matching procedure is both challenging and important,7,9,15–17 especially given the increased emphasis on the appearance of patients seeking dental treatment.
In the oral cavity, a composite restoration is subjected to intrinsic and extrinsic staining.18–21 Staining can be related to polymerization depth, filler particles size, photo-initiator, and the resin matrix.22–27 Change in temperature, water sorption or adsorption, food, drinks, smoking as well as oral hygiene are some of the oral environment contributing factors.20,22,28,29 Extrinsic staining can be managed by maintaining polished composite surface initially and later through periodic re-polishing.30–34
Resin-based composite materials represent the most commonly used restorative material in aesthetic restorations. These materials are composed of a polymeric matrix based on dimethacrylate monomers and inorganic fillers and are coated with methyl methacrylate-functional silanes to bond the particles to the matrix, as well as a photo-initiator system that permits photoactivation using a light source.32,35–42 Most resin-based composites utilize camphorquinone (CQ) as the initiator system. CQ is a stable yellow compound with an unbleachable chromophore group. The presence of CQ in the resin leads to an undesirably yellowish effect in the final cured resin-based material; to complicate the problem further, this yellowing effect increases with aging.32,43–48 Later on, a different photo-initiator (monoacylphosphine oxide (Lucirin TPO)) system has been introduced that is designed to improve polymerization kinetics and reduce photo-yellowing effects.45,49–53 The use of Lucirin eliminated the amine group, thereby increasing color stability upon aging.25,45,48,54–56
IPS Empress Direct (Ivoclar Vivadent, Schaan, Liechtenstein), a resin-based material, was introduced to the market. Utilizing the latest technology, the manufacturer of IPS composite added nano-fillers and the Lucirin TPO-initiator system to the compound to reduce the amount of CQ used, thereby reducing its yellowing effect. According to the manufacturer, the new resin composite material IPS Empress Direct (Ivoclar Vivadent) can mimic the optical characteristics of teeth, including color, opalescence, and translucency, while expressing superior mechanical properties.57,58 However, few studies have investigated the properties of IPS Empress Direct. To the best of our knowledge, limited study has specifically investigated the ability of IPS Empress resin composite to match the color and optical properties of teeth.12,52
Study objectives
The study objectives were as follows: 1) to evaluate the ability of IPS Empress Direct to mimic tooth appearance and color, and 2) to evaluate specifically the color stability of IPS Empress Direct resin composite.
Study design
Preparation of tooth samples
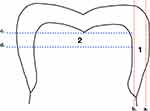
Fifteen intact, freshly extracted human teeth representing common shads (3 each shade; A1, A2, A3, B1, and B2) were collected; teeth were indicated for extraction for orthodontics treatment and a signed written informed consent was obtained from each patient. The tooth roots were embedded in transparent epoxy resin (Coltene, Altstätten, Switzerland). Two discs were cut from each tooth (n=30, 15 enamel and 15 dentin). These discs were subsequently sectioned according to 2 different planes. First, a superficial slice on the widest enamel plane was removed, and a 1-mm (±0.05 mm) disc was prepared following this axis. Next, a second 1-mm (±0.05 mm) disc was obtained by sectioning in a perpendicular direction along the long axis of the tooth 1.5 mm below the deepest point in the occlusal surface. The first disc was used to measure the enamel shade from its inner side, while the second disc was used to measure the dentinal shades from the occlusal side.8 (Figure 1)
IPS Empress Direct composite samples preparation
Thirty acrylic discs with holes (1 mm thick, 12 mm in diameter) were used as molds to construct the resin composite samples (3 samples of each shade, A1E, A2E, A3E, B1E, B2E, A1D, A2D, A3D, B1D, B2D). These discs were placed on top of a glass slide covered with a Mylar sheet. Next, the discs were slightly overfilled with the materials to be tested. After the molds were filled, they were covered with a second Mylar sheet and a glass slide to remove any excess material. Each specimen was examined with the naked eye against natural light to check for any internal porosities or defects. A light-curing unit [QHL 75 (505 mW/cm2) (Dentsply, York, PA, USA)] was used to photoactivate the resin composite. Curing was performed with each specimen in overlapping sections each for 30 s. Both upper and lower surfaces were cured until the whole specimen was irradiated for a total of 8 curing cycles. After curing, each specimen was polished from both surfaces using a mechanical polisher (MetaServ 250 Grinder-Polisher, Buehler, IL, USA) with 2000- and 3000-grit silica carbide abrasive papers under constant water cooling.38 The thickness of samples was then measured using a digital caliber to ensure thickness of 1 mm.
Solution immersion
Randomly resin discs were divided into 10 groups (n=6) to be immersed into 10 different staining solutions (coffee, coffee with sugar, coffee with milk, red tea, red tea with sugar, red tea with milk, tomato juice, pomegranate juice, coke, and distilled water as a control group).
After polishing of composite samples, samples were washed and stored in distilled water for 24 hrs in a 37°C incubator prior immersion for the polymerization to be completed, then samples were immersed separately in 10-mL containers filled with the corresponding solution. (Table 1) Samples were kept in a thermostatically controlled incubator at 37°C±1°C for 4 weeks. The solution was stirred 3 times a day to prevent sedimentation; solutions were changed weekly to prevent fungal colonization.22,59,60
 |
Table 1 Different staining solutions used in the investigation |
At the end of the 4 weeks, samples were removed from the solution with forceps and ultrasonically cleaned (UCP-10 Ultrasonic Cleaner, 230V UCP, Crest Ultrasonics Corp., NJ, USA) under distilled water for 1 min each. Samples were then blotted dry with tissues, and color measurements were obtained before and after 4 weeks of immersion.
Color measurements
The color was measured according to the CIE L* a* b* color scale relative to D65 on a Crystaleye Handheld Dental Spectrophotometer (Olympus America Inc., USA)61–63 Three color coordinate readings at three different parts were obtained from each specimen.
Color coordinates were calculated as below:
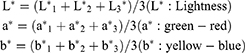
L* 1, L* 2, L3, a*1, a*2, a*3, b*1, b*2, and b*3 were the color coordinates of the 3 parts of each specimen.
Color difference was measured following the equation:
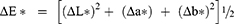
Translucency parameter was calculated as the difference in color between the resin composite specimens as they appeared against a white background and as they appeared against a black background according to the following equation:
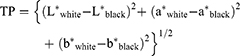
Statistical analysis
Color reproducibility
The color coordinate measurements were computed for both natural tissues (e.g., enamel or dentin) and IPS Empress. A linear regression analysis was performed to investigate the relationships between the colors of natural tooth tissues and those of IPS Empress. This approach enabled the evaluation of the significance of the correlation coefficient between the values of L*, a*, and b*. A paired t-test was conducted to analyze the difference in translucency between the enamel and dentinal shades of the IPS resin composite.
Color stability
Statistical analysis was performed using multifactorial ANOVA followed by Tukey test to evaluate differences in ΔE, ΔL, Δa, and Δb before and after immersion in staining solution. The data analysis was carried out using IBM SPSS statistics for Windows version 24.0. Armonk, NY: IBM Corp., with a significance set at (p00.05).
Results
Color reproducibility
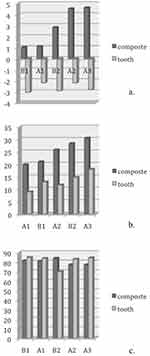
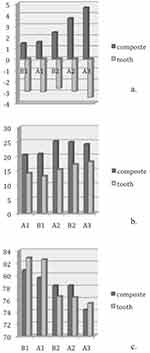
The L*a*b* data of natural teeth and IPS Empress composite are presented in (Table 2). The translucency parameters of the IPS Empress composite are presented in (Table 2). Linear regression was plotted to investigate the correlations between the color parameters of IPS Empress Direct and natural tooth tissues. (Figures 2–4)
 |
Table 2 The CIE L* a* b* color parameter for natural teeth tissues (tooth) and the IPS Empress Direct resin composite (composite) |
There was a significant correlation between a* and b*; between a* and the material type and between b* and the material type. All three correlation coefficients had positive values. For a* and b* when the value of b* increased, the value of a* also increased and vice versa, which indicates an increase in the chroma toward red and yellow. The composite specimens exhibited the same changes and correlations as did the tooth specimens.
The results in Figures 1 and 2 showed an increase in the values of a* and b* (ie, positive in the composite and negative in the tooth tissues) with increases in the enamel shade. Conversely, B1E manifested the smallest amount of chroma followed by A1E, B2E, A2E, and A3E. It is worth noting that the value (L*) decreased as the shades increased, while B1E showed the highest value followed by A1E, B2E, A2E, and A3E. These observations coincided with dentinal shade observations. The L* values of the enamel shades were higher than those for the dentinal shades, which was expected because the enamel is characterized by high translucency and value.
There was a significant (p=0.0001) decrease in the values of translucency between the enamel shades and dentinal shades of the IPS Empress Direct resin composite (Tables 3 and 4).
 |
Table 3 Translucency parameter values for the different shades of the IPS Empress Direct resin composite |
 |
Table 4 The CIE L* a* b* color parameters for the IPS Empress composite samples against white background then black background |
Color stability
The color change was determined by evaluating the color difference (ΔE) (Tables 5 and 6). Although there is a detected change in color, there was no significance found in ΔE between various solutions including the control group (distilled water) (Table 7).
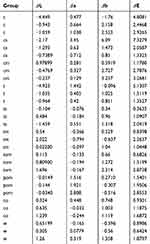 |
Table 5 Color stability and change evaluation |
 |
Table 6 Color stability and change evaluation ANOVA results |
 |
Table 7 Color stability and change evaluation Tukey results |
The clinically acceptable color difference when ΔE ˂ 3, which means color change blow 2 cannot be detected by the naked eye.59,64 Three solutions showed a change in color higher than 3, coffee, coffee with sugar, and red tea. All other solution showed changes in color that are acceptable clinically. Different coffee and the red tea groups showed decrees in the lightness and increase in the yellowness (Table 5). Tomato juice groups showed an increase in redness, while the pomegranate juice showed reduce in lightness (Figure 5).
Discussion
In the CIE (an international organization devoted to color standardization), three-dimensional color space involves three axes: L*, a*, and b*. The value of L* is a measure of the lightness of an object (value) where zero represents perfect black, and 100 represents a perfect reflecting diffuser. a* and b* both measure chroma (color saturation) and hue (color). The a* value is the measure of redness (positive a*) or greenness (negative a*). The b* value is the measure of yellowness (positive b*) or blueness (negative b*). The a* and b* values approach zero for achromatic colors (e.g., white and gray) but increase in magnitude with more intense colors (chroma).9,65,66
The color of natural teeth is a complex combination of different layers (e.g., pulp chamber and its content, as well as dentin and enamel), with each layer having its unique optical properties and structure. Discussing tooth color matching is important in clinical settings, as a wide range of tooth shades and colors occupy only a narrow area in the broader color schematic. In other words, the difference between the lightest and darkest tooth shades, although crucial in clinical dentistry, is but a minor difference regarding shades in the real world. As such, even a minor difference in the color coordinates can result in crucial differences in tooth appearance.9,67,68
This study aimed to evaluate the color properties and stability of IPS Empress Direct resin composite. To minimize the number of variables, a standardized thickness was used for both resin and the tooth samples. The color measurements were obtained under controlled viewing conditions using the same background and corrected sources of light. The Crystal-eye spectrophotometer used in the study has been used in several published studies, one of which supported its reliability and accuracy.8,61,66,69–71
In the oral environment, the surrounding soft tissues, the darkness of the oral cavity and the internal pulpal tissues all influence the color of teeth, and they may also influence the detection of color perception regarding differences between natural tissue and restorative materials. The a* values of non-vital tooth tissues have shown a shift toward green (negative values), while the b* values have shown a shift toward blue. Thus, a*, b*, and L* values of restorative materials have to be adjusted to obtain better matching to vital tooth structures (in vivo). This characteristic explains why no attempts have been made to evaluate the differences in the values of a*, b*, and TP between composite and natural tissues statistically.7,8 Instead, a linear regression was plotted, and correlations were subsequently investigated (Figures 2 and 3).
 |
Figure 5 One of the samples after immersion in tomato solution. |
Significant correlations were detected between a* and b*; between a* and the material type; and between b* and the material type. All three correlation coefficients had positive values. In other words, when the value of b* increased, the value of a* also increased and vice versa. The correlation between the material and changes in a* and b* is affected by the arrangement and choices of shades that were tested, as the shades were selected and arranged from lower chroma and higher values to higher chroma and lower values. Chroma increased from the lighter shades to the darker shades (A1 to A4 or B1 to B4). Conversely, values decreased from the lighter shades to the darker shades. This increase in chroma and decrease in value were shown clearly when the data were plotted for both the natural tooth tissues and IPS Empress (Figures 2 and 3). The negative value of a* did not reflect the decrease in the amount of chroma, as it reflected the direction toward green instead of red. The same can be applied for b* as the negative value indicated a shift toward blue.
The values tended to be smaller when the samples were examined against a black background, as the composite resin revealed a darker and greenish and bluish appearance when viewed against dark backing. This finding explains the grayish appearance commonly seen when the composite is used to restore a through-and-through cavity (classes III and IV), which helps to mimic natural appearance of the tooth more effectively by reproducing the translucent outer enamel layer reflecting the darker oral cavity from underneath.15,39,72,73
The concept of using both enamel and dentinal shades utilizes a layering technique,8,74 which results in a more optical and anatomically accurate reproduction of tooth appearance. The difference in translucency between enamel and dentinal shades has helped mimic the natural appearance of teeth (Table 3). Natural enamel is characterized by a high translucency and low chroma, while the dentin exhibits relatively opaque characteristics and higher chroma.8,67,72,75 The translucency of composite is a result of not only chroma increases, but also of the filler content and pigment additions that diffuse the light spectrum.76,77 IPS Empress Direct contains nano-fillers that improve light diffusivity and color properties. The use of opaquer dentin helps to block the dark color of the oral cavity in through-and-through cavity restorations. The darkness of the oral cavity also results in a reduction in the value of the relatively translucent composite, rendering a perfectly matched colored restoration unacceptable.
The staining solution used in this study was coffee, coffee with sugar, coffee with milk, red tea, red tea with sugar, red tea with milk, tomato juice, pomegranate juice, and coke. These solutions were chosen as the most common daily beverages known to have staining potentials and cover a wide variety of staining shades.32,78,79 Distilled water was chosen as a control group (Figure 6). Four weeks were chosen in according to previous studies, which is equivalent to 2.5 years of clinical aging.19,80 Control group showed acceptable color (ΔE˂3) after the immersion period indicating that the water sorption by itself causes no staining of composite restoration.81 Although there was no significant difference detected between the groups, black coffee showed the highest change in color, followed by the red tea, the coffee with sugar, and the last was the coffee with milk (Figure 7). The addition of the sugar and milk group was added as some studies showed a difference in staining potentials of coffee when sugar or milk was added.56 The higher staining potential of the coffee can be contributed to the presence of yellow staining molecules with high affinity to polymer molecules.82–84 For the tea, the staining could be due to the presence of tannic acid and stains.28,29,85–87 Coke despite its acidity which might deteriorate the restoration surface, proven to have low potential to stain resin restorations as the yellow stain particles have low polarity.88–91 It was expected that the pomegranate and the tomato juices would show the heist staining potentials which was not the case in the study as they showed the lowest change in color.
 |
Figure 6 Two samples after immersion in distilled water. |
 |
Figure 7 One of the samples after immersion in coffee with sugar solution. |
It is safe to say that the IPS Empress Direct composite resin can resist staining through aging it might be due to the use of different photoinitiator which eliminates the amine group, improve polymerization kinetic as well as reduce the yellowing effect of polymerization.
In conclusion, the optical properties and translucency of IPS Empress Direct resin composite displayed high potential in mimicking the optical properties of natural tooth color according to the CIE color space. IPS Empress Direct manifests high stability of color resisting staining upon clinical aging.
Clinical relevance
The desire to have a more pleasant smile has become an essential esthetic need among today’s patients. Tooth color appearance can dramatically affect a patient’s social life and confidence, while it can have adverse effects if the patient is not comfortable with his or her smile. In the oral cavity, teeth are prone to decay, discoloration, trauma, or fracture. Restoring anterior teeth has always been a challenge, as the restoration must restore tooth contour, texture, and optical characteristics.
Teeth have a complex, layered structure, and their colors are the net result of the combined colors of each layer. As patients are increasingly interested in esthetic dental treatment, color-matching procedures are becoming more challenging and essential.
Although color stability and staining of camphorquinone-photo-activated resin composite have been widely investigated, the color stability and staining of a Lucirin-photo-activated composite have not been investigated. Though this study is an in vitro study, it can predict the ability of the IPS Empress Direct resin composite (Lucirin-photo-activated composite) to reproduce teeth color and resist staining upon aging.
Ethics
Consent form was signed by patients from where teeth were extracted for orthodontics treatment.
Acknowledgment
The study was funded and approved by the Department of Scientific Research at Imam Abdulrahman Bin Faisal University (previously known as Dammam University) # 2011-036-Dent.
Disclosure
The author confirms that she has no conflict or involvement with any organization that have a financial or non-financial interest in the materials discussed in this manuscript.
References
1. Yılmaz B, Irmak Ö, Yaman BC. Outcomes of visual tooth shade selection performed by operators with different experience. J Esthet Restor Dent. 2019. doi:10.1111/jerd.12507
2. Narayan VK, Varsha VK, Girish HC, Murgod S. Stereomicroscopic study on unsectioned extracted teeth. J Forensic Dent Sci. 2017;9(3):157–164. doi:10.4103/jfo.jfds_43_16
3. Yu H, Cheng SL, Jiang NW, Cheng H. Effects of cyclic staining on the color, translucency, surface roughness, and substance loss of contemporary adhesive resin cements. J Prosthet Dent. 2018;120(3):462–469. doi:10.1016/j.prosdent.2017.10.009
4. Gupta S, Chandra A, Agnihotri A, Gupta OP, Maurya N. Age estimation by dentin translucency measurement using digital method: an institutional study. J Forensic Dent Sci. 2017;9(1):42.
5. Pereira Sanchez N, Powers JM, Paravina RD. Instrumental and visual evaluation of the color adjustment potential of resin composites. J Esthet Restor Dent. 2019. doi:10.1111/jerd.12488
6. Culic C, Varvara M, Tatar G, et al. In vivo evaluation of teeth shade match capabilities of a dental intraoral scanner. Curr Health Sci J. 2018;44(4):337–341. doi:10.12865/CHSJ.44.04.02
7. Mourouzis P, Koulaouzidou EA, Palaghias G, Helvatjoglu-Antoniades M. Color match of resin composites to intact tooth structure. J Appl Biomater Funct Mater. 2015;13. doi:10.5301/jabfm.5000228
8. Dietschi D, Ardu S, Krejci I. A new shading concept based on natural tooth color applied to direct composite restorations. Quintessence Int. 2006;27(2):91–102.
9. Joiner A. Tooth colour: a review of the literature. J Dent. 2004;32:3–12.
10. Santos SMM, Silva PD, Faria ESAL. Alternative to Avoid Tooth Discoloration after Regenerative Endodontic Procedure: A Systematic Review. Braz Dent J. 2018;29(5):469–474. doi:10.1590/0103-6440201802394
11. Miotti LL, Santos IS, Nicoloso GF, Pozzobon RT, Susin AH, Durand LB. The use of resin composite layering technique to mask discolored background: a CIELAB/CIEDE2000 analysis. Oper Dent. 2017;42(2):165–174. doi:10.2341/15-368-L
12. Perez BG, Miotti LL, Susin AH, Durand LB. The use of composite layering technique to mask a discolored background: color analysis of masking ability after aging-part II. Oper Dent. 2019. doi:10.2341/18-016-L
13. Powers JM, Sakaguchi RL. Craig’s Restorative Dental Materials. St. Louis, MO: Mosby Inc.; 2006.
14. Anusavice K. Philips’ Science of Dental Materials. Philadelphia, PA: W.B. Saunders co; 2003.
15. Hattab FN, Qudeimat MA, Al-Rimawi HS. Dental discoloration: an overview. J Esthet Dent. 1999;11:291–310.
16. Loiner A. Tooth colour: a review of the literature. J Dent. 2004;32:3–12.
17. Sugii MM, Caldas RA, Gouvea THN, Leite Lima DAN, Marchi GM, Baggio Aguiar FH. Utilizing the optical properties of composite resins to improve esthetics: a layering technique for anterior restorations. Gen Dent. 2019;67(1):55–60.
18. Ardu S, Duc O, Di Bella E, Krejci I, Daher R. Color stability of different composite resins after polishing. Odontology. 2018;106(3):328–333. doi:10.1007/s10266-017-0337-y
19. Arocha MA, Mayoral JR, Lefever D, Mercade M, Basilio J, Roig M. Color stability of siloranes versus methacrylate-based composites after immersion in staining solutions. Clin Oral Investig. 2013;17(6):1481–1487. doi:10.1007/s00784-012-0837-7
20. Ozera EH, Pascon FM, Correr AB, et al. Color stability and gloss of esthetic restorative materials after chemical challenges. Braz Dent J. 2019;30(1):52–57. doi:10.1590/0103-6440201902263
21. Duc O, Di Bella E, Krejci I, Betrisey E, Abdelaziz M, Ardu S. Staining susceptibility of resin composite materials. Am J Dent. 2019;32(1):39–42.
22. Khatri A, Nandlal B. Staining of a conventional and a nanofilled composite resin exposed in vitro to liquid ingested by children. Int J Clin Pediatr Dent. 2010;3(3):183–188. doi:10.5005/jp-journals-10005-1074i
23. Shintani H, Satou N, Yukihiro A, et al. Water sorption, solubility and staining properties of microfilled resins polished by various methods. Dent Mater J. 1985;4(1):54–62.
24. Prieto LT, Pimenta de Araújo CT, Araujo Pierote JJ, Salles de Oliveira DCR, Coppini EK, Sartini Paulillo LAM. Evaluation of degree of conversion and the effect of thermal aging on the color stability of resin cements and flowable composite. J Conserv Dent. 2018;21(1):47–51. doi:10.4103/JCD.JCD_128_17
25. Shin DH, Rawls HR. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent Mater. 2009;25(8):1030–1038. doi:10.1016/j.dental.2009.03.004
26. Soygun K, Varol O, Ozer A, Bolayir G. Investigations on the effects of mouthrinses on the colour stability and surface roughness of different dental bioceramics. J Adv Prosthodont. 2017;9(3):200–207. doi:10.4047/jap.2017.9.3.200
27. Rodrigues CS, Nora BD, Mallmann A, May LG, Jacques LB. Repolishing resin composites after bleaching treatments: effects on color stability and smoothness. Oper Dent. 2019;44(1):54–64. doi:10.2341/17-107-L
28. Barutcigil Ç, Barutcigil K, Özarslan MM, Dündar A, Yilmaz B. Color of bulk-fill composite resin restorative materials. J Esthet Restor Dent. 2018;30(2):E3–E8. doi:10.1111/jerd.12340
29. Alberton Da Silva V, Alberton Da Silva S, Pecho OE, Bacchi A. Influence of composite type and light irradiance on color stability after immersion in different beverages. J Esthet Restor Dent. 2018;30(5):390–396. doi:10.1111/jerd.12383
30. Deljoo Z, Sadeghi M, Azar MR, Bagheri R. The effect of different polishing methods and storage media on discoloration of resin composites. J Dent Biomater. 2016;3(2):226–232.
31. Spina DR, Grossi JR, Cunali RS, et al. Evaluation of discoloration removal by polishing resin composites submitted to staining in different drink solutions. Int Sch Res Notices. 2015;2015:853975. doi:10.1155/2015/853975
32. Schroeder T, Da Silva PB, Basso GR, Franco MC, Maske TT, Cenci MS. Factors affecting the color stability and staining of esthetic restorations. Odontology. 2019. doi:10.1007/s10266-019-00421-x
33. Corcodel N, Hassel AJ, Sen S, et al. Effects of staining and polishing on different types of enamel surface sealants. J Esthet Restor Dent. 2018;30(6):580–586. doi:10.1111/jerd.12423
34. Mara Da Silva T, Barbosa Dantas DC, Franco TT, Franco LT, Rocha Lima Huhtala MF. Surface degradation of composite resins under staining and brushing challenges. J Dent Sci. 2019;14(1):87–92. doi:10.1016/j.jds.2018.11.005
35. Puetzfeldt A. Resin composite in dentistry: the monomer systems. Eur J Oral Sci. 1997;105:97–116.
36. Kalachandra STD, McGrath JE, Sankarapandian M, Shobha HK. Structure-property relationship in dental composites based on polydimethacrylates. Polymer Paper. 1997;38:94–95.
37. Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resin. Biomaterials. 2002;23:1819–1829. doi:10.1016/s0142-9612(01)00308-8
38. Salgado VE, Albuquerque PP, Cavalcante LM, Pfeifer CS, Moraes RR, Schneider LF. Influence of photoinitiator system and nanofiller size on the optical properties and cure efficiency of model composites. Dent Mater. 2014;30(10):e264–e271. doi:10.1016/j.dental.2014.05.019
39. Salgado VE, Rego GF, Schneider LF, Moraes RR, Cavalcante LM. Does translucency influence cure efficiency and color stability of resin-based composites? Dent Mater. 2018;34(7):957–966. doi:10.1016/j.dental.2018.03.019
40. Bacchi A, Yih JA, Platta J, Knight J, Pfeifer CS. Shrinkage/stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. J Mech Behav Biomed Mater. 2018;78:235–240. doi:10.1016/j.jmbbm.2017.11.011
41. Mada DC, Gasparik C, Irimie AI, Mada MD, Dudea D, Campian RS. Evaluation of chromatic changes of a nanocomposite resin using the new whitness index. Clujul Med. 2018;91(2):222–228. doi:10.15386/cjmed-893
42. Shirinzad M, Rezaei-Soufi L, Mirtorabi MS, Vahdatinia F. Effect of accelerated artificial aging on translucency of methacrylate and silorane-based composite resins. J Dent (Tehran). 2016;13(2):92–100.
43. Rueggeberg FA, Ergle JW, Lockwood PE. Effect of photoinitiator level on properties of a light-cured and post-cure heated model resin system. Dent Mater. 1997;13:360–364.
44. Studer K, KÖNIGER R. Initial photoyellowing of photocrosslinked coatings. Eur Coat J. 2001;1:26–37.
45. Park YJ, Chae K, Rawls HR. Development of a new photoinitiation system for dental light-cure composite resin. Dent Mater. 1999;15:120–127.
46. Tanoue N, Matsumura H, Atsuta M. The influence of ultraviolet radiation intensity on curing depth of photoactivated composite veneering materials. J Oral Rehab. 1998;25:770–775.
47. Teshima W, Nomura Y, Tanaka N, Urabe H, Okazaki M, Nahara Y. ESR study camphorquinone/amine photoinitiator system using blue light-emitting diodes. Biomaterials. 2003;24:2097–2103. doi:10.1016/s0142-9612(02)00636-1
48. Smith DS, Vandewalle KS, Whisler G. Color stability of composite resin cements. Gen Dent. 2011;59(5):390–394.
49. Suh B. Controlling and understanding the polymerization shrinkage-induced stresses in light-cured composites. Compendium. 1999;20(Suppl. 25):34–41.
50. Burtscher PRV. Efficiency of various light initiators after curing with different light-curing units. J Dent Res. 2003; 81: Abstr.No. 42.
51. Sun GJ, Chae K. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer Paper. 2000;41:6205–6212. doi:10.1016/S0032-3861(99)00832-0
52. Lai G, Zhao L, Wang J, Kunzelmann KH. Surface properties and color stability of incrementally-filled and bulk-fill composites after in vitro toothbrushing. Am J Dent. 2017;30(5):262–266.
53. Macedo MGFP, Volpato CAM, Henriques BAPC, Vaz PCS, Silva FS, Silva CFCL. Color stability of a bis-acryl composite resin subjected to polishing, thermocycling, intercalated baths, and immersion in different beverages. J Esthet Restor Dent. 2018;30(5):449–456. doi:10.1111/jerd.12404
54. Allen N. Photoinitiators for UV and visible curing of coatings: mechanism and properties. J Photochem Photobiol A Chem. 1996;100:101–107. doi:10.1016/S1010-6030(96)04426-7
55. Rutsch WDK, Leppard D, Kohler M. Recent developments in photoinitiators. Prog Org Coat. 1996;27:227–239. doi:10.1016/0300-9440(95)00539-0
56. Piccoli YB, Lima VP, Basso GR, Salgado VE, Lima GS, Moraes RR. Optical stability of high-translucent resin-based composites. Oper Dent. 2019. doi:10.2341/18-025-L
57. Ramsey C. IPS empress direct. Dent Today. 2010;29(5):44.
58. Azad E, Atai M, Zandi M, Shokrollahi P, Solhi L. Structure-properties relationships in dental adhesives: effect of initiator, matrix monomer structure, and nano-filler incorporation. Dent Mater. 2018;34(9):1263–1270. doi:10.1016/j.dental.2018.05.013
59. Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95(2):137–142. doi:10.1016/j.prosdent.2005.11.019
60. Poggio C, Ceci M, Beltrami R, Mirando M, Wassim J, Colombo M. Color stability of esthetic restorative materials: a spectrophotometric analysis. Acta Biomater Odontol Scand. 2016;2(1):95–101. doi:10.1080/23337931.2016.1217416
61. Shigemi Ishikawa-Nagai AY, John D, Silva DA, Miller L. Spectrophotometric analysis of tooth color reproduction on anterior all-ceramic crowns: part 1: analysis and interpretation of tooth color. J Esthet Restor Dent. 2010;22(1):42–52. doi:10.1111/j.1708-8240.2009.00311.x
62. Park SKLY. Shade distribution of commercial resin composites and color difference with shade guide tabs. Am J Dent. 2007;20(5):335–339.
63. LeeYK LB, Kim CW, Powers JM. Comparison of color of resin composites of white and translucent shades with two shade guides. J Esthet Restor Dent. 2001;13(3):179–186.
64. Schneider LF, Cavalcante LM, Silikas N, Watts DC. Degradation resistance of silorane, experimental ormocer and dimethacrylate resin-based dental composites. J Oral Sci. 2011;53(4):413–419.
65. King KA, deRijk WG. Variation of L*a*b* values among vitapan classical shade guide. J Prosthodont. 2007;16(2):352–356. doi:10.1111/j.1532-849X.2007.00207.x
66. Kopperud HM, Johnsen GF, Lamolle S, Kleven IS, Wellendorf H, Haugen HJ. Effect of short LED lamp exposure on wear resistance, residual monomer and degree of conversion for Filtek Z250 and Tetric EvoCeram composites. Dent Mater. 2013;29(8):824–834. doi:10.1016/j.dental.2013.04.022
67. Miller L .Organizing color in dentistry. J Am Dent Assoc.1987;115:26E–40E. Spec No:26E-40E. doi:10.14219/jada.archive.1987.0315
68. Al-Dwairi Z, Shaweesh A, Kamkarfar S, Kamkarfar S, Borzabadi-Farahani A, Lynch E. Tooth shade measurements under standard and nonstandard illumination and their agreement with skin color. Int J Prosthodont. 2014;27(5):458–460. doi:10.11607/ijp.3826
69. Da Silvaa JD, Park SE, Hans-Peter Weber I-NS. Clinical performance of a newly developed spectrophotometric system on tooth color reproduction. J Prosthet Dent. 2008;99(5):361–368. doi:10.1016/S0022-3913(08)60083-9
70. YOSHIDA AKI, MILLER LLOYD, DA SILVA JOHND, ISHIKAWA-NAGAI S. Spectrophotometric analysis of tooth color reproduction on anterior all-ceramic crowns: part 2: color reproduction and its transfer from in vitro to in vivo. J Esthetic Dent. 2010;22(1):53–63. doi:10.1111/j.1708-8240.2009.00312.x
71. Lee YK, Yu B, Lee SH, Cho MS, Lee CY, Lim HN. Shade compatibility of esthetic restorative materials–A review. Dent Mater. 2010;26(12):1119–1126. doi:10.1016/j.dental.2010.08.004
72. Hasegawa A, Ikeda I, Kawaguchi S. Color and translucency of in vivo natural central incisors. J Prosthet Dent. 2000;83(4):418–423. doi:10.1016/S0022-3913(00)70036-9
73. Hickel R, Dasch W, Janda R, Tyas M, Anusavice K. New direct restorative materials. FDI commission project. Int Dent J. 1998;48:3–16.
74. Dietschi D. Free-hand composite resin restoration: a key to anterior aesthetics. Pract Periodontics Aesthet Dent. 1995;7:15–25.
75. Browning WD, Contreras-Bulnes R, Brackett MG, Brackett WW. Color differences: polymerized composite and corresponding Vitapan classical shade tab. J Dent. 2009;37(Suppl 1):e34–e39. doi:10.1016/j.jdent.2009.05.008
76. Ikeda T, Sidhu SK, Omata Y, Fujita M, Sono H. Colour and translucency of opaque-shades and body-shades of resin composite. Eur J Oral Sci. 2005;113:170–173. doi:10.1111/j.1600-0722.2005.00205.x
77. ElSayad II. Color and translucency of finished and unfinished esthetic restorative materials after staining and bleaching. Saudi Dent J. 2018;30(3):219–225. doi:10.1016/j.sdentj.2018.02.002
78. Silva TMD, Sales ALLS, Pucci CR, Borges AB, Torres CRG. The combined effect of food-simulating solutions, brushing and staining on color stability of composite resins. Acta Biomater Odontol Scand. 2017;3(1):1–7. doi:10.1080/23337931.2016.1276838
79. Tavangar M, Bagheri R, Kwon TY, Mese A, Manton DJ. Influence of beverages and surface roughness on the color change of resin composites. J Investig Clin Dent. 2018;9(3):e12333. doi:10.1111/jicd.12333
80. Ertas E, Guler AU, Yucel AC, Koprulu H, Guler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25(2):371–376.
81. Arregui M, Giner L, Ferrari M, Vallés M, Mercadé M. Six-month color change and water sorption of 9 new-generation flowable composites in 6 staining solutions. Braz Oral Res. 2016;30(1):e123. doi:10.1590/1807-3107bor-2016.vol30.0123
82. Ardu S, Braut V, Gutemberg D, Krejci I, Dietschi D, Feilzer AJ. A long-term laboratory test on staining susceptibility of esthetic composite resin materials. Quintessence Int. 2010;41(8):695–702.
83. Da Silva TM, Da Silva NY, Gonçalves LL, Alves LP, Fernandes AU, Gonçalves SEP. Staining beverages and cigarette smoke on composite resin and human tooth fluorescence by direct spectrometry. J Contemp Dent Pract. 2017;18(5):352–357.
84. Guler AU, Yilmaz F, Kulunk T, Guler E, Kurt S. Effects of different drinks on stainability of resin composite provisional restorative materials. J Prosthet Dent. 2005;94(2):118–124. doi:10.1016/j.prosdent.2005.05.004
85. Esmaeili B, Afkhami S, Abolghasemzadeh F. The effect of time between curing and tea immersion on composite resin discoloration. Gen Dent. 2018;66(2):64–68.
86. Um CM, Ruyter IE. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991;22(5):377–386.
87. Dinç Ata G, Gokay O, Müjdeci A, Kivrak TC, Mokhtari Tavana A. Effect of various teas on color stability of resin composites. Am J Dent. 2017;30(6):323–328.
88. Alharbi A, Ardu S, Bortolotto T, Krejci I. Stain susceptibility of composite and ceramic CAD/CAM blocks versus direct resin composites with different resinous matrices. Odontology. 2017;105(2):162–169. doi:10.1007/s10266-016-0258-1
89. Ardu S, Duc O, Di Bella E, Krejci I. Color stability of recent composite resins. Odontology. 2017;105(1):29–35. doi:10.1007/s10266-016-0234-9
90. Gregor L, Krejci I, Di Bella E, Feilzer AJ, Ardu S. Silorane, ormocer, methacrylate and compomer long-term staining susceptibility using ΔE and ΔE 00 colour-difference formulas. Odontology. 2016;104(3):305–309. doi:10.1007/s10266-015-0212-7
91. Reddy PS, Tejaswi KL, Shetty S, Annapoorna BM, Pujari SC, Thippeswamy HM. Effects of commonly consumed beverages on surface roughness and color stability of the nano, microhybrid and hybrid composite resins: an in vitro study. J Contemp Dent Pract. 2013;14(4):718–723.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.