Back to Journals » Research and Reports in Urology » Volume 10
Colony-stimulating factors detected in tumor cells and voided urine are potential prognostic markers for patients with muscle-invasive bladder cancer undergoing radical cystectomy
Authors Morizawa Y , Miyake M , Shimada K , Hori S , Tatsumi Y, Nakai Y , Tanaka N, Fujii T, Fujimoto K
Received 24 February 2018
Accepted for publication 31 May 2018
Published 21 September 2018 Volume 2018:10 Pages 103—111
DOI https://doi.org/10.2147/RRU.S166497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Yosuke Morizawa,1 Makito Miyake,1 Keiji Shimada,2 Shunta Hori,1 Yoshihiro Tatsumi,1 Yasushi Nakai,1 Nobumichi Tanaka,1 Tomomi Fujii,3 Kiyohide Fujimoto1
1Department of Urology, Nara Medical University, Nara, Japan; 2Department of Pathology, Nara City Hospital, Nara, Japan; 3Department of Diagnostic Pathology, Nara Medical University, Nara, Japan
Background: The clinical use of macrophage colony-stimulating factor, granulocyte colony-stimulating factor (G-CSF), and granulocyte macrophage colony-stimulating factor (GM-CSF) has improved the safety of cytotoxic chemotherapy. However, the overexpression of these CSFs in cancers has been reported to be associated with a poor prognosis in various malignancies. We evaluated the potential of CSF expression as a predictor of clinical outcome in patients with muscle-invasive bladder cancer (MIBC).
Methods: Consecutive patients (n=58) with MIBC who underwent radical cystectomy (RC) were included in this retrospective study. Treatment-naïve tumor specimens obtained by initial transurethral resection of bladder tumors prior to RC were immunostained with antibodies against macrophage colony-stimulating factor, G-CSF, and GM-CSF. We compared the clinicopathological variables and survival between these groups. Baseline levels of CSFs in the serum and voided urine were quantified using an enzyme-linked immunosorbent assay and compared with the expression of CSFs in the tumor lesions.
Results: Low expression of GM-CSF in the tumor cells was significantly correlated with a pathological T4 category (vs T2–3; P=0.02). In univariate survival analysis, high G-CSF and low GM-CSF expression in the tumor lesion were associated with poor outcomes. Furthermore, Cox proportional regression analysis revealed that high G-CSF and low GM-CSF expression in the tumor were independent predictors of shorter recurrence-free survival, cancer-specific survival, and overall survival. The levels of CSFs in voided urine were associated with the expression of CSFs in the tumor lesions.
Conclusion: GM-CSF and G-CSF expression in the tumor lesions obtained by initial transurethral resection are independent predictors of poor outcome in MIBC after RC. Levels of G-CSF and GM-CSF in urine before treatment could be useful in prognostication.
Keywords: colony-stimulating factor, M-CSF, G-CSF, GM-CSF, muscle-invasive bladder cancer, radical cystectomy
Background
Cisplatin-based combination chemotherapy has been the standard of care for treating patients with locally advanced and metastatic urothelial carcinoma in neoadjuvant, adjuvant, and metastatic settings.1–3 Chemotherapy causes not only hematopoietic damage but also immunological dysfunction. Impaired immunological function is a serious problem in cancer treatment from the viewpoint of infectious disease prevention, later recurrence, and long-term prognosis. The clinical use of macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), and granulocyte macrophage colony-stimulating factor (GM-CSF) has improved the safety of chemotherapy.3–5 G-CSF is widely used to increase the production of granulocytes for the treatment of neutropenia during chemotherapy.3 The clinical use of M-CSF during intensive chemotherapy for ovarian cancer has been approved in Japan.6 GM-CSF has been approved for the treatment of neutropenia during stem cell transplantation.7
Overexpression of CSFs (M-CSF, G-CSF, and GM-CSF) in many malignant cancers has been reported. M-CSF expression in type II papillary renal cell carcinoma and breast cancers has been associated with a poor prognosis.8–10 G-CSF is frequently associated with aggressive tumor cell growth and a poor clinical outcome.11 GM-CSF expression in colorectal cancer was found to be an independent predictor of a favorable outcome.12
In the present study, we retrospectively reviewed the records of 58 patients with newly diagnosed muscle-invasive bladder cancer (MIBC). We aimed to conclusively demonstrate the clinicopathological parameters and expression of CSFs in patients with MIBC.
Methods
Patients and data collection
Between 2002 and 2013, 121 patients with urothelial carcinoma of the bladder without evidence of distant metastasis underwent radical cystectomy (RC) at the Department of Urology, Nara Medical University. Medical interventions including transurethral resection of bladder tumor (TURBT), intravesical treatment, neoadjuvant chemotherapy, and RC could influence the population of immune-related cells and cytokines in the tumor microenvironment. To investigate the baseline prognostic factors that are available before RC, we selected patients with MIBC who underwent initial TURBT at Nara Medical University before any other treatment in order to obtain treatment-naïve bladder specimens. Among the 121 patients, 44 (36%) underwent TURBT at other hospitals, seven (6%) were diagnosed with non-muscle-invasive bladder cancer (NMIBC) including T1 and Tis, and 12 (10%) patients received neoadjuvant chemotherapy, leaving 58 (48%) who were included in the present study.
All of the hematoxylin and eosin (H&E)-stained specimens were reevaluated by two experienced uro-pathologists (KS and TF). The analyzed tumor variables included pathological T category, histological subtypes, lymph node involvement, and lymphovascular invasion (LVI).13–17
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB) of the Nara Medical University (NMU-1256) and complied with the 1964 Helsinki Declaration and its later amendments. As the data for the study were obtained through retrospective chart review, a waiver of informed consent was approved by the IRB. Personal information linked to research subjects and donors was anonymized (when necessary, the information was labeled with an identifying code to make it possible to distinguish between the individuals). Then, de-identified patient data were analyzed.
Immunohistochemical (IHC) staining
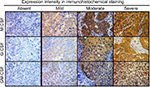
Paraffin-embedded tumor specimens from the initial TURBT when the patients were diagnosed with MIBC were immunostained as previously described.18 Briefly, IHC staining was performed with a streptavidin-biotin (SAB) complex method using the Histofine SAB-PO kit (Nichirei Co., Tokyo, Japan) according to the manufacturer’s directions. For antigen retrieval, the sections were routinely autoclaved for 10 minutes in 0.01 M citrate buffer (pH 6.0). The primary antibodies were monoclonal rabbit anti-M-CSF (ab52864; Abcam, Cambridge, MA, USA), monoclonal mouse antihuman G-CSF (#11041; IBL Japan, Gunma, Japan), and polyclonal rabbit GM-CSF (PP1101P1; Acris Antibodies, San Diego, CA, USA). The human tonsil and bone marrow tissues were used as positive controls for CSF staining. The sections were counterstained with Meyer’s hematoxylin and mounted with malinol, and then were examined alongside H&E stained specimens to identify the precise locations of the lesions. Three independent areas in the sections were selected and saved as pictures. IHC evaluation was carried out independently by two investigators (YM and YT). Evaluations were performed blindly without the knowledge of the patients’ outcome or other clinicopathological characteristics. Percentages of positive tumor cells were evaluated with a high-power field. Staining intensities were classified as absent (0), mild (1), moderate (2), and severe (3) (Figure 1). A histoscore was calculated as the product of the percentage of positive cells times the intensity of staining with a range of values from 0 to 300 and staining intensity. Expression level of CSFs was categorized as low or high according to the median value of histoscore. We compared the clinicopathological variables and survival between the high expression group and low expression group.
Enzyme-linked immunosorbent assay (ELISA) for M-CSF, G-CSF, and GM-CSF
Serum and urine were analyzed with an ELISA to measure CSF levels. Peripheral blood and voided urine were taken at initial TURBT before any treatment. In addition, no patients had a lower renal function (less than estimate glomerular filtration rate 30), cardiovascular diseases, hematological malignancies, and acute inflammatory diseases, which influence CSF levels. We stored all samples frozen at –80°C and used fresh samples to avoid repeated free-thaw cycles according to manufacturer’s directions. We compared CSF levels in patients with MIBC between patients with NMIBC and healthy controls (Table S1). Cytokine levels were measured using commercially available kits (M-CSF; Human M-CSF Immunoassay DMC00B, R&D Systems Inc., Minneapolis, MN, USA, G-CSF; Human G-CSF ELISA Development Kit 900-K77, PeproTech, Gumma, Japan, and GM-CSF; Human GM-CSF ELISA Kit 873.040.192, Diaclone, Besancon, France) according to the manufacturer’s instructions. Standard curves for each cytokine were generated using the reference concentrations provided in each kit. The detection range for each ELISA kit (M-CSF, G-CSF, and GM-CSF) was 11.2–5,000 pg/mL, 16–2,000 pg/mL, and 4.9–500 pg/mL, respectively.
Statistical analyses
The clinical outcomes were evaluated by recurrence-free survival (RFS), cancer-specific survival (CSS), and overall survival (OS) measured in months from the date of RC. The cutoff date of the last follow-up was December 31, 2016. The clinicopathological characteristics were compared using Student’s t-test, chi-square test, and analysis of variance. All tests were two-sided. The CSF levels in serum and urine were compared using Mann–Whitney U test. Univariate and multivariate analyses for RFS, CSS, and OS were performed using Cox proportional hazards models. RFS and CSS were examined using the Kaplan–Meier method. The results of Cox model analysis are reported with relative risks and 95% confidence intervals (CIs). IBM SPSS version 21 (IBM Corporation, Armonk, NY, USA) and PRISM software version 5.00 (GraphPad Software, Inc., La Jolla, CA, USA) were used for statistical analyses and data plotting, respectively. A P-value <0.05 was defined as statistically significant.
Results
Patient characteristics and immunohistochemistry
The clinicopathological variables of the patients are shown in Table 1. We determined the association of CSF expression determined by IHC staining with various clinicopathological parameters. Staining intensities were classified as absent (0), mild (1), moderate (2), and severe (3) (Figure 1). M-CSF expression intensity in tumor cells was scored as low (0) in six patients (10%), mild (1) in 18 patients (30%), moderate (2) in 17 patients (30%), and severe (3) in 17 patients (30%). G-CSF expression intensity in tumor cells was scored as low (0) in 14 patients (24%), mild (1) in 17 patients (30%), moderate (2) in 20 patients (34%), and severe (3) in six patients (12%). GM-CSF expression intensity in tumor cells was scored as absent (0) in 12 patients (21%), mild (1) in 16 patients (28%), moderate (2) in 26 patients (45%), and severe (3) in four patients (7%). A histoscore was calculated as the product of the percentage of positive cells times the intensity of staining with a range of values from 0 to 300. Overall, M-CSF expression in tumor cells was low in 38 patients (66%) and high in 20 patients (34%). G-CSF expression in tumor cells was low in 25 patients (43%) and high in 33 patients (57%). GM-CSF expression in tumor cells was low in 30 patients (52%) and high in 28 patients (48%).
The median values of histoscore of M-CSF, G-CSF, and GM-CSF were 52.5, 100, and 80, respectively. We compared the clinicopathological variables and survival between the high expression group and low expression group. Low expression of GM-CSF significantly correlated with pathological T stage (Table 1). G-CSF and M-CSF were not significantly associated with any clinicopathological variables.
M-CSF, G-CSF, and GM-CSF by ELISA
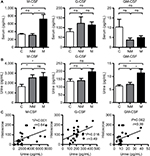
M-CSF levels in the serum of patients with MIBC were higher than those in patients with NMIBC and controls. There were no significant differences in G-CSF and GM-CSF in the serum among the three groups (Figure 2A). G-CSF and GM-CSF levels in the urine of patients with MIBC were higher than in those with NMIBC and controls (Figure 2B). Spearman rank correlation coefficient analysis revealed significant correlations between CSF expression in the tumor and urine for M-CSF (P=0.001), G-CSF (P=0.016), and GM-CSF (P=0.023) (Figure 2C).
Clinical outcomes
Overall, 21 (36%) patients had cancer recurrence at a median of 22.5 months after RC, and 20 (34%) died at a median of 39.5 months after RC, of whom 13 (22%) died of bladder cancer at a median of 39.5 months. In univariate analyses, M-CSF expression in bladder cancer cells was not associated with cancer outcomes (Figure 3A). G-CSF expression (high vs low) was associated with the probability of recurrence (5-year RFS: 52% vs 21%) and cancer-specific mortality (5-year CSS: 38% vs 11%) (Figure 3B). GM-CSF expression (low vs high) was associated with the probability of recurrence (5-year RFS: 57% vs 17%) and cancer-specific mortality (5-year CSS: 44% vs 10%) (Figure 3C). In multivariate Cox models, pathological T stage, high G-CSF expression, and low GM-CSF expression were significantly associated with an increased risk of recurrence. Pathological T stage, high G-CSF expression, and low GM-CSF expression were significantly associated with cancer-specific mortality. Pathological T stage and low GM-CSF expression were significantly associated with overall mortality (Table 2).
Discussion
In the management of MIBC, many predictors of a poor outcome such as age, sex, performance status, preoperative low hemoglobin, C-reactive protein, neutrophil–lymphocyte ratio, pathological T stage, lymph node metastasis, LVI, and tumor growth pattern have been used.13–17,19–21 In the present study, we investigated the expression of CSFs in treatment-naïve bladder specimens from 58 patients with MIBC. To our knowledge, this is the first study to analyze the expression of CSFs in MIBC.
CSFs are growth factors regulating the growth, proliferation, and differentiation of cells of hematopoietic lineages. Overexpression of CSFs in many malignant cancers has been reported. M-CSF expression in type II papillary renal cell carcinoma and breast cancers has been associated with a poor prognosis.8–10. In this study, M-CSF expression in bladder cancer cells detected by IHC staining was not associated with cancer outcomes for patients with MIBC after RC.
G-CSF expression in bladder cancer cells detected by IHC staining was significantly associated with an increased risk of recurrence and cancer-specific mortality of patients with MIBC after RC. G-CSF-producing malignant tumors have been reported to occur in many organs, most of which have been associated with extremely poor clinical outcomes.22 Mizutani et al23 reported that 9.2% of bladder tumors had elevated G-CSF levels, which was positively associated with an increase in grade and progression of stage of cancer, more so in patients with distant metastasis. Patients with G-CSF-producing bladder cancers have poor disease-specific survival rates at 5 years follow-up.23
The mechanism responsible for overexpression of G-CSF in bladder cancer has yet to be fully elucidated. An in vitro study found that G-CSF/G-CSF receptors exhibit high affinity binding, and this biological pathway increases the proliferation of bladder cancer cells.24 This autocrine mechanism of growth may be associated with aggressive tumor growth and adverse clinical outcomes.
GM-CSF expression in bladder cancer cells detected by IHC staining was significantly associated with a decreased risk of recurrence and cancer-specific mortality. Similar to our results, Nebiker et al reported that GM-CSF expression in colorectal cancer was an independent favorable prognostic factor.12 GM-CSF plays a key role in the differentiation and functional maturation of different myeloid populations and has the ability to activate antigen-presenting cells. Some researchers have reported that immunotherapy with GM-CSF was effective in animal models of bladder cancer.19,20 GM-CSF enhances the activity of antitumor immune cells such as granulocytes, macrophages, and dendritic cells in tumors and may lead to successful tumor treatment.25 GM-CSF expression in bladder tumors may activate immune cells.
GM-CSF level in the urine of patients with MIBC was higher than that in those with NMIBC and controls. Similar to our results, Kumari et al reported that urine level of GM-CSF was significantly higher in high-grade bladder cancer patients compared to low-grade bladder cancer patients and controls.26 In the present study, all the patients were diagnosed with high-grade bladder cancer. GM-CSF in the urine of patients with bladder cancer may indicate poor prognosis. But in patients with MIBC, levels of GM-CSF in urine before treatment could be also useful in prognostication because levels of GM-CSF in the urine significantly correlated with GM-CSF expression in the tumor which was associated with cancer-specific mortality.
G-CSF administration to patients with cancer during chemotherapy is effective in reducing neutropenia duration and the risk of neutropenia-related negative events and in reducing the risk of febrile neutropenia and early deaths, including infection-related mortality.3,27,28 However, Perez et al reported a patient with rapid clinical deterioration and leukemoid reaction, treatment of bladder cancer with G-CSF along with chemotherapy should be considered.29 Recombinant G-CSF may have both direct and indirect stimulatory effects on the growth of bladder cancer cells.30 Trials on GM-CSF administration with chemotherapy for patients with cancer produced less convincing data. However, for recipients of allogeneic hematopoietic stem-cell transplantation, compared with G-CSF, prophylactic GM-CSF was associated with lower transplantation-related mortality.31 Administration of GM-CSF with chemotherapy to patients with bladder cancer could improve outcomes compared with that of G-CSF because GM-CSF enhances the activity of antitumor immune cells.
Limitations of this study include a small sample size and the fact it is a retrospective analysis. Future studies such as in vivo study or animal study, will be necessary to determine the regulatory effects of CSFs on bladder cancer and to evaluate the expression of CSF receptors and immune cells in the tumor microenvironment. Another limitation is that the specificity of three antibodies was not assessed. It is not easy to confirm the specificity because none of the blocking peptides was commercially available. The supplier has confirmed the specificity and provided the recommended staining protocol for each antibody in the datasheets.
Conclusion
High G-CSF and low GM-CSF expression in the tumor are independent predictors of a poor outcome in MIBC after RC. Levels of G-CSF and GM-CSF in the urine before treatment could be useful in prognostication.
Availability of data and materials
Please contact the authors for data requests.
Acknowledgment
This work was supported in part by Fiscal Years 2015–2016 Nara Medical University Grant-in-Aid for Collaborative Research Projects (KF and MM).
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117(2):276–282. | ||
Froehner M, Koch R, Heberling U, et al. Decreased overall and bladder cancer-specific mortality with adjuvant chemotherapy after radical cystectomy: multivariable competing risk analysis. Eur Urol. 2015;69(6):984–987. | ||
Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors. J Clin Oncol. 2001;19(10):2638–2646. | ||
Heaney ML, Toy EL, Vekeman F, et al. Comparison of hospitalization risk and associated costs among patients receiving sargramostim, filgrastim, and pegfilgrastim for chemotherapy-induced neutropenia. Cancer. 2009;115(20):4839–4848. | ||
Hidaka T, Akada S, Teranishi A, et al. Mirimostim (macrophage colony-stimulating factor; M-CSF improves chemotherapy-induced impaired natural killer cell activity, Th1/Th2 balance, and granulocyte function. Cancer Sci. 2003;94(9):814–820. | ||
Ohno R, Miyawaki S, Hatake K, et al. Human urinary macrophage colony-stimulating factor reduces the incidence and duration of febrile neutropenia and shortens the period required to finish three courses of intensive consolidation therapy in acute myeloid leukemia: a double-blind controlled study. J Clin Oncol. 1997;15(8):2954–2965. | ||
Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in neutropenia. J Immunol. 2015;195(4):1341–1349. | ||
Behnes CL, Bremmer F, Hemmerlein B, Strauss A, Strobel P, Radzun HJ. Tumor-associated macrophages are involved in tumor progression in papillary renal cell carcinoma. Virchows Arch. 2014;464(2):191–196. | ||
Kacinski BM, Scata KA, Carter D, et al. FMS (CSF-1 receptor) and CSF-1 transcripts and protein are expressed by human breast carcinomas in vivo and in vitro. Oncogene. 1991;6(6):941–952. | ||
Ramakrishnan S, Xu FJ, Brandt SJ, Niedel JE, Bast RC Jr, Brown EL. Constitutive production ofmacrophage colony-stimulating factor by human ovarian and breast cancer cell lines. J Clin Invest. 1989;83(3):921–926. | ||
Hirasawa K, Kitamura T, Oka T, Matsushita H. Bladder tumor producing granulocyte colony-stimulating factor and parathyroid hormone related protein. J Urol. 2002;167(5):2130. | ||
Nebiker CA, Han J, Eppenberger-Castori S et al. GM-CSF production by tumor cells is associated with improved survival in colorectal cancer. Clin Cancer Res. 2014;20(12):3094–3106. | ||
Kang M, Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Prognostic factors for conditional survival in patients with muscle-invasive urothelial carcinoma of the bladder treated with radical cystectomy. Sci Rep. 2015;5:12171. | ||
Hinata N, Miyake H, Miyazaki A, Nishikawa M, Tei H, Fujisawa M. Performance status as a significant prognostic predictor in patients with urothelial carcinoma of the bladder who underwent radical cystectomy. Int J Urol. 2015;22(8):742–746. | ||
Türkölmez K, Tokgöz H, Reşorlu B, Köse K, Bedük Y. Muscle-invasive bladder cancer: predictive factors and prognostic difference between primary and progressive tumors. Urology. 2007;70(3):477–481. | ||
Sejima T, Morizane S, Yao A, et al. Prognostic impact of preoperative hematological disorders and a risk stratification model in bladder cancer patients treated with radical cystectomy. Int J Urol. 2014;21:52–57. | ||
Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol. 2013;189:1275–1281. | ||
Morizawa Y, Miyake M, Shimada K, et al. Extended resection including adjacent organs and Ki-67 labeling index are prognostic factors in patients with retroperitoneal soft tissue sarcomas. World J Surg Oncol. 2016;14(1):43. | ||
Yei S, Bartholomew RM, Pezzoli P, et al. Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine. Gene Ther. 2002;9(19):1302–1311. | ||
Zhang X, Shi X, Li J, et al. A novel therapeutic vaccine of mouse GM-CSF surface modified MB49 cells against metastatic bladder cancer. J Urol. 2012;187(3):1071–1079. | ||
Morizawa Y, Miyake M, Shimada K, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol Oncol. 2016;34(6):11–17. | ||
Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc. 2010;85(7):656–663. | ||
Mizutani Y, Okada Y, Terachi T, Kakehi Y, Yoshida O. Serum granulocyte colony-stimulating factor levels in patients with urinary bladder tumor and various urological malignancies. Br J Urol. 1995;76(5):580–586. | ||
Tachibana M, Miyakawa A, Tazaki H, et al. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor-1. Cancer Res. 1995;55(15):3438–3443. | ||
Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48(7):e242. | ||
Kumari N, Agrawal U, Mishra AK, et al. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumour Biol. 2017;39(4):1–14. | ||
Barnes G, Pathak A, Schwartzberg L. G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med. 2014;3(6):1477–1484. | ||
Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167. | ||
Perez FA, Fligner CL, Yu EY. Rapid clinical deterioration and leukemoid reaction after treatment of urothelial carcinoma of the bladder: possible effect of granulocyte colony-stimulating factor. J Clin Oncol. 2009;27(34):215–217. | ||
Shameem IA, Kurisu H, Matsuyama H, Shimabukuro T, Naito K. Direct and indirect effects of recombinant human granulocyte-colony stimulating factor on in vitro colony formation of human bladder cancer cells. Cancer Immunol Immunother. 1994;38(6):353–357. | ||
Wan L, Zhang Y, Lai Y, et al. Effect of granulocyte-macrophage colony-stimulating factor on prevention and treatment of invasive fungal disease in recipients of allogeneic stem-cell transplantation: a prospective multicenter randomized phase IV trial. J Clin Oncol. 2015;33(34):3999–4006. |
Supplementary materials
We randomly extracted nine patients with low-grade non-muscle-invasive bladder cancer who underwent TURBT at the Department of Urology, Nara Medical University between 2002 and 2013. The control cohort consisted of 61 healthy volunteers (Table S1). Eight healthy controls underwent routine medical checkup once a year and revealed no evidence of malignant disease including urogenital cancer and chronic kidney disease, which could cause proteinuria.
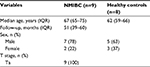  | Table S1 Clinicopathological features in patients with NMIBC and healthy controls Abbreviations: NMIBC, non-muscle-invasive bladder cancer; IQR, interquartile range. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.