Back to Journals » International Journal of General Medicine » Volume 14
COL2A1 Mutation (c.611G>C) Leads to Early-Onset Osteoarthritis in a Chinese Family
Authors Li P , Wang A, Li J, Li X, Sun W, Liu Q
Received 8 March 2021
Accepted for publication 12 May 2021
Published 16 June 2021 Volume 2021:14 Pages 2569—2574
DOI https://doi.org/10.2147/IJGM.S310050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Pengyu Li,1,2 Anran Wang,2 Jiangxia Li,2 Xi Li,2 Wenjie Sun,2 Qiji Liu2
1Department of Interventional Radiology and Vascular Surgery, Peking University First Hospital, Beijing, People’s Republic of China; 2Department of Medical Genetics, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
Correspondence: Qiji Liu
Department of Medical Genetics, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, People’s Republic of China
Email [email protected]
Abstract: Mutations in the gene coding collagen type II α 1 chain (COL2A1) are associated with a series of human disorders mainly involving the skeletal system. Here, we describe the second family with COL2A1 mutation, c.611G>C, Gly204Ala, leading to a replacement of glycine in the core triple helical (Gly-X-Y) domain of COL2A1 gene. The replacements of glycine in every third position of the triple with other amino acids will cause failure in the structure of type II collagen. The affected family members manifested early-onset osteoarthritis involving multiple joints. We propose that the COL2A1 gene should be taken into consideration for genetic counseling for patients with hereditary premature osteoarthritis and individuals carrying this mutation should receive early interventions for osteoarthritis.
Keywords: COL2A1, mutation, early-onset osteoarthritis
Introduction
Type II collagen is the predominant structural collagen of hyaline cartilage as well as intervertebral disc nucleus pulposus, and a critical component of vitreous humor.1,2 It is characterized by three indispensable homologous α1-peptide chains which are encoded by procollagen type II gene (COL2A1), located at 12q13.3 Because of the abundance of type II collagen in articular cartilage, molecular defects in COL2A1 lead to type II collagenopathies which involve a wide spectrum of phenotypes of skeletal dysplasia ranging from perinatally lethal mutations such as lethal achondrogenesis type II/hypochondrogenesis (OMIM 200610) and platyspondylic lethal skeletal dysplasia (OMIM 151210), to severe diseases related to short-trunk dwarfism, such as spondyloepiphyseal dysplasia congenital (SEDC; OMIM 183900), to disorders presenting with premature osteoarthritis, such as Stickler dysplasia (OMIM 108300), and disorders involving the femoral head such as avascular necrosis of femoral head (ANFH; OMIM 608805) and Legg-Calvé-Perthes disease (LCPD; OMIM 150600).4,5
Early-onset osteoarthritis due to defects in COL2A1 has been reported to be related with a series of mutations.6,7 A heterozygous missense variant in COL2A1 (NM_001844.4: c.611G>C, Gly204Ala) leading to a glycine-alanine substitution at position 204 was reported to be associated with early-onset arthritis involving multiple joints in an Australian family in 2010.8 Here we describe another Chinese family and 13 patients with the same variant (c.611G>C) who presented similar symptoms. Our findings not only further add to proof of the specific phenotype caused by this mutation, but indicate that this site might be a potential mutational hotspot in the COL2A1 gene.
Case
Ethics Statement
This study was approved by the Medical Ethics Committees of Shandong University, China. Written informed consent according to the Declaration of Helsinki was obtained from all participating individuals.
Case Presentation and Pedigree Analysis
The proband, a 37-year-old male (III-10) was diagnosed with osteoarthritis of hip joint in a local hospital together with his father, a 65-year-old male (II-5) who had similar symptoms. They came to Qilu hospital (Jinan, China) for genetic counseling with regard to bone disease. Detailed investigation of medical history and physical and laboratory examinations were performed to obtain comprehensive information. The chief complaints of the proband were characterized by groin pain, limping, limited movement of bilateral hip joints and walking distance. The proband first reported post-exercise pain in left hip at age 25 years, after two years or so, pain generalized in bilateral hip joints. Physical examination also revealed restricted neck flexion and elbow extension.
The proband’s father (II-5) presented with hip joint pain in his twenties and physical examination showed stiffness and limited motion in multiple joints including hip, neck, elbow, shoulder, wrist and hand. Though having suffered pain for more than 30 years, patient II-5 had never received regular medication nor surgery. He explained that his pain was endurable and significantly relieved after rest. No obvious abnormalities in limbs were found and their facial features were normal. Neurological examinations revealed no significant findings. The results of all the laboratory tests including rheumatology were within normal ranges.
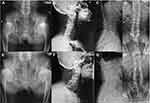
We offered a thorough radiological examination but the two patients only consented to take X-rays for hip joint and spine. Illustrative radiographs of the two patients (II-5 and III-10) are shown in Figure 1. Radiographic findings showed significant osteoarthritic changes in bilateral hip joints and cervical spine: joint spaces narrowing, osteophytes formation and osteosclerosis. Degenerative changes were shown in lumbar spine. Of note, anteroposterior radiograph of spine of patient III-10 presented scoliosis which was attributed to long-term limping and was not shown in his father.
Besides the two patients, a total of 13 members in this family were also believed to be affected and 11 were clinically diagnosed with hip osteoarthritis and cervical spondylopathy (Figure 2). There was no difference in height between the affected and non-affected members in this family. The family members I-1 and II-7 were already dead and we were not able to obtain related clinical materials. Given the characteristics of the pedigree, the disease clearly followed a pattern of autosomal dominant inheritance.
Genetic Analysis
Genomic DNA was extracted from peripheral venous blood obtained from each participating individual using standard procedures with RBC Lysis Solution, WBC Lysis Solution, and Protein Precipitation Solution. DNA for II-5 and III-10 in this family was sent for whole-exome sequencing (YIN FENG GENE, China). Rare variants were filtered in the databases of genomic (1000g_all), esp6500siv2_all, gnomAD, ExAC and the Chinese Gene Mutation Database (CNGMD). Variations were screened according to their scores using SIFT, PolyPhen-2 and Mutation Taster software. Based on the results of filtering and genetic analysis according to phenotype, the heterozygous c.611C>G, p.Gly204Ala (G204A) (NM_001844.4) mutation in exon 9 of COL2A1 was speculated to be the most possible pathogenic mutation. The p.Gly204Ala mutation was predicted to be a detrimental mutation in protein function by SIFT (deleterious; score: 0.005), PolyPhen-2 (probably damaging; score: 0.998) and Mutation Taster (disease causing; score: 1). We then used Sanger sequencing to detect the mutation in COL2A1 gene in this family. Primers for Sanger sequencing were based on the sequences of exon 9 of COL2A1 gene. The primer sequences were as follows: forward, 5ʹ-GCTGTTGGCTGGGAGAAGAT-3ʹ; reverse, 5ʹ-TCATCTGCGACACGATGGAG-3ʹ. In total, 13 family members (II-1, II-3, II-5, II-6, II-9, II-11, II-13, III-3, III-7, III-8, III-10, III-11, III-12) were able to be reached and agreed to undergo genetic testing. This heterozygous mutation co-segregated with osteoarthritis phenotype in all affected family members by Sanger sequencing (Figure 3). Protein sequence alignment of COL2A1 in multiple species showed that the glycine at position 204 was highly conserved (Figure 4).
Discussion
This is a report of the second family with COL2A1 mutation, c.611C>G, which was related with early-onset osteoarthritis. The same mutation site was reported in a Caucasian family in Australia by Kannu et al.8 and this is the first time that it is observed in Mongolians. The appropriate age of early-onset osteoarthritis based on literatures is considered to be under 50 years.9,10 The Australian patients presented with similar symptoms including generalized stiffness, pain and restricted motion affecting multiple joints.8 Two patients received hip replacements at age 23 and 33 years respectively and one patient underwent an ankle arthroscopy at age 16 years.8 Compared with that Australian family, none of these Chinese patients has received surgeries because of osteoarthritis. It could be partly attributed to the Western people more eagerly pursuing a higher quality of life. Nevertheless, the onset of osteoarthritis started significantly later in the Chinese patients. Most of the Australian patients presented joint stiffness and pain when children and one patient recalled that his symptoms appeared at age 3 years.8 However, in our cases, all patients reported clinical symptoms after age 20 years and no one reported joint discomfort in their teens. Patients III-7 and III-8 reported that stiffness of hip joint first appeared at age 40 years. Of note, all affected family members in the Australian family were also HLA-B27 positive but Kannu et al. deemed this not to be a risk factor predisposing to their arthritis for no sign of ankylosis was shown in those patients.8 In addition to racial differences, we speculate that HLA-B27 positivity might contribute to the earlier onset of osteoarthritis.
Type II collagen consists of three homologous α1 peptide chains coded by COL2A1 that twist together in the endoplasmic reticulum and Golgi of chondrocytes to form a triple helix structure, which integrates into fibril networks in the extracellular matrix.11,12 The triple helical domain is characterized by about 330 repeated triplets of glycine followed by two other amino acids (AAs), constituting a repeated Gly-X-Y sequence.4 The Gly-X-Y triple-helix motif is pivotal for the stable crosslink of α1 peptide chain to form functional type II collagen.13 The glycine in every third position of the triple is strictly conserved and the replacements of glycine with other AAs will cause failure in the structure of type II collagen.4,14 Type II collagen is the predominant structural collagen of hyaline cartilage. Disturbances of Type II collagen would alter fibril structure and interfere with the interactions with other extracellular matrix components, thus affecting cartilage stability and accelerating the wear of cartilage. Therefore, the onset of osteoarthritis occurs at a young age . COL2A1 mutations result in various human disorders collectively termed type II collagenopathies of which most are related to substitution of the triple-helical glycine residues.4,15 Glycine substitutions at different sites in COL2A1 have been frequently reported to be associated with hypochondrogenesis, SEDC, and avascular necrosis of the femoral head.7,8 However, for COL2A1-related early-onset osteoarthritis, the most common mutation was arginine to cysteine (Arg-to-Cys) substitutions.7 The changes of glycine elicit structural alterations leading to destabilization with more chance of severe phenotypes. Compared with this, the Arg-to-Cys substitutions are more frequently associated with milder phenotypes.7 Whereas, Lopponen et al. raised a point that neither mutation sites nor AA substitutions could be regarded as a consistent predictor of disease severity.16 We think the relationship between genotype and phenotype still needs further studies. Our findings merely provide more evidence and information of premature onset of osteoarthritis caused by glycine substitution of COL2A1. Though whole-exome sequencing has been increasingly used for genetic analysis in recent years, it is still out of reach in some areas due to technical or economic reasons. For patients with hereditary premature osteoarthritis, COL2A1 mutation should be taken into consideration.
In conclusion, this is a report of the second family with COL2A1 mutation c.611G>C resulting in early onset of degenerative joint disease. Our findings propose that racial differences and concomitant HLA-B27 positivity may contribute to earlier occurrence of osteoarthritis. Individuals who carry this mutation should be followed up at regular intervals and considered for preventive treatment or early intervention for osteoarthritis.
Data Sharing Statement
All data analyzed during this study are included in this published article.
Consent for Publication
Written informed consent for the publication of any associated data and accompanying images was obtained from all individuals involved in this study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Cheah KS, Lau ET, Au PK, Tam PP. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991;111(4):945–953. doi:10.1242/dev.111.4.945
2. Kannu P, Irving M, Aftimos S, Savarirayan R. Two novel COL2A1 mutations associated with a Legg-Calvé-Perthes disease-like presentation. Clin Orthop Relat Res. 2011;469(6):1785–1790. doi:10.1007/s11999-011-1850-x
3. Chen WM, Liu YF, Lin MW, et al. Autosomal dominant avascular necrosis of femoral head in two Taiwanese pedigrees and linkage to chromosome 12q13. Am J Hum Genet. 2004;75(2):310–317. doi:10.1086/422702
4. Li N, Yu J, Cao X, et al. A novel p. Gly630Ser mutation of COL2A1 in a Chinese family with presentations of Legg-Calve-Perthes disease or avascular necrosis of the femoral head. PLoS One. 2014;9(6):e100505. doi:10.1371/journal.pone.0100505
5. Lankisch P, Honscheid A, Schaper J, Borkhardt A, Laws HJ. COL2A1 mutation as a cause of premature osteoarthritis in a 13-year-old child. Joint Bone Spine. 2014;81(1):83–85. doi:10.1016/j.jbspin.2013.06.007
6. Barat-Houari M, Dumont B, Fabre A, et al. The expanding spectrum of COL2A1 gene variants IN 136 patients with a skeletal dysplasia phenotype. Eur J Hum Genet. 2016;24(7):992–1000. doi:10.1038/ejhg.2015.250
7. Barat-Houari M, Sarrabay G, Gatinois V, et al. Mutation update for COL2A1 gene variants associated with Type II collagenopathies. Hum Mutat. 2016;37(1):7–15. doi:10.1002/humu.22915
8. Kannu P, Bateman JF, Randle S, et al. Premature arthritis is a distinct type II collagen phenotype. Arthritis Rheum. 2010;62(5):1421–1430. doi:10.1002/art.27354
9. Ruault V, Yauy K, Fabre A, et al. Clinical and molecular spectrum of nonsyndromic early-onset osteoarthritis. Arthritis Rheum. 2020;72(10):1689–1693. doi:10.1002/art.41387
10. Kemp J, Moore K, Fransen M, Russell T, Freke M, Crossley KM. A pilot randomised clinical trial of physiotherapy (manual therapy, exercise, and education) for early-onset hip osteoarthritis post-hip arthroscopy. Pilot Feasibility Stud. 2018;4:16. doi:10.1186/s40814-017-0157-4
11. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–35. doi:10.1186/ar380
12. Kuivaniemi H, Tromp G, Prockop DJ. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi:10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9
13. Liu F, Xiong Z, Liu Q, Hu J, Li W, Zhang N. COL2A1 mutation (c.3508G>A) leads to avascular necrosis of the femoral head in a Chinese family: a case report. Mol Med Rep. 2018;18(1):254–260. doi:10.3892/mmr.2018.8984
14. Arnold WV, Fertala A. Skeletal diseases caused by mutations that affect collagen structure and function. Int J Biochem Cell Biol. 2013;45(8):1556–1567. doi:10.1016/j.biocel.2013.05.017
15. Gelse K, Pöschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. doi:10.1016/j.addr.2003.08.002
16. Lopponen T, Korkko J, Lundan T, Seppanen U, Ignatius J, Kaariainen H. Childhood-onset osteoarthritis, tall stature, and sensorineural hearing loss associated with Arg75-Cys mutation in procollagen type II gene (COL2A1). Arthritis Rheum. 2004;51(6):925–932. doi:10.1002/art.20817
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.