Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Cognitive Performance is Associated with Altered Cerebral Hemodynamics Assessed by Transcranial Ultrasound in Parkinson’s Disease
Authors Ge YL, Gong SY, Wang PZ, Yan JH, Li W, Zhang JR, Jin H, Zhuang S, Hu L, Ding CW, Yang YP, Wang F , Li D, Chen J, Mao CJ , Zhang YC, Li K, Liu CF
Received 12 January 2022
Accepted for publication 18 May 2022
Published 12 July 2022 Volume 2022:18 Pages 1421—1431
DOI https://doi.org/10.2147/NDT.S358150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yi-Lun Ge,1,* Si-Yi Gong,1,* Pu-Zhi Wang,1 Jia-Hui Yan,1 Wen Li,1 Jin-Ru Zhang,1 Hong Jin,1 Sheng Zhuang,1 Lei Hu,2 Chang-Wei Ding,2 Ya-Ping Yang,1 Fen Wang,3 Dan Li,4 Jing Chen,1 Cheng-Jie Mao,1,4 Ying-Chun Zhang,2 Kai Li,1 Chun-Feng Liu1,3,4
1Department of Neurology, the Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 2Department of Ultrasound, the Second Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 3Institute of Neuroscience, Soochow University, Suzhou, Jiangsu, People’s Republic of China; 4Department of Neurology, Suqian First Hospital, Suqian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kai Li, Department of Neurology, the Second Affiliated Hospital of Soochow University, No. 1055 Sanxiang Road, Suzhou, Jiangsu, 215004, People’s Republic of China, Tel +86-512-6778-4179, Fax +86-512-6828-4303, Email [email protected]
Purpose: Cognitive impairment (CI) is a common but debilitating non-motor symptom in Parkinson’s disease (PD). Although cerebrovascular functions are related to cognitive performance in healthy individuals, such a relation in PD remains elusive. This study aims to assess the association between cerebrovascular function and cognitive performance in PD individuals.
Patients and Methods: Two-hundred-and-one PD individuals were retrospectively included. They were subsequently divided into two groups: PD with normal cognition (PD-NC) and PD with CI (PD-CI). Cerebral hemodynamic characteristics of the middle cerebral arteries were assessed by transcranial ultrasound. The association between scores in each cognitive domain and cerebral hemodynamic parameters was further analyzed using regression analyses. Additionally, a binary logistic regression model with backward stepwise procedure was applied to build the model for discriminating CI in PD individuals. An independent dataset of additional 46 PD individuals was used further.
Results: The PD-CI group showed a relatively lower end-diastolic blood flow velocity (EDV, p < 0.05) and a higher resistive index (RI, p < 0.05) compared to the PD-NC group. RI showed significant associations with the memory item score of Montreal Cognitive Assessment (p < 0.05). A model combining clinical and hemodynamic variables was established with optimal efficiency (area under the curve, AUC = 0.651). Further replication of the model in an independent dataset yielded a great consistency (AUC = 0.704).
Conclusion: In our study, cerebrovascular functions were significantly associated with the cognitive performance in PD individuals, especially with the memory task. The established model was effective in identifying CI in PD individuals, which might be a potentially useful tool to screen the cognitive decline in PD individuals at an early stage of the disease. Further studies with larger sample sizes in different populations are warranted.
Keywords: Parkinson’s disease, cognitive impairment, transcranial ultrasound, resistive index
Introduction
Although Parkinson’s disease (PD) is mainly characterized by motor symptoms, cognitive impairment (CI) in PD can develop at an early stage or even precede the development of motor symptoms with a high prevalence and considerably reduces patients’ quality of life.1–3 Large cross-sectional cohorts have unveiled that the prevalence rate of mild cognitive impairment (MCI) is 20.3~64.3%,4,5 and approximately 20% of PD individuals with MCI will convert to dementia within three years.6 83% of PD individuals will end up with dementia after 20 years of follow-up, which poses enormous challenges for families and society.7
Several studies showed that vascular pathologies frequently occurred in PD individuals, which may contribute to the development of CI.8,9 Additionally, a growing body of studies has shed light on the importance of modifiable vascular risk factors on cognitive decline in PD.10–12 Therefore, exploring cerebrovascular hemodynamic changes in PD individuals with CI is of vital importance.
Transcranial ultrasound is a commonly used method for disease assessment in PD. Previous studies revealed that PD individuals with cognitive deficits exhibited the enlarged third ventricle13 and enhanced substantia nigra echogenicity.14 Researchers have also found that cerebral small-vessel disease is associated with cognitive decline and motor deterioration in PD.15,16 PD patients with vascular risk factors or vascular comorbidities tend to have higher rates of CI, postural instability, and some other non-motor symptoms.10 However, few studies have demonstrated the relationship between cognitive dysfunctions and cerebrovascular characteristics in PD.
Transcranial ultrasound is a non-invasive and economical tool with accuracy for measuring cerebral hemodynamics in major cerebral arteries.17 The present study aims to delineate the potential associations between CI in PD individuals and cerebrovascular features. Furthermore, we investigated the relationship between each cognitive domain score of the cognitive assessments and cerebral hemodynamic parameters. Finally, a model for differentiating CI in PD was constructed with logistic regression analyses, and further replication of the model was conducted using an independent dataset.
Methods
Subjects
Two hundred and seventy PD individuals, who fulfilled either the 2015 Movement Disorder Society clinical diagnostic criteria18 or UK Brain Bank criteria for PD,19 were recruited consecutively from the Department of Neurology of the Second Affiliated Hospital of Soochow University (Suzhou, China) from April 2012 to May 2019. Thirty-one individuals were excluded from the study due to the insufficient bone window. Six individuals with major depressive symptoms, as assessed by the Hamilton Depression Rating Scale (HAM-D), were excluded.20 All participants went through magnetic resonance imaging on a 3.0-T scanner (Achieva, Philips Healthcare, Best, the Netherlands) and 21 participants with extended white matter lesions were also excluded. Individuals with a medical history of stroke were not enrolled in this study. Eleven PD individuals at an advanced stage with poor compliance were also excluded from the study. In total, a total of 201 individuals were included in the study. In the replication stage, additional 60 PD individuals were recruited to validate our model. Eleven of 60 individuals were excluded due to depressive symptoms, and 3 were excluded because of insufficient bone window. This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Soochow University. Data were collected after written informed consent was obtained from all participants.
Clinical Assessment
Motor symptoms were evaluated with Unified Parkinson’s Disease Rating Scale (UPDRS) Part III in the “on” state.21 Disease severity was assessed with a modified Hoehn & Yahr (H&Y) scale in the “off” state.22 Levodopa equivalent doses (LEDs) of prescribed dopaminergic medications were calculated as previously described.23 Cognitive performance was assessed by the Mini-Mental State Examination (MMSE)24 and Montreal Cognitive Assessment (MoCA, Beijing version).25 Participants were divided into two groups based on MoCA scores:26 PD with normal cognition (PD-NC) and PD with CI (PD-CI). HAM-D was used for the evaluation of depression in PD individuals.20 All evaluations were performed by trained movement-disorder neurologists who were blinded to the diagnosis of the patients.
Transcranial Ultrasound
Transcranial ultrasound was conducted by our experienced ultrasound practitioners within one week after clinical assessments. The individuals were examined in the supine position. The brain was insonated through the bilateral temporal acoustic bone window in the orbitomeatal line using a transducer (Acuson Sequoia 512, Siemens, Erlangen, Germany) with an emitting frequency of 2 MHz, a wall filter of 150 Hz, and a Doppler angle of 48°.27,28 Blood flow velocities of the middle cerebral arteries (MCAs), including peak systolic velocity (PSV) and end-diastolic velocity (EDV), were obtained at the depth of 52 to 64 mm, where the hemodynamic signals were the most stable.28 Resistive indexes (RIs) were calculated automatically by the internal algorithm according to the formula RI = (PSV-EDV)/PSV.27 The angle of the transducer was varied when needed. These parameters were obtained bilaterally over at least 10 cardiac cycles after a 30s stable period with a clear signal. Transcranial ultrasound was performed in a darkened room by the same experienced clinician who was blinded to the individuals’ clinical status to eliminate any bias in the examination results.
Statistical Analysis
All statistical analyses were conducted with IBM SPSS Statistics, version 25.0, 64-bit (IBM Corporation, Armonk, NY, USA). Normality tests were verified using Kolmogorov–Smirnov test or Shapiro–Wilk’s test. Quantitative data were compared using Student’s t-test if normally distributed or non-parametric Mann–Whitney U-test if not normally distributed. Categorical variables were compared using chi-square test. Unary linear regression was used to assess the association between scores of specific cognitive domains and cerebral hemodynamic parameters. A binary logistic regression model with backward stepwise procedure was applied to build the model for discriminating CI. Hosmer-Lemeshow test was performed to assess the goodness of fit in logistic regression analyses. Areas under the curves (AUCs) were calculated to estimate the efficiency of our model. All the statistical tests were 2-sided, and p < 0.05 was considered statistically significant.
Results
Demographics
In total, 201 PD individuals were included in the study. Of them, 147 were male. The average age was 61.99 ± 10.04 years, and the mean disease duration was 47.46 ± 39.80 months. The mean age at onset was 57.99 ± 10.25 years. No statistically significant difference regarding demographics was found between the PD-NC and PD-CI groups (Table 1).
 |
Table 1 Demographic Characteristics of PD Individuals with/Without Cognitive Impairment |
Cerebral Hemodynamics Were Associated with Cognitive Performance in PD Individuals
We calculated the maximum, minimum and average values of PSV, EDV and RI of bilateral MCAs, and compared these values between the PD-NC and PD-CI groups (Table 2). There was no statistically significant difference between the two groups regarding all the PSV parameters. However, the maximum, minimum and average EDV values were significantly lower in the PD-CI group than in the PD-NC group (p = 0.008 for maximum, p = 0.032 for minimum and p = 0.008 for average, respectively) (Table 2). Additionally, the PD-CI group showed a significantly higher minimum and average RI than the PD-NC group (p = 0.026 for minimum, p = 0.031 for average, respectively) (Table 2).
 |
Table 2 Cerebral Hemodynamic Parameters in PD Patients with/Without Cognitive Impairment |
Cerebral Hemodynamics Were Associated with Short-Term Memory in PD Individuals
For cognitive domains, all item scores were poorer in the PD-CI group, while the most significant difference was in the memory task (delayed recall) score (Supplementary Material 1). Negative correlations were observed between RI and memory task score of MoCA (p = 0.002 for minimum RI of the both sides; p = 0.003 for average) (Table 3). However, there was no association between RI and other cognitive domains assessed with MoCA (Table 3).
 |
Table 3 Unary Linear Regression Analyses Between RI and 7 Independent Cognitive Domains of MoCA in PD Patients |
There was no statistically significant difference between EDV values and each cognitive domain evaluated with MoCA in PD individuals (Table 4). However, a potentially positive correlation was observed between the memory task score of MoCA and average EDV, albeit without reaching statistical significance (p = 0.068 for average bottom velocity) (Table 4).
 |
Table 4 Unary Linear Regression Analyses Between EDV and 7 Independent Cognitive Domains of MoCA in PD Individuals |
Development and Validation of the Model in Identifying Cognitive Impairment in PD Individuals
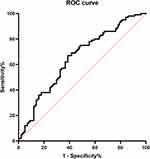
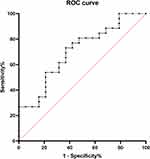
We used the five hemodynamic parameters, respectively, as independent predictors for CI but yielded limited effectiveness (Figure 1). We then developed a model using binary logistic regression analyses for discriminating CI in PD individuals. Additionally, the stepwise (backward) logistic regression procedure was applied to exclude covariates with less significance or collinearity (Table 5). Finally, the model exhibited acceptable goodness of fit (Hosmer-Lemeshow, p > 0.05) with an AUC of 0.651 (95% CI 0.576–0.727) (Figure 2). Further validation of this model on an additional independent dataset of 46 PD individuals yielded optimal efficiency with an AUC of 0.704 (95% CI 0.551–0.858) (Figure 3).
 |
Table 5 Binary Logistic Regression Analyses of Cognitive Impairment in 201 PD Individuals |
 |
Figure 1 The Receiver operating characteristic plots using five hemodynamic parameters respectively as independent predictors for CI. Abbreviation: CI, cognitive impairment. |
Discussion
In this study, we evaluated the association between cerebrovascular functions assessed by transcranial Doppler (TCD) and cognitive performance in PD individuals. Our findings showed that PD individuals with cognitive impairment exhibited significantly lower EDV values and higher RI values compared to the PD-NC group. Furthermore, our study showed an inverse correlation between RI and memory index score of MoCA in PD individuals. Additionally, a model combining the clinical and hemodynamic parameters was constructed for discriminating CI in PD.
Our study demonstrated that the PD-CI group exhibited lower EDV values than the PD-NC group, while there was no significant difference in PSV parameters between the two groups. The different changes in EDV and PSV reflect increased blood flow velocity variability. The alteration of blood flow velocity variability might be a cause or consequence of cognitive impairments related to vascular pathology or neuropathology. In fact, it remains elusive whether the result is secondary to the decreased metabolic demand in PD individuals with cognitive deficits or whether the altered variation in blood flow velocity leads to the recurrent and chronic cerebral hypoperfusion, which further causes neuronal damage or dysfunction in vulnerable areas like the hippocampus, thereby causing cognitive decline in PD. However, the latter one, also known as the vascular hypothesis, is supported by longitudinal studies showing that increasing volume of white matter hyperintensities was associated with a performance decline in cognitive tests in the healthy elderly.29,30 Of note, previous research has provided other potential mechanisms responsible for the cognitive decline in PD, including energy deficiency in neurons,31,32 free radical mediated injury,33–36 synaptic changes37–40 and microinfarcts.41,42
Meanwhile, some studies reported that the autonomic denervation associated with parkinsonism further led to compromised vascular autoregulatory capacity.43 Therefore, our findings may support the hypothesis that the decreased capacity of cerebrovascular autoregulation could lead to cognitive decline through cerebral hypoperfusion in PD.
Our investigation showed that RI values in the PD-CI group were significantly higher than those in the PD-NC group. RI, a conclusive parameter that incorporates systolic and diastolic blood flow velocities, represents the stiffness of arterial vessel walls and vascular compliance or responsiveness to dilatory stimulation in the microvascular bed.27,44 With the increase in peripheral resistance as implicated by higher RI, the EDV values should show a decline theoretically, which is consistent with our findings. RI can be a proxy marker for hypoperfusion that causes neuronal damage or dysfunction and further contributes to cognitive decline, which has been suggested as a possible mechanism for CI concerning atherosclerosis.45,46
In addition, this study reported that RI was significantly associated with the memory index score of MoCA in PD individuals, concurring with prior studies that found higher RI was associated with steeper decline in intelligence.44 Most research has reported that PD individuals commonly exhibited fronto-striatal deficits with executive dysfunctions being the most prominent manifestation,47 while 40% of PD individuals presented cognitive impairments in memory, language and visuospatial abilities.48 In this study, we failed to find an association between cerebral hemodynamic parameters and executive dysfunction in PD individuals. One possible explanation for this might be the limited sample size available. Moreover, executive functions and visuospatial abilities were assessed together with MoCA scale, which may impact statistical results. Furthermore, the mechanisms that probably underlie the deficits in memory and executive functions in PD may differ.
Moreover, our study indicated that age, age at onset, education, LED, UPDRS-III, H&Y stage, maximum EDV, average EDV and minimum RI were independently associated with cognitive impairment in PD. Previous studies showed that mild cognitive impairment in PD was associated with older age, lower education, longer disease duration, higher LED and more severe motor symptoms,49 which is in line with our results. However, for the first time, our study showed that cerebral hemodynamic parameters were associated with cognitive decline in PD and further constructed a concise and precise model combining the clinical and hemodynamic parameters for discriminating or predicting of cognitive impairment in PD, which might be a potentially practical tool for detection of cognitive decline in PD.
To our knowledge, this is the first study investigating the association between cerebrovascular characteristics indicated by transcranial ultrasound and CI in PD. This study has confirmed the hypothesis that vascular factors are associated with cognition in order to explain the difference in PD phenotypes, which is in accordance with previous studies.17 In addition, we provided an effective tool that is helpful for the discrimination of CI in PD.
However, our study has some limitations. First, the major drawback of our study is the lack of longitudinal data, which may provide evidence for a potentially causal relationship between cerebral hemodynamics and CI. Secondly, the sample size is relatively small. Further studies with larger sample sizes in different populations are warranted.
Conclusion
In our study, cerebrovascular functions were significantly associated with cognitive performance in PD individuals, especially with memory performance. The established model was effective in identifying CI in PD individuals, which may be a potentially useful tool to screen cognitive decline in PD individuals at an early stage of the disease. Our observations collectively suggest that subsequent studies should include vascular parameters to balance the effect of vascular factors. Further longitudinal studies with larger sample sizes in different populations are warranted.
Acknowledgment
We express our gratitude to all the individuals who participated in the study.
Funding
This work was supported by the Jiangsu Provincial Key R&D Program (BE2018658), Jiangsu Provincial Medical Key Discipline Project (ZDXKB2016022), Discipline Construction Program of the Second Affiliated Hospital Soochow University (XKTJ-XK202001), National Natural Science Foundation of China (81801258), National Natural Science Foundation of China (81801120), talent support project for science and teaching of the Second Affiliated Hospital of Soochow University (XKTJ-RC202004), pre-research project for doctors of the Second Affiliated Hospital of Soochow University (SDFEYBS1702) and pre-research project for doctors of the Second Affiliated Hospital of Soochow University (SDFEYBS2014).
Disclosure
None of the authors have any conflicts of interest to report in this work.
References
1. Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11(8):697–707. doi:10.1016/S1474-4422(12)70152-7
2. Pont-Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord. 2015;30(2):229–237. doi:10.1002/mds.26077
3. Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi:10.1016/S1474-4422(14)70287-X
4. Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70(5):580–586. doi:10.1001/jamaneurol.2013.2110
5. Lawrence BJ, Gasson N, Loftus AM. Prevalence and subtypes of mild cognitive impairment in Parkinson’s disease. Sci Rep. 2016;6:33929. doi:10.1038/srep33929
6. Saredakis D, Collins-Praino LE, Gutteridge DS, Stephan BCM, Keage HAD. Conversion to MCI and dementia in Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2019;65:20–31. doi:10.1016/j.parkreldis.2019.04.020
7. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi:10.1002/mds.21956
8. Rektor I, Goldemund D, Sheardova K, Rektorova I, Michalkova Z, Dufek M. Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(1):24–29. doi:10.1016/j.parkreldis.2008.02.007
9. Schwartz RS, Halliday GM, Cordato DJ, Kril JJ. Small-vessel disease in patients with Parkinson’s disease: a clinicopathological study. Mov Disord. 2012;27(12):1506–1512. doi:10.1002/mds.25112
10. Malek N, Lawton MA, Swallow DM, et al. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov Disord. 2016;31(10):1518–1526. doi:10.1002/mds.26698
11. Stojkovic T, Stefanova E, Soldatovic I, et al. Exploring the relationship between motor impairment, vascular burden and cognition in Parkinson’s disease. J Neurol. 2018;265(6):1320–1327. doi:10.1007/s00415-018-8838-3
12. Chahine LM, Dos Santos C, Fullard M, et al. Modifiable vascular risk factors, white matter disease and cognition in early Parkinson’s disease. Eur J Neurol. 2019;26(2):246–e18. doi:10.1111/ene.13797
13. Behnke S, Pilotto A, Liepelt-Scarfone I, et al. Third ventricular width assessed by transcranial ultrasound correlates with cognitive performance in Parkinson’s disease. Parkinsonism Relat Disord. 2019;66:68–73. doi:10.1016/j.parkreldis.2019.07.005
14. Yilmaz R, Behnke S, Liepelt-Scarfone I, et al. Substantia nigra hyperechogenicity is related to decline in verbal memory in healthy elderly adults. Eur J Neurol. 2016;23(5):973–978. doi:10.1111/ene.12974
15. Shibata K, Sugiura M, Nishimura Y, Sakura H. The effect of small vessel disease on motor and cognitive function in Parkinson’s disease. Clin Neurol Neurosurg. 2019;182:58–62. doi:10.1016/j.clineuro.2019.04.029
16. Wan Y, Hu W, Gan J, et al. Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 2019;9(4):e01219. doi:10.1002/brb3.1219
17. Tymko MM, Ainslie PN, Smith KJ. Evaluating the methods used for measuring cerebral blood flow at rest and during exercise in humans. Eur J Appl Physiol. 2018;118(8):1527–1538. doi:10.1007/s00421-018-3887-y
18. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi:10.1002/mds.26424
19. Daniel SE, Lees AJ. Parkinson’s disease society brain bank, London: overview and research. J Neural Transm Suppl. 1993;39:165–172.
20. Leentjens AF, Verhey FR, Lousberg R, Spitsbergen H, Wilmink FW. The validity of the Hamilton and Montgomery-Asberg depression rating scales as screening and diagnostic tools for depression in Parkinson’s disease. Int J Geriatr Psychiatry. 2000;15(7):644–649. doi:10.1002/1099-1166(200007)15:7<644:AID-GPS167>3.0.CO;2-L
21. Fahn S. Unified Parkinson’s Disease Rating Scale. Vol. 2. Macmillan Healthcare Information; 1987.
22. Goetz CG, Poewe W, Rascol O, et al. Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020–1028. doi:10.1002/mds.20213
23. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–2653. doi:10.1002/mds.23429
24. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
25. Yu J, Li J, Huang X. The Beijing version of the Montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry. 2012;12:156. doi:10.1186/1471-244X-12-156
26. Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population-based study. J Geriatr Psychiatry Neurol. 2011;24(4):184–190. doi:10.1177/0891988711422528
27. Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211(2):411–417. doi:10.1148/radiology.211.2.r99ma48411
28. Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000;31(11):2641–2647. doi:10.1161/01.STR.31.11.2641
29. Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol. 2005;58(4):610–616. doi:10.1002/ana.20630
30. van den Heuvel DM, Ten Dam VH, de Craen AJet al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77(2):149–153. doi:10.1136/jnnp.2005.070193
31. Pathak D, Berthet A, Nakamura K. Energy failure: does it contribute to neurodegeneration? Ann Neurol. 2013;74(4):506–516. doi:10.1002/ana.24014
32. Muddapu VR, Chakravarthy VS. Influence of energy deficiency on the subcellular processes of Substantia Nigra Pars Compacta cell for understanding Parkinsonian neurodegeneration. Sci Rep. 2021;11(1):1754. doi:10.1038/s41598-021-81185-9
33. Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25(10):2504–2512. doi:10.1523/JNEUROSCI.0035-05.2005
34. Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi:10.2174/157015909787602823
35. Kumar H, Lim HW, More SV, et al. The role of free radicals in the aging brain and Parkinson’s Disease: convergence and parallelism. Int J Mol Sci. 2012;13(8):10478–10504. doi:10.3390/ijms130810478
36. Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol. 2013;106–107:17–32. doi:10.1016/j.pneurobio.2013.04.004
37. Gajera CR, Fernandez R, Postupna N, et al. Mass synaptometry: high-dimensional multi parametric assay for single synapses. J Neurosci Methods. 2019;312:73–83. doi:10.1016/j.jneumeth.2018.11.008
38. Matuskey D, Tinaz S, Wilcox KC, et al. Synaptic changes in Parkinson disease assessed with in vivo Imaging. Ann Neurol. 2020;87(3):329–338. doi:10.1002/ana.25682
39. Gajera CR, Fernandez R, Montine KS, et al. Mass-tag barcoding for multiplexed analysis of human synaptosomes and other anuclear events. Cytometry A. 2021;99(9):939–945. doi:10.1002/cyto.a.24340
40. Gajera CR, Fernandez R, Postupna N, et al. Mass synaptometry: applying mass cytometry to single synapse analysis. Methods Mol Biol. 2022;2417:69–88.
41. Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42(11):3183–3189. doi:10.1161/STROKEAHA.111.623462
42. Hatate J, Miwa K, Matsumoto M, et al. Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat Disord. 2016;26:29–34. doi:10.1016/j.parkreldis.2016.02.011
43. Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21(10):1318–1331. doi:10.1038/s41593-018-0234-x
44. Alhusaini S, Karama S, Nguyen TV, et al. Association between carotid atheroma and cerebral cortex structure at age 73 years. Ann Neurol. 2018;84(4):576–587. doi:10.1002/ana.25324
45. Sztriha LK, Nemeth D, Sefcsik T, Vecsei L. Carotid stenosis and the cognitive function. J Neurol Sci. 2009;283(1–2):36–40. doi:10.1016/j.jns.2009.02.307
46. Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: similarities and differences. Atherosclerosis. 2013;227(2):193–200. doi:10.1016/j.atherosclerosis.2012.11.008
47. Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi:10.1093/brain/awm111
48. Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi:10.1212/01.wnl.0000180516.69442.95
49. Baiano C, Barone P, Trojano L, Santangelo G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: a meta-analysis. Mov Disord. 2020;35(1):45–54. doi:10.1002/mds.27902
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.