Back to Journals » Journal of Blood Medicine » Volume 10
Cold agglutinin disease: current challenges and future prospects
Authors Berentsen S , Röth A , Randen U, Jilma B, Tjønnfjord GE
Received 10 January 2019
Accepted for publication 1 March 2019
Published 9 April 2019 Volume 2019:10 Pages 93—103
DOI https://doi.org/10.2147/JBM.S177621
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Sigbjørn Berentsen,1 Alexander Röth,2 Ulla Randen,3 Bernd Jilma,4 Geir E Tjønnfjord5–7
1Department of Research and Innovation, Haugesund Hospital, Haugesund, Norway; 2Department of Hematology, West German Cancer Center, University Hospital Essen, University of Duisburg-Essen, Essen, Germany; 3Department of Pathology, Akershus University Hospital, Lørenskog, Norway; 4Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria; 5Department of Haematology, Oslo University Hospital, Oslo, Norway; 6KG Jebsen’s Center for B-cell Malignancies, University of Oslo, Oslo, Norway; 7Institute of Clinical Medicine, University of Oslo, Oslo, Norway
Abstract: Cold agglutinin disease (CAD) is a complement-dependent, classical pathway-mediated immune hemolytic disease, accounting for 15–25% of autoimmune hemolytic anemia, and at the same time, a distinct clonal B-cell lymphoproliferative disorder of the bone marrow. The disease burden is often high, but not all patients require pharmacological treatment. Several therapies directed at the pathogenic B-cells are now available. Rituximab plus bendamustine or rituximab monotherapy should be considered first-line treatment, depending on individual patient characteristics. Novel treatment options that target the classical complement pathway are under development and appear very promising, and the C1s inhibitor sutimlimab is currently being investigated in two clinical Phase II and III trials. These achievements have raised new challenges and further prospects, which are discussed. Patients with CAD requiring therapy should be considered for clinical trials.
Keywords: autoimmune hemolytic anemia, cold agglutinin disease, lymphoproliferative, complement, complement inhibitors, therapy
Introduction
Over the last 15 years, considerable progress has been made in the understanding and treatment of chronic cold agglutinin disease (CAD). CAD accounts for 15–25% of cases of autoimmune hemolytic anemia (AIHA) and affects mainly elderly or middle-aged people, but has been reported in patients as young as 30.1–4 The prevalence in Northern Europe has been estimated to be 16 cases per million inhabitants and the incidence rate to be 1 per million per year, both of which are probably slight underestimations.3
CAD, also known as primary CAD, is a clonal B-cell lymphoproliferative disorder (LPD).5–8 This well-defined clinico-pathological entity should be distinguished from secondary cold agglutinin syndrome (CAS), a similar cold hemolytic syndrome that occasionally complicates specific infections (typically Mycoplasma pneumoniae pneumonia or Epstein–Barr virus infection) or malignancies (especially aggressive lymphoma).9 Only CAD will be further addressed here.
The involved autoantibodies, known as cold agglutinins (CAs), bind to their antigen at an optimum temperature of 3–4°C, but are also able to react at a higher temperature, depending on the thermal amplitude (TA). The TA is defined as the highest temperature at which the CA will bind to its antigen.10–12 CA activity is assessed by the titer, expressed as the inverse value of the highest serum dilution at which agglutination can be detected. CAs in CAD are monoclonal, usually of the immunoglobulin (Ig) Mκ class and, in the vast majority of cases, specific for the erythrocyte surface carbohydrate antigen termed I.3,12–15 Complement activation via the classical pathway is essential for subsequent hemolysis.16–21
Recent advances in CAD include the demonstration of a distinct clonal B-cell LPD, the documentation of effective B-cell-directed therapies, and the development of novel complement-directed therapies. This review will focus on these achievements, which also raise some challenges.
Clinical presentation
According to a descriptive study, the median age of patients with CAD is 76 years and the median age at presentation is 67 years (lower range, 30 years).3 In the same cohort, median hemoglobin (Hb) level was 8.9 g/dL with a lower and upper tertile of 8.0 g/dL and 10.4 g/dL, respectively. There are several reports of Hb levels as low as 4 g/dL.3,4,10,22 At the other end of the spectrum, compensated hemolysis with a normal Hb is not uncommon.3,4,22 Another large series found hemoglobinuria in 15% of the patients.4 An association between low ambient temperatures and worsening of hemolysis has been documented in individual patients.8,23 According to our data, exacerbations occur in 70% of patients during febrile infections and, at least in some cases, following major trauma or major surgery.3,24–26 Literature estimates on transfusion dependency show a high degree of variability; the two largest series of unselected patients with well-documented CAD found that 40–50% had received transfusions for a shorter or longer period of time. Cold-induced, agglutination-mediated symptoms from the capillary circulation have been recorded in up to 90% of the patients, ranging from slight acrocyanosis to disabling Raynaud phenomena.3 Less typical clinical features include gangrene, which is uncommon, and cutaneous disorders, sometimes described as livedo reticularis and sometimes as livedo racemosa.20,27,28 Such atypical vascular manifestations should warrant the exclusion of cryoglobulinemia as a differential diagnosis or a concomitant property of the cold-reactive IgM.3,27,29
Diagnostic criteria
Patients with biochemical evidence of chronic hemolysis are diagnosed with CAD if the direct antiglobulin test (DAT) is strongly positive for complement protein C3d and negative (or only weakly positive) for IgG, combined with a CA titer of ≥64.3,4,12,30 Usually, the CA titer is much higher, but it should nonetheless be acknowledged that occasional patients with otherwise typical CAD may have a CA titer <64.4,30,31 An additional diagnostic criterion is the absence of known causes of secondary CAS, for example, specific infections or aggressive lymphoma. A bone marrow low-grade clonal LPD (discussed below) can be demonstrated in a majority of patients, but confirmation of an LPD is sometimes a matter of sensitivity and not required for diagnosis.5,8,32 A spuriously high mean corpuscular volume and false reduction in red blood cell counts are regularly seen, making estimated hematocrit values unreliable, but these are not diagnostic criteria.33
Polyclonal CAs with TA <25–28°C and, mostly, in low titers are detectable in some healthy individuals, probably as remnants of a phylogenetically ancient, primitive adaptive immune system.30,34,35 In one study, CAs were found in 0.3% of a cohort with unrelated disorders.36 Subjects with detectable CA without hemolysis or circulatory symptoms do not have CAD.30,32
It is of critical importance that blood samples for CA titration, TA determination, Ig quantification, electrophoresis, and immunofixation are kept at 37–38°C from sampling to separation of serum or plasma to avoid false low values and low sensitivity (Table 1).12,30,32 Even automated cell counts and Hb levels in ethylene diamine tetraacetate (EDTA) blood are sometimes difficult to obtain because of agglutination in the tube. Prewarming at 37°C for up to 2 hrs will usually overcome this problem; if not, a short preheating at 41°C for 1 min may be tried.37
 | Table 1 Cold agglutinin disease: handling of samples |
Pathogenesis
A clonal lymphoproliferative B-cell disorder
In 1957, Christenson et al characterized the first monoclonal protein ever described, which was a CA from a patient with CAD.38 Monoclonal IgM with κ light chain restriction was recurrently found in subsequent studies.13 During the 1990s, a British group demonstrated selective usage of the IGHV4-34 gene segment, followed by the description by a Norwegian group of an abnormal bone marrow B-cell clone in a majority of patients.39,40 In a subsequent multicenter cohort of 86 Norwegian patients, a clonal LPD was found by histology in 50 (76%) of 66 bone marrow biopsy samples and by flow cytometry in 36 (90%) of 40 bone marrow aspirates.3 A retrospective single-center study from the USA confirmed a clonal disorder in 69 (78%) of 89 patients.4 More recently, an Austrian group was able to show that even patients with no electrophoretic, flow cytometric, or histologic evidence of an abnormal B-cell clone had a clonal rearrangement of their heavy or light Ig chain genes.8
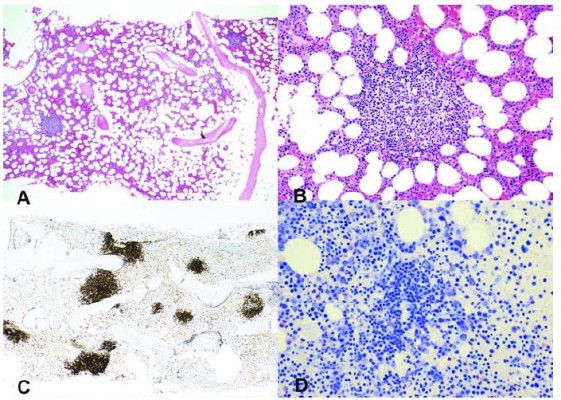
The two large retrospective studies interpreted these findings as consistent with a heterogeneous group of clonal disorders.3,4 The LPD was most often classified as lymphoplasmacytic lymphoma (LPL)/Waldenström’s macroglobulinemia (WM), followed by marginal zone lymphoma (MZL) and unclassified indolent LPD.3 In a more recent, comprehensive study, however, bone marrow samples from 54 patients with CAD were systematically re-examined by a group of lymphoma pathologists, using a standardized panel of morphologic, immune histochemical, flow cytometric, and molecular methods.5 The findings were consistent with a surprisingly homogeneous bone marrow disorder that we termed “primary CAD-associated LPD” and found to be distinct from LPL, MZL, and other previously recognized lymphoma entities (Figure 1). Nodular B-cell aggregates were found in biopsy specimens from 40 of 54 patients, whereas the bone marrow of 14 patients showed only scattered clonal B-cells. In the samples showing aggregates, median lymphoid infiltration was 10% of the intertrabecular surface (range, 5–80%). Specific features of LPL, such as paratrabecular growth, fibrosis, lymphoplasmacytoid cell morphology, or an increased number of mast cells surrounding the lymphoid aggregates, were not seen. Differences between CAD and LPL were also observed by immunohistochemical and flow cytometric analysis. It is noteworthy that the MYD88 L265P mutation, previously shown to be present in approximately 90% of cases of WM/LPL,41 could not be demonstrated in CAD-associated LPD.5 Three subsequent studies have confirmed the absence or infrequent occurrence of this mutation in CAD.6,42,43
 | Figure 1 Cold agglutinin-associated lymphoproliferative bone marrow disorder. Bone marrow trephine biopsy showing intraparenchymatous nodular lymphoid lesions (panels A and B, H&E staining, 40× and 200×, respectively). Immunoperoxidase staining for CD20 highlights intraparenchymatous nodular B-cell infiltration (panel C, 200×). Mast cells are not discerned around the nodular lymphoid lesions (panel D, Giemsa staining, 200×). Notes: Reproduced with permission from Randen U, Trøen G, Tierens A, et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99(3):497–504.5 Copyright © 2014 Ferrata Storti Foundation. |
A British flow cytometry study confirmed significant immune phenotypic differences in clonal B-cells between CAD and WM/LPL.6 In addition to the well-known, selective IGHV4-34 heavy chain gene usage in primary CAD, a recent study found a highly restricted light chain gene usage.42 Clonal rearrangement of the IGKV3-20 gene sequence was seen in 16 (59%) of 27 patients; seven of these displayed highly homologous CDR3 regions. In another study of 16 patients with well-characterized primary CAD, using flow cytometry–assisted cell sorting of bone marrow followed by whole-exome and targeted sequencing, recurrent mutations of KMT2D and CARD11 were detected in 11 (69%) and five patients (31%), respectively.7 In contrast, KMT2D mutations have been found in 26% of patients with WM/LPL and 5% of individuals with monoclonal IgM-gammopathy of undetermined significance (IgM-MGUS).44
These findings confirm that CAD is a clonal LPD of the bone marrow in probably every case. This LPD should be regarded as a distinct entity, well characterized by a total of morphological, immune histochemical, flow cytometric, and molecular genetic findings.
A complement-mediated immune hemolytic anemia
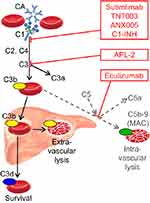
CA binds to its antigen at the erythrocyte surface during passage through the cooler parts of the peripheral circulation, causing agglutination of red blood cells and complement activation by the classical pathway.12,16,17,45,46 IgM is a potent classical pathway trigger, and the antigen-IgM complex binds complement protein C1q. C1 esterase then activates C2 and C4, generating C3 convertase which cleaves C3 into C3a and C3b. These steps are critical for the hemolytic process; if they are blocked, there is no hemolysis.19,21,47 Upon warming to 37°C in the central circulation, CA detaches from the cells, allowing agglutinated erythrocytes to separate, while C3b remains bound. C3b-opsonized cells are prone to phagocytosis by the mononuclear phagocytic system, mainly in the liver, a process known as extravascular hemolysis18,45,46 On the surface of the surviving erythrocytes, C3b is cleaved, leaving high numbers of C3d molecules which can be detected by the DAT. Because there are no C3d receptors in the mononuclear phagocyte system, these erythrocytes are protected from phagocytosis.18 This is probably part of the explanation why most patients do not develop life-threatening anemia.
In most patients with CAD, the C3b-mediated phagocytosis is considered the predominant hemolytic mechanism during steady-state disease.16,18,48 The absence, or at least modest role, of terminal pathway activation in stable patients can probably be attributed to the protective effect of CD55 and CD59, which, unlike in paroxysmal nocturnal hemoglobinuria (PNH), are intact in CAD.16,20 At least in some patients and situations, however, C3 is supposed to initiate the terminal complement cascade by binding and splitting C5, resulting in the formation of the C5b-9 complex (membrane attack complex) and intravascular hemolysis.20,49 The occurrence of hemoglobinuria in about 15% of the patients4 and the rather frequent finding of hemosiderinuria29 support this notion, as does the beneficial effect of C5 inhibition with eculizumab.20,26,49,50
Due to continuous consumption, most patients have low C3 and C4 serum levels, which is probably rate-limiting for the complement-mediated hemolysis.12,25 During febrile infections and following major surgery or major trauma, acute phase reaction will increase the production of complement proteins. The low levels are replete and complement activity is enhanced,25 which explains the exacerbations seen in a majority of CAD patients in such situations.3,24–26
In general, AIHA as well as other diseases with complement-mediated intravascular hemolysis are associated with an increased risk of venous and arterial thrombosis.51,52 Such a risk has been well documented in severely affected CAD patients,20 but it has been difficult to show this in unselected cohorts. Registry studies do indicate an increased risk, but these studies have been based on hospitals' diagnosis rather than hard diagnostic criteria and have not distinguished between CAD and CAS.53,54
Each of the two main components of the pathogenesis – clonal B-cell lymphoproliferation and classical complement pathway-dependent hemolysis – provide a rationale for therapeutic approaches.
Therapy
Watchful waiting and non-pharmacological management
The beneficial effect of keeping warm has been recognized for more than 50 years.13,30 Although the observed benefit is moderate and largely based on clinical experience, there is no reason to change existing recommendations on protection against cold exposure, in particular of the head, face, and extremities, and of avoidance of cold infusions.30,32 To avoid an acute phase reaction-induced exacerbation of hemolysis, any bacterial infection should be treated promptly.24,25 Transfusions, when indicated, can safely be given, provided the patient and the extremity used for infusion are kept warm and an in-line blood warmer is used.30,32 It may be necessary to perform the screening for irregular antibodies and crossmatching (if required) at 37°C, but the compatibility problems typical for warm-AIHA are not encountered in CAD. Transfusion of complement-rich blood products, eg, plasma, should probably be avoided as this may replete the low C4 and C3 levels and, at least in theory, enhance complement-mediated hemolysis.25
Traditionally, it has been recommended to rely on non-pharmacological management in most cases and reserve drug therapy for CAD patients with severe hemolytic anemia.10,55 Although these recommendations have been based on the poor effect of older therapies and an underestimation of the clinical symptoms, there is still no hard evidence to support pharmacological treatment in patients with only mild anemia and tolerable cold-induced circulatory symptoms. Descriptive studies have shown, however, that drug therapy has been tried in 70–80% of unselected patients, indicating that previous recommendations may have been too restrictive.3,4 Increasingly, pharmacological treatment is considered in patients with symptomatic anemia, severe cold-induced circulatory symptoms, and/or thrombosis.20,30,32,56,57
Unspecific and supportive therapies
At least in some countries, therapy with corticosteroids remains frequently used in clinical practice.51,57 No trials have been published, but retrospective series have described low response rates and heterogeneous and weak response criteria.3,4,51,57 Furthermore, the poor efficacy will often lead to the use of unacceptably high maintenance doses in the few responders.3,55 It is, therefore, widely agreed that corticosteroids should not be used to treat CAD.30,32,55,56,58,59 Other unspecific immune suppression, for example, with azathioprine, has not proved beneficial.3,60 Therapy with interferon-α was ineffective in a cohort of four patients although some conflicting evidence does exist.61–63
Based on theoretical considerations and clinical experience, folic acid supplementation has often been recommended as an adjuvant.30 In a small prospective trial, erythropoietin was seen to produce a favorable effect in all of 12 AIHA patients, five of whom had CAD.64 It is not clear whether prophylaxis against thrombosis is indicated in CAD and, if so, for which patients. Until more data have been collected, it seems reasonable to consider antithrombotic prophylaxis in the presence of additional risk factors, severe intravascular hemolysis, or acute exacerbation until the hemolytic process is under control.
B-cell-directed therapies
The first treatment shown to be effective in CAD was rituximab monotherapy, 375 mg/m2 once a week for four weeks.65–67 A Norwegian prospective uncontrolled trial described 37 courses of therapy in 27 patients, using strict response criteria.66 The overall response (OR) rate was 54% and the responders achieved a median increase in Hb levels of 4.0 g/dL. There was only one complete response (CR), and the median time to response (TTR) was 1.5 months. The median response duration was modest (11 months), but six of 10 relapsed patients responded to re-treatment. The study drug was well tolerated. A subsequent Danish prospective study reported similar results.67 Rituximab and fludarabine in combination, investigated in a prospective trial in 2010, resulted in a 76% OR rate with 20% CR, 56% partial responses (PR), and an estimated median response duration of more than 66 months, however with some toxicity.68
In 2017, a Nordic group published a prospective, non-randomized study of rituximab (375 mg/m2 day 1) plus bendamustine (90 mg/m2 day 1 and 2) for four cycles at 28-day interval in 45 patients.22 The OR rate was 71% and the CR rate 40%, with a median Hb increase of 4.4 g/dL in the CR group and 3.9 g/dL among those who achieved PR. The observed response duration was long (less than 10% relapses after a median of 32-month observation). Median TTR was 1.9 months (upper range, 12 months) with an even longer time to optimal response in some patients. The toxicity profile was favorable as compared with the rituximab plus fludarabine regimen; neutropenia grade 3–4 was seen in 33% of the patients; however, only 11% experienced infection with or without neutropenia.
In a recent Italian prospective trial, six of 19 patients responded to bortezomib monotherapy, administered as a single cycle.69 Although this response rate may seem low, the results should be considered a “proof of principle,” and an extended duration and/or bortezomib-based combination therapy may yield higher response rates.
In an early report, four patients treated with chlorambucil achieved a reduction in CA titer and IgM serum concentration, but no significant increase in Hb levels.40,70 Another study described five patients who prospectively received cladribine monotherapy, none of whom responded.71 There is a strong theoretic rationale for the novel B-cell targeted therapies, of which ibrutinib has become an established treatment for WM/LPL, showing favorable results even in MYD88 L265P-negative cases.72,73 However, there are no published studies regarding the use of these drugs in CAD.
Complement-directed therapies
Complement modulation for treatment of CAD was first described in 2009 and is undergoing rapid development (Figure 2).16,49,74 The anti-C5 monoclonal antibody, eculizumab, has proved able to inhibit intravascular hemolysis and reduce lactate dehydrogenase levels and transfusion requirements in a prospective study of 13, mostly transfusion-dependent patients with CA-mediated hemolytic anemia (12 CAD and one severe CAS).20 Only a marginal increase in Hb level was observed. This is not unexpected, as the C5 inhibition will not affect the C3b-mediated, extravascular hemolysis. Eculizumab has also proved useful as a rapidly acting rescue therapy for severe CAD/CAS and, according to a case report, as prophylaxis against cardiac surgery-induced exacerbation in CAD.20,26,75,76
Classical pathway inhibition would be expected to do better. The first available drug for blocking the classical pathway was plasma-derived C1 esterase inhibitor (C1-INH). Although approved only for hereditary angioedema, which is not a complement-mediated disorder, pharmacological doses of C1-INH were shown to stop complement activation and hemolysis and efficiently improve anemia when used as rescue therapy in a patient with a severe, IgM-mediated warm-AIHA.77,78 Recently, a similar effect was observed in a patient with acute, severe CAS.21 However, levels of endogenous C1-INH are normal in CAD and CAS; frequently repeated high doses are likely to be required to maintain the effect, and, therefore, C1-INH is probably not attractive as a long-term therapy.
Sutimlimab (previously known as TNT009 or BIVV009) is a humanized monoclonal antibody against C1s.79 An in vitro study of TNT003, the murine antibody from which sutimlimab is derived, found complete inhibition of complement activation, C3 deposition, and phagocytosis of erythrocytes in the presence of patient sera as a source of CA and normal human serum as a source of complement.19 In a recent clinical Phase IB trial describing 10 patients with CAD, weekly intravenous administration of sutimlimab increased Hb levels by a median of 1.6 g/dL within the first week of treatment and by 3.9 g/dL within 6 weeks.80 The anti-C1s antibody immediately abrogated extravascular hemolysis, normalizing bilirubin levels within 24 hrs in most patients, and all of six previously transfusion-dependent patients became transfusion-free. Pharmacodynamics and pharmacokinetics were favorable. Hemolytic anemia recurred 3–4 weeks after discontinuation, but re-introduction of sutimlimab restored the control. Drug-related adverse events were not observed. The patients were vaccinated against Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae.80 Sutimlimab is now undergoing Phase II and III trials in patients with CAD (ClinicalTrials.gov, NCT03347396 and NCT03347422).
In vitro experiments have shown that ANX005, a humanized monoclonal antibody that inhibits C1q, prevents C4 and C3 deposition on human erythrocytes exposed to CAD sera, resulting in a dose-dependent reduction in hemolysis.81,82 A safety trial in healthy volunteers has just been completed but has not yet been published (ClinicalTrials.gov, NCT03010046).
APL-2 is a compstatin analog, a pegylated cyclic peptide that inhibits C3 and thereby prevents the formation of C3b.83,84 Thus, APL-2, which is developed for subcutaneous administration, has the potential to block the entire complement system. A safety study in healthy volunteers has not shown a risk of infection thus far, but the experience is limited and the participants were vaccinated.83 High efficacy and safety have recently been reported in a Phase IB trial in PNH.85 APL-2 is currently being studied in a Phase II trial comprising patients with AIHA, including CAD (ClinicalTrials.gov, NCT03226678).
Challenges and future perspectives
Concepts and definitions
Definitions found in the literature are not uniform, which may create some confusion.30,32,34 The terms CAD (or cold hemagglutinin disease, CHAD) and CAS (or cold hemagglutinin syndrome, CHAS) have previously been used interchangeably, but these entities should be distinguished and defined as explained in the “Introduction“ section.9,20,56 The criteria for CR and PR used in this review are identical to those applied in most prospective trials of B-cell-directed therapies for CAD,22,66,68,71 but these response definitions are not applicable to treatment with complement inhibitors.16,20,80 Literature- or consensus-based definitions would be appreciated.
Diagnosis and awareness
In 1970, only 13 patients had been diagnosed with CAD in the whole population of Denmark, which was then 4.93 million inhabitants.70 More recent Nordic population-based estimates suggest a prevalence of 12–16 cases per million inhabitants.3,54 These data, together with a discrepancy between prevalences found even in recent studies, may indicate that CAD is still underdiagnosed.3,54,86 Misdiagnosis has therapeutic consequences because different types of AHIA should be treated differently. Furthermore, even in large centers, CA titration, Ig quantification, and electrophoresis may yield misleading low values or even false negative results due to incorrect handling of blood samples. Knowledge of the clinical features seen in many patients may help increase doctors’ awareness and facilitate correct diagnosis, as may the correct handling of samples (Table 1).
Descriptive studies
In order to improve knowledge of epidemiology as well as clinical and pathogenetic features, we should recognize the strengths and weaknesses of different methodologies used in descriptive studies of CAD. Good single institution or multicenter studies have the advantage of exact diagnostic criteria, whereas it may be difficult to design such studies to be population-based and selection bias may occur.3,4,34 This issue might be avoided by performing registry-based studies, but until now, these studies have utilized insurance-based or hospital diagnosis registries.53,54,57 Therefore, patient inclusion has relied on doctors’ or hospitals’ diagnoses rather than well-defined diagnostic criteria. The conflicting data on mortality and frequency of thromboembolic incidents are typical examples that highlight these methodology issues. Establishing an international CAD patient registry would probably help overcome these problems.
Evidence-based therapy for CAD: a challenge?
Because CAD is a rare disease, it is difficult to design and conduct randomized studies with sufficient statistical power. Even uncontrolled prospective trials and large retrospective series are relatively few, and recommendations found in the literature have often been based on pooled case reports or very small cohorts.
Papers published between 1998 and 2003 illustrate the problem. Rituximab monotherapy was first described during this period, and several single-case observations were published.65,87–89 A small, prospective trial was reported in 2001;90 two Phase II trials appeared in 2004–2006,66,67 and a larger, retrospective series was published in 2006.3 Altogether, of the 23 cases reported by 2003, responses had been observed in 21 patients (91%);91,92 the two non-responders were described in 2001 in the only prospective cohort.90 In the three larger studies (2004–2006), however, OR rates were between 45% and 58%.66,67 Remissions were often classified as CR in the case reports, whereas the systematic studies show that CR following rituximab monotherapy is an uncommon event. The obvious explanation for these discrepancies is the well-known fact that response rates calculated from pooled case reports are likely to be influenced by publication bias and heterogeneous or poorly defined response criteria.
Several publications have shown that it is possible to perform prospective trials using a multicenter approach.20,22,66–69,71,80 As spontaneous remissions of chronic CAD are rare,3,4 a given therapy modality can be considered evidence-based if supported by good non-randomized trials. Recent experience indicates that even randomized trials are feasible when performed on an international scale (ClinicalTrials.gov, NCT03347396 and NCT03347422). We should not accept response data derived from single or pooled case reports as a basis for treatment recommendations.
Due to these unmet needs, patients with CAD requiring treatment should preferably be included in prospective trials whenever possible.
Implementation of achievements
Many patients with CAD requiring therapy are still being treated with corticosteroids. When reading case reports and descriptive studies, we appreciate that clinicians should be more familiar with the existing B-cell-directed therapies in CAD. Although such therapy options should be considered even when no B-cell clone has been demonstrated,8,32 the consequent use of relevant diagnostic procedures (serum electrophoresis, immunofixation, bone marrow flow cytometry, and biopsy) in all patients with AIHA would help to reveal the clonal nature of CAD in a higher proportion of patients in clinical practice.5,59
Perspectives on B-cell-directed therapies
Rituximab plus bendamustine combination therapy has proved safe and efficacious and may be used in the first line for severely affected patients without major comorbidities.22,32 For other patients requiring treatment, rituximab monotherapy should be preferred. Like all other therapies described here, these drugs are off-label for CAD in most countries. Several options are available in the second-line setting: rituximab plus bendamustine (if not used in the first line or if the patient has enjoyed remission for more than a couple of years after having received the same therapy); repeat rituximab monotherapy in relapsed patients; bortezomib-based therapy; and rituximab plus fludarabine in selected, fit elderly patients.30,32 Despite this, we should aim at developing less toxic and, if possible, still more efficacious therapies. The safety and efficacy of novel targeted B-cell-suppressive agents, such as ibrutinib, second-generation Bruton tyrosine kinase inhibitors, and venetoclax should be explored.72 It would also be worthwhile to study proteasome inhibitor–based combination therapies, hoping to improve on response rates achieved by bortezomib monotherapy.69
Future perspectives on complement modulation
nhibition of the classical complement pathway in CAD is still investigational but very promising, and, currently, the most developed option is therapy with sutimlimab. Further trials and experience can be expected to clarify any remaining safety issues, but until now, studies have not shown a risk of severe infection provided the patients are properly vaccinated.80 Likewise, a theoretical risk of developing systemic lupus erythematosus as a consequence of proximal complement inhibition has not been confirmed.80,93
However, complement modulation will not improve the cold-induced circulatory symptoms, which are caused by agglutination and not by complement activation.9 The novel complement inhibitors will probably be expensive. It may also be argued that complement inhibition will have to be continued indefinitely to maintain its effect, in contrast to B-cell-directed therapies, which are temporary.22 On the other hand, at least 25% of the patients will fail on chemoimmunotherapy, and some patients have contraindications to such treatment or are reluctant to receive cytotoxic drugs. Furthermore, rapidly acting therapies will be required in the most severe cases and in some patients with acute exacerbation, whereas the TTR can be long following chemoimmunotherapy.20,22 In such patients, proximal complement inhibition will possibly be used as a “bridge” that can allow the transition to B-cell-directed therapy when remission has been achieved and the condition is stabilized .75,80,94
Conclusion
“Primary” CAD is a complement-driven AIHA and, at the same time, a clonal B-cell LPD of the bone marrow. The disease burden is often high, but not all patients require treatment. For those who do, several B-cell-directed therapeutic options are available. Rituximab plus bendamustine or rituximab monotherapy may be used in the first line, depending on patient characteristics. The novel complement-directed therapies are still investigational but very promising, and the monoclonal anti-C1s antibody, sutimlimab, has entered clinical Phase II and III trials. Patients with CAD requiring treatment should be included in prospective trials whenever possible.
Acknowledgment
We thank Sarah Ely for review of the language.
Disclosure
S Berentsen has received research support from Mundipharma, travel support from Alexion and Apellis, lecture honoraria from Alexion, Bioverativ, and Janssen-Cilag, and has consulted for Apellis, Bioverativ, Momenta Pharmaceuticals, and True North Therapeutics, outside the submitted work. A Röth has received research support from Alexion and Roche, travel support from Alexion and AbbVie, lecture honoraria from Alexion, Roche, and Novartis, and has consulted for Alexion, Bioverativ, Novartis, and True North Therapeutics, outside the submitted work. U Randen reports no conflicts of interest. B Jilma has received reimbursement for travel for presentations and scientific advice from True North Therapeutics (a Bioverativ Company), outside the submitted work. GE Tjønnfjord has received research support from Mundipharma, Janssen-Cilag and Alexion Pharma, and lecture honoraria from Janssen-Cilag, Alexion Pharma, and Roche Pharma, outside the submitted work.
References
1. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4(6):607–618. doi:10.1586/ehm.11.60
2. Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre. Br Med J (Clin Res Ed). 1981;282(6281):2023–2027.
3. Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466.
4. Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114–1121. doi:10.1182/blood-2013-02-474437
5. Randen U, Trøen G, Tierens A, et al. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99(3):497–504. doi:10.3324/haematol.2013.091702
6. de Tute R, Rawstron A, Evans P, Owen R. Cold agglutinin disease is a phenotypically distinct clonal B-cell disorder. 15 International Myeloma Workshop, Rome, Italy: sept 23–26, 2015. Clin Lymphoma Myeloma Leuk. 2015;15:e184.
7. Malecka A, Trøen G, Tierens A, et al. Frequent somatic mutations of KMT2D (MLL2) and CARD11 genes in primary cold agglutinin disease. Br J Haematol. 2018;183(5):838–842. doi:10.1111/bjh.15063
8. Arthold C, Skrabs C, Mitterbauer-Hohendanner G, et al. Cold antibody autoimmune hemolytic anemia and lymphoproliferative disorders: a retrospective study of 20 patients including clinical, hematological, and molecular findings. Wien Klin Wochenschr. 2014;126(11–12):376–382. doi:10.1007/s00508-014-0547-z
9. Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29(3):455–471. doi:10.1016/j.hoc.2015.01.002
10. Schubothe H. The cold hemagglutinin disease. Semin Hematol. 1966;3(1):27–47.
11. Rosse WF, Adams JP. The variability of hemolysis in the cold agglutinin syndrome. Blood. 1980;56(3):409–416.
12. Ulvestad E, Berentsen S, Bo K, Shammas FV. Clinical immunology of chronic cold agglutinin disease. Eur J Haematol. 1999;63(4):259–266.
13. Harboe M, van Furth R, Schubothe H, Lind K, Evans RS. Exclusive occurrence of K chains in isolated cold haemagglutinins. Scand J Haematol. 1965;2(3):259–266.
14. Stone MJ, McElroy YG, Pestronk A, Reynolds JL, Newman JT, Tong AW. Human monoclonal macroglobulins with antibody activity. Semin Oncol. 2003;30(2):318–324. doi:10.1053/sonc.2003.50077
15. Issitt PD. I blood group system and its relationship to disease. J Med Lab Technol. 1968;25(1):1–6.
16. Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141–149. doi:10.1053/j.seminhematol.2018.04.002
17. Jonsen J, Kåss E, Harboe M. Complement and complement components in acquired hemolytic anemia with high titer cold antibodies. Acta Med Scand. 1961;170:725–729.
18. Jaffe CJ, Atkinson JP, Frank MM. The role of complement in the clearance of cold agglutinin-sensitized erythrocytes in man. J Clin Invest. 1976;58(4):942–949. doi:10.1172/JCI108547
19. Shi J, Rose EL, Singh A, et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123(26):4015–4022. doi:10.1182/blood-2014-02-556027
20. Röth A, Bommer M, Hüttmann A, et al. Eculizumab in cold agglutinin disease (DECADE): an open-label, prospective, bicentric, nonrandomized phase 2 trial. Blood Adv. 2018;2(19):2543–2549. doi:10.1182/bloodadvances.2018024190
21. Tesfaye A, Broome C. A novel approach for treatment of cold agglutinin syndrome-related severe hemolysis. J Hematol. 2016;5(1):30–33. doi:10.14740/jh242w
22. Berentsen S, Randen U, Oksman M, et al. Bendamustine plus rituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood. 2017;130(4):537–541. doi:10.1182/blood-2017-04-778175
23. Lyckholm LJ, Edmond MB. Images in clinical medicine. Seasonal hemolysis due to cold-agglutinin syndrome. N Engl J Med. 1996;334(7):437. doi:10.1056/NEJM199606133342404
24. Ulvestad E. Paradoxical haemolysis in a patient with cold agglutinin disease. Eur J Haematol. 1998;60(2):93–100.
25. Ulvestad E, Berentsen S, Mollnes TE. Acute phase haemolysis in chronic cold agglutinin disease. Scand J Immunol. 2001;54(1–2):239–242.
26. Tjønnfjord E, Vengen OA, Berentsen S, Tjønnfjord GE. Prophylactic use of eculizumab during surgery in chronic cold agglutinin disease. BMJ Case Rep. 2017. doi:10.1136/bcr-2016-219066
27. Rørvik K. The syndrome of high-titre cold haemagglutination; a survey and a case report. Acta Med Scand. 1954;148(4):299–308.
28. Shiiya C, Ota M. Cold agglutinin disease presenting as livedo racemosa. Cmaj. 2017;189(22):E781. doi:10.1503/cmaj.171389
29. Stone MJ, Berentsen S. Hyperviscosity syndrome, cold agglutinin hemolytic anemia, and cryoglobulinemia. In: Leblond V, Treon SP, Dimopoulos M, editors. Waldenström’s Macroglobulinemia. Switzerland: Springer International Publishing; 2017:171–183.
30. Hill QA, Stamps R, Massey E, et al. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176(3):395–411. doi:10.1111/bjh.14478
31. Bendix BJ, Tauscher CD, Bryant SC, Stubbs JR, Jacob EK. Defining a reference range for cold agglutinin titers. Transfusion. 2014;54(5):1294–1297. doi:10.1111/trf.12453
32. Berentsen S. How I manage patients with cold agglutinin disease. Br J Haematol. 2018;181(3):320–330. doi:10.1111/bjh.15109
33. Bessman JD, Banks D. Spurious macrocytosis, a common clue to erythrocyte cold agglutinins. Am J Clin Pathol. 1980;74(6):797–800.
34. Dacie J. Auto-immune haemolytic anaemia (AIHA): cold-antibody syndromes I: idiopathic types: clinical presentation and haematological and serological findings. In: Dacie J, editor. The Haemolytic Anaemias. Vol. 3. London: Churchill Livingstone; 1992:210–239.
35. Litman GW. Sharks and the origins of vertebrate immunity. Sci Am. 1996;275(5):67–71.
36. Jain MD, Cabrerizo-Sanchez R, Karkouti K, Yau T, Pendergrast JM, Cserti-Gazdewich CM. Seek and you shall find–but then what do you do? Cold agglutinins in cardiopulmonary bypass and a single-center experience with cold agglutinin screening before cardiac surgery. Transfus Med Rev. 2013;27(2):65–73. doi:10.1016/j.tmrv.2012.12.001
37. La Gioia A, Fumi M, Fiorini F, et al. Short preheating at 41 degrees C leads to a red blood cells count comparable to that in RET channel of Sysmex analysers in samples showing cold agglutination. J Clin Pathol. 2018;71(8):729–734. doi:10.1136/jclinpath-2017-204954
38. Christenson WN, Dacie JV, Croucher BE, Charlwood PA. Electrophoretic studies on sera containing high-titre cold haemagglutinins: identification of the antibody as the cause of an abnormal gamma 1 peak. Br J Haematol. 1957;3(3):262–275.
39. Pascual V, Victor K, Spellerberg M, Hamblin TJ, Stevenson FK, Capra JD. VH restriction among human cold agglutinins. The VH4-21 gene segment is required to encode anti-I and anti-i specificities. J Immunol. 1992;149(7):2337–2344.
40. Berentsen S, Bø K, Shammas FV, Myking AO, Ulvestad E. Chronic cold agglutinin disease of the “idiopathic” type is a premalignant or low-grade malignant lymphoproliferative disease. APMIS. 1997;105(5):354–362.
41. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi:10.1056/NEJMoa1200710
42. Malecka A, Trøen G, Tierens A, et al. Immunoglobulin heavy and light chain gene features are correlated with primary cold agglutinin disease onset and activity. Haematologica. 2016;101(9):e361–364. doi:10.3324/haematol.2016.146126
43. Cao XX, Meng Q, Cai H, et al. Detection of MYD88 L265P and WHIM-like CXCR4 mutation in patients with IgM monoclonal gammopathy related disease. Ann Hematol. 2017. doi:10.1007/s00277-017-2968-z
44. Varettoni M, Zibellini S, Defrancesco I, et al. Pattern of somatic mutations in patients with Waldenstrom macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica. 2017;102(12):2077–2085. doi:10.3324/haematol.2017.172718
45. Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest. 1972;51(3):575–582. doi:10.1172/JCI106846
46. Schreiber AD, Frank MM. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Invest. 1972;51(3):583–589. doi:10.1172/JCI106847
47. Jäger U, Gilbert JC, Panicker S, et al. The anti C1s complement antibody TNT009 induces rapid complete remissions of anaemia in patients with primary cold agglutinin disease.
48. Baines AC, Brodsky RA. Complementopathies. Blood Rev. 2017;31(4):213–223. doi:10.1016/j.blre.2017.02.003
49. Röth A, Hüttmann A, Rother RP, Dührsen U, Philipp T. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113(16):3885–3886. doi:10.1182/blood-2009-01-196329
50. Gupta N, Wang ES. Long-term response of refractory primary cold agglutinin disease to eculizumab therapy. Ann Hematol. 2014;93(2):343–344. doi:10.1007/s00277-013-1800-7
51. Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. doi:10.1182/blood-2014-06-583021
52. Yusuf HR, Hooper WC, Grosse SD, Parker CS, Boulet SL, Ortel TL. Risk of venous thromboembolism occurrence among adults with selected autoimmune diseases: a study among a U.S. cohort of commercial insurance enrollees. Thromb Res. 2015;135(1):50–57. doi:10.1016/j.thromres.2014.10.012
53. Broome C, Cunningham JM, Mullins M, et al. Incidence of thromboembolic events is increased in a retrospective analysis of a large cold agglutinin disease (CAD) Cohort. 59th annual meeting of the American Society of Hematology, Atlanta, GA. Blood. 2017;130:928.
54. Bylsma L, Ording AG, Frøslev T, et al. The occurrence and survival of cold agglutinin disease in Denmark. 23rd congress of the Eurpopean Hematology Association, Stockholm, June 2018. HemaSphere. 2018;2(S1):513.
55. Dacie J. Treatment and prognosis of cold-antibody AIHA. In: Dacie J, editor. The Haemolytic Anaemias. Vol. 3. London: Churchill Livingstone; 1992:502–520.
56. Michel M, Jäger U. Autoimmune hemolytic anemia. In: Hoffman R, Benz EJ, Silberstein LE, Heslop H, Weitz J, Anastasi J, editors. Hematology: Basic Principles and Practice.
57. Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839–848. doi:10.1182/bloodadvances.2017004390
58. Gertz MA. Management of cold haemolytic syndrome. Br J Haematol. 2007;138(4):422–429. doi:10.1111/j.1365-2141.2007.06664.x
59. Barcellini W, Fattizzo B, Zaninoni A. Current and emerging treatment options for autoimmune hemolytic anemia. Expert Rev Clin Immunol. 2018;14(10):857–872. doi:10.1080/1744666X.2018.1521722
60. Worlledge SM, Brain MC, Cooper AC, Hobbs JR, Dacie JV. Immmunosuppressive drugs in the treatment of autoimmune haemolytic anaemia. Proc R Soc Med. 1968;61(12):1312–1315.
61. Hillen HF, Bakker SJ. Failure of interferon-alpha-2b therapy in chronic cold agglutinin disease. Eur J Haematol. 1994;53(4):242–243.
62. O’Connor BM, Clifford JS, Lawrence WD, Logue GL. Alpha-interferon for severe cold agglutinin disease. Ann Intern Med. 1989;111(3):255–256.
63. Rordorf R, Barth A, Nydegger U, Tobler A. Treatment of severe idiopathic cold-agglutinin diseases using interferon-alpha 2b. Schweiz Med Wochenschr. 1994;124(1–2):56–61.
64. Salama A, Hartnack D, Lindemann HW, Lange HJ, Rummel M, Loew A. The effect of erythropoiesis-stimulating agents in patients with therapy-refractory autoimmune hemolytic anemia. Transfus Med Hemother. 2014;41(6):462–468. doi:10.1159/000366244
65. Lee EJ, Kueck B. Rituxan in the treatment of cold agglutinin disease. Blood. 1998;92(9):3490–3491.
66. Berentsen S, Ulvestad E, Gjertsen BT, et al. Rituximab for primary chronic cold agglutinin disease: a prospective study of 37 courses of therapy in 27 patients. Blood. 2004;103(8):2925–2928. doi:10.1182/blood-2003-10-3597
67. Schöllkopf C, Kjeldsen L, Bjerrum OW, et al. Rituximab in chronic cold agglutinin disease: a prospective study of 20 patients. Leuk Lymphoma. 2006;47(2):253–260. doi:10.1080/10428190500286481
68. Berentsen S, Randen U, Vågan AM, et al. High response rate and durable remissions following fludarabine and rituximab combination therapy for chronic cold agglutinin disease. Blood. 2010;116(17):3180–3184. doi:10.1182/blood-2010-06-288647
69. Rossi G, Gramegna D, Paoloni F, et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood. 2018;132(5):547–550. doi:10.1182/blood-2018-03-835413
70. Hippe E, Jensen KB, Olesen H, Lind K, Thomsen PE. Chlorambucil treatment of patients with cold agglutinin syndrome. Blood. 1970;35(1):68–72.
71. Berentsen S, Tjønnfjord GE, Shammas FV, et al. No response to cladribine in five patients with chronic cold agglutinin disease. Eur J Haematol. 2000;65(1):88–90.
72. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–1440. doi:10.1056/NEJMoa1501548
73. Abeykoon JP, Paludo J, King RL, et al. MYD88 mutation status does not impact overall survival in Waldenstrom macroglobulinemia. Am J Hematol. 2018;93(2):187–194. doi:10.1002/ajh.24955
74. Risitano AM. Current and future pharmacologic complement inhibitors. Hematol Oncol Clin North Am. 2015;29(3):561–582. doi:10.1016/j.hoc.2015.01.009
75. Shapiro R, Chin-Yee I, Lam S. Eculizumab as a bridge to immunosuppressive therapy in severe cold agglutinin disease of anti-Pr specificity. Clin Case Rep. 2015;3(11):942–944. doi:10.1002/ccr3.399
76. Makishima K, Obara N, Ishitsuka K, et al. High efficacy of eculizumab treatment for fulminant hemolytic anemia in primary cold agglutinin disease. Ann Hematol. 2018 Oct 15. doi: 10.1007/s00277-018-3521-4. [Epub ahead of print]
77. Wouters D, Stephan F, Strengers P, et al. C1-esterase inhibitor concentrate rescues erythrocytes from complement-mediated destruction in autoimmune hemolytic anemia. Blood. 2013;121(7):1242–1244. doi:10.1182/blood-2012-11-467209
78. Wouters D, Zeerleder S. Complement inhibitors to treat IgM-mediated autoimmune hemolysis. Haematologica. 2015;100(11):1388–1395. doi:10.3324/haematol.2015.128538
79. Bartko J, Schoergenhofer C, Schwameis M, et al. A randomized, first-in-human, healthy volunteer trial of sutimlimab, a humanized antibody for the specific inhibition of the classical complement pathway. Clin Pharmacol Ther. 2018;104(4):655–663. doi:10.1002/cpt.1111
80. Jäger U, D’Sa S, Schörgenhofer C, et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood. 2019;133(9):893–901. doi:10.1182/blood-2018-06-856930
81. Gertz MA, Qiu H, Kendall L, Saltarelli M, Yednock T, Sankaranarayanan S. ANX005, an inhibitory antibody against C1q, blocks complement activation triggered by cold agglutinins in human disease. 58th meeting of the American Society of Hematology, San Diego, CA. Blood. 2016;128:1265. doi:10.1182/blood-2016-06-724161
82. Lansita JA, Mease KM, Qiu H, Yednock T, Sankaranarayanan S, Kramer S. Nonclinical development of ANX005: a humanized anti-C1q antibody for treatment of autoimmune and neurodegenerative diseases. Int J Toxicol. 2017;36(6):449–462. doi:10.1177/1091581817740873
83. Grossi FV, Bedwell P, Deschatelets P, et al. APL-2, a complement C3 inhibitor for the potential treatment of paroxysmal nocturnal hemoglobinuria (PNH): phase I data from two completed studies in healthy volunteers. 58th annual meeting of the American Society of Hematology, San Diego, CA. Blood. 2016;128:1251. doi:10.1182/blood-2016-06-724161
84. Risitano AM, Ricklin D, Huang Y, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123(13):2094–2101. doi:10.1182/blood-2013-11-536573
85. Wong RSM, Pullon RWH, Deschatelets P, et al. Inhibition of C3 with APL-2 results in normalisation of markers of intravascular and extravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH). 60th annual meeting of the American Society of Hematology, San Diego, CA. Blood. 2018;132:2314.
86. Hadnagy C. Agewise distribution of idiopathic cold agglutinin disease. Z Gerontol. 1993;26(3):199–201.
87. Sparling TG, Andricevic M, Wass H. Remission of cold hemagglutinin disease induced by rituximab therapy. Cmaj. 2001;164(10):1405.
88. Engelhardt M, Jakob A, Ruter B, Trepel M, Hirsch F, Lubbert M. Severe cold hemagglutinin disease (CHD) successfully treated with rituximab. Blood. 2002;100(5):1922–1923.
89. Gharib M, Poynton C. Complete, long-term remission of refractory idiopathic cold haemagglutinin disease after Mabthera. Br J Haematol. 2002;117(1):248–249.
90. Berentsen S, Tjønnfjord GE, Brudevold R, et al. Favourable response to therapy with the anti-CD20 monoclonal antibody rituximab in primary chronic cold agglutinin disease. Br J Haematol. 2001;115(1):79–83.
91. Finazzi G. Rituximab in autoimmune cytopenias: for which patients? Haematologica. 2002;87(2):113–114.
92. Camou F, Viallard JF, Pellegrin JL. Rituximab in cold agglutinin disease. Rev Med Interne. 2003;24(8):501–504.
93. Aggarwal R, Sestak AL, D’Souza A, Dillon SP, Namjou B, Scofield RH. Complete complement deficiency in a large cohort of familial systemic lupus erythematosus. Lupus. 2010;19(1):52–57. doi:10.1177/0961203309346508
94. Berentsen S. Cold agglutinins: fending off the attack. Blood. 2019;133(9):885–886. doi:10.1182/blood-2019-01-894303
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.