Back to Journals » OncoTargets and Therapy » Volume 13
Coexistence of Low-Grade Fetal Adenocarcinoma and Adenocarcinoma in situ of the Lung Harboring Different Genetic Mutations: A Case Report and Review of Literature
Authors Liu S, Wang J, Luo X, Li X, Miao Y , Wang L , Li Q, Qiu X, Wang EH
Received 2 May 2020
Accepted for publication 26 June 2020
Published 7 July 2020 Volume 2020:13 Pages 6675—6680
DOI https://doi.org/10.2147/OTT.S260993
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Shuli Liu,1 Jinping Wang,1 Xue Luo,1 Xiaoman Li,2 Yuan Miao,1 Liang Wang,1 Qingchang Li,1 Xueshan Qiu,1 En-Hua Wang1
1Department of Pathology, College of Basic Medical Sciences and the First Affiliated Hospital, China Medical University, Shenyang, People’s Republic of China; 2Key Laboratory of Medical Cell Biology, Ministry of Education, China Medical University, Shenyang, People’s Republic of China
Correspondence: Liang Wang
Department of Pathology, College of Basic Medical Sciences and the First Affiliated Hospital, China Medical University, Shenyang 110001, People’s Republic of China
Tel/ Fax +86 24 23261638
Email [email protected]
Abstract: Low-grade fetal lung adenocarcinoma (L-FLAC) is an exceptionally rare pulmonary tumor, presenting with unclear histological and molecular features. In particular, the potential driver genes of L-FLAC remain elusive. To date, only five reports have documented genetic aberrations in L-FLAC. In the current study, we describe an unusual case of L-FLAC coexisting with adenocarcinoma in situ (AIS) of the lung, harboring different genetic mutations. A 39-year-old non-smoker female patient was referred to our hospital with the chief complaint of dyspnea for 20 days. Chest computed tomography (CT) revealed a 2.5× 1.5× 1.5 cm nodule in the right middle lobe, with no mediastinal lymph node enlargement or distant metastases. Thoracoscopic surgery was performed to remove the nodules. Histopathological analysis of the tissue sections, based on findings from immunohistochemical staining, confirmed a diagnosis of L-FLAC coexisting with AIS of the lung. Next-generation sequencing revealed L-FLAC-based mutations in DICER1 and CTNNB1, and AIS harboring KRAS mutations. Currently, the patient remains recurrence-free 17 months after the initial diagnosis. This report presents the first case demonstrating the coexistence of L-FLAC and AIS in the same pulmonary nodule, harboring different genetic mutations. Based on the literature review, this is the second reported case of L-FLAC bearing DICER1 mutations.
Keywords: low-grade fetal adenocarcinoma, CTNNB1, DICER1, KRAS, NGS
Introduction
Fetal lung adenocarcinoma is markedly rare and was first reported in 1982.1 An extremely very rare lung cancer, it accounts for only 0.1–0.5% of all lung neoplasms. In 1999, this tumor was classified as a variant of lung adenocarcinoma by the World Health Organization (WHO) and is currently termed as “fetal adenocarcinoma of the lung” (FLAC).2 The tumor mass is generally solitary, well-circumscribed, and located peripherally in the lung. Typically, FLAC causes clinical symptoms such as chest pain, dyspnea, cough, and hemoptysis. Additionally, some asymptomatic patients are diagnosed with incidental findings on chest radiographs. According to the histological patterns, the tumor was further classified into two subtypes: low-grade FLAC (L-FLAC) and high-grade FLAC (H-FLAC).3 Furthermore, these two subtypes present different clinical characteristics and outcomes. Patients with H-FLAC generally present advanced-stage diseases and a poorer prognosis. Nakatani et al have reported that L-FLAC, histologically, is pure in pattern and may present an even better prognosis than stage-matched general pulmonary adenocarcinomas.4 Unlike its high-grade counterpart, L-FLAC histologically resembles the fetal lung without transition to conventional adenocarcinomas. Therefore, the precise diagnosis of L-FLAC is critical for the treatment and management of patients. Currently, utilizing immunohistochemistry (IHC), histological studies remain the most accurate examination for this tumor. However, the molecular features of FLAC require further elucidation. Here, we present a case of L-FLAC coexisting with adenocarcinoma in situ (AIS) in two distinct regions of the same pulmonary nodule, and distinct genetic mutations were revealed by analyzing the two lesions using next-generation sequencing (NGS).
Case Report
A 39-year-old non-smoker female patient, predominantly complaining of dyspnea for 20 days, was referred to our hospital. Hematological, biochemical, and sputum tests showed no abnormalities. Chest computed tomography (CT) revealed a 2.5×1.5×1.5cm nodule in the right middle lobe, with no mediastinal lymph node enlargement or distant metastases (Figure 1). Following lobectomy and mediastinal lymph node dissection, an intraoperative frozen section was examined and diagnosed as lung adenocarcinoma.
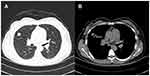 |
Figure 1 Computed tomography (CT) scans of the chest. (A and B) Photographs present a well-circumscribed and lobulated 2.5-cm nodule in the right middle lobe. |
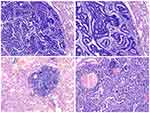
The resected tumor presented a smooth, whitish, and firm surface, with small hemorrhagic areas, and appeared to be relatively well-circumscribed. No obvious necrosis or cystic changes were identified. Microscopically, the tumor tissue contained two distinct non-adjacent lesions. One lesion demonstrated complex glandular and tubular structures, with prominent morule formation, resembling the developing fetal lung in its pseudo glandular stage. The tumor cells displayed low nuclear atypia, and no necrosis was detected (Figure 2A and B). Based on these findings, a diagnosis of L-FLAC was proposed. In the second lesion, the tumor cells were distributed alongside the alveolar structures, without stromal, vascular, and alveolar space invasion, presenting a maximum diameter of only 2 mm (Figure 2C and D). Therefore, this lesion was deemed an AIS of the lung. Although these two lesions were located within the same nodule, they were individually present in different tissue sections. After screening all tissue sections, no spindle-shaped tumor cells or sarcomatous structures were observed. Furthermore, no vessel invasion or pleural involvement was detected.
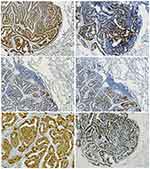
Immunohistochemically, the L-FLAC cells presented diffuse staining for cytokeratin, thyroid transcription factor-1 (TTF-1, Figure 3A), epithelial membrane antigen (EMA), and sal-like protein 4 (SALL4, Figure 3B). Chromogranin A (CgA, Figure 3C) and synaptophysin (Figure 3D) were only focally stained. The tumor cells were negative for cytokeratin 7, α-fetoprotein, CD56, and glypican 3. Notably, β-catenin showed diffuse staining on the cell membrane, with only sporadic nuclear/cytoplasmic expression (Figure 3E). Additionally, the β-catenin expression pattern was similar in the morule and non-morule structures. The Ki-67 index was approximately 40% (Figure 3F).
To further identify the genetic mutation profile, we performed NGS on both the L-FLAC and AIS regions, using adjacent normal lung tissue as the germline control. Molecular studies have revealed that these two lesions harbored distinct genetic mutations (Supplementary Figure 1). L-FLAC revealed somatic mutations in DICER1 frameshift mutation in the exon 23 (c.4407_4410delTTCT (p.S1470Lfs*19)) with an abundance of 31.9%, and missense mutations in exon 24 (c.5125G>A p.D1709N) with an abundance of 38.3%. Simultaneously, missense mutations were identified in CTNNB1 in exon 3 (c.98C>G p.S33C) with an abundance of 41.2%. However, AIS demonstrated a K-RAS missense mutation in exon 2 (c.35G>C p.G12A). Finally, the diagnosis of L-FLAC coexisting with AIS stage 1A (pT1bN0M0) was confirmed. Postoperatively, no evidence of recurrence was observed for 17 months.
Discussion
As a distinct entity according to the WHO classification, FLAC is a malignant tumor with extremely low incidence, accounting for less than 0.5% of all lung tumors.2 The two FLAC subtypes, L-FLAC and H-FLAC, differ in multiple aspects, including histopathology and clinical characteristics.5 L-FLAC is commonly detected in female patients in the third-fourth decade of their lives. Typically, the initial diagnosis of L-FLAC occurs during the early stages (I–II).6 H-FLAC mostly occurs in elderly male smokers, presenting advanced-stage disease and severe symptoms.7
L-FLAC showed a pure histological pattern, with prominent morule formation and low nuclear atypia. However, H-FLAC, generally displays fetal morphology (over 50%), together with other common types of lung adenocarcinomas.8 Moreover, H-FLAC commonly demonstrates prominent nuclear atypia and nucleoli, accompanied by necrosis and a high frequency of mitosis. In the current case, no prominent nuclear atypia, necrosis, or a direct transition to conventional adenocarcinoma was observed. Accordingly, a diagnosis of L-FLAC, rather than H-FLAC, was confirmed.
In addition to histopathological patterns, these two subtypes demonstrate distinct immunohistochemical staining. Owing to the activation of the WNT signaling pathway, typically, but not in all cases, L-FLAC can display aberrant cytoplasm/nuclear distribution of β-catenin.9 In H-FLAC, the β-catenin expression is usually localized on the cell membranes. L-FLAC presents lower expression of p53, which in contrast, is commonly overexpressed in H-FLAC. Morita et al and Suzuki et al have reported that α-fetoprotein, glypican 3, and SALL4 are frequently expressed in H-FLAC, especially in non-fetal-like components.7,8 However, in the current case, SALL4 also demonstrated diffuse expression. Therefore, immune profiles can be distinct in different cases. As few studies have documented SALL4 expression in L-FLAC, further research is crucial to elucidate whether SALL4 can be utilized as a useful marker in the differential diagnosis of H-FALC and L-FLAC. We speculated that SALL4 expression was associated with the DICER1 mutation, as shown in the current study. Several studies have reported that DICER1-associated malignancies, especially with blastomaoid components, show SALL4 expression.10,11 However, owing to the limited data available, this interpretation needs further supportive evidence.
In the current case, the β-catenin demonstrated only sporadic cytoplasmic/nuclear localization in tumor cells and was mostly present on cell membranes. Similar expression patterns of β-catenin have been reported in L-FLAC in a 16-year-old female patient by both Wu et al12 and de Kock et al.13 It worth noticing that, in that case, the L-FLAC also harbored DICER1 mutations in addition to CTNNB1 mutations. L-FLAC is considered as a putative precursor lesion of pulmonary blastoma (PB), which is a biphasic tumor consisting of FLAC (typically low-grade) and primitive mesenchymal stroma. Both L-FLAC and PB are frequently associated with mutations of CTNNB1.9 Interestingly, till date, mutations in both DICER1 and CTNNB1 have only been identified in two cases of PB.14 In these two cases, β-catenin also demonstrated predominantly membranous expression with multifocal cytoplasmic/nuclear localization. Collectively, these cases further support that L-FLAC and PB shared partially overlapping genetic abnormalities, and mutations in DICER1 may be associated with aberrant β-catenin expression patterns.
Besides mutations in CTNNB1, genomic mutation profiles in L-FLAC remain elusive owing to extremely low incidences and reports. To date, only six reports have documented the genetic mutations in L-FLAC, including the current study, and these findings are summarized in Table 1.4,12,13,15,16 By reviewing the literature, although uncommon mutations in BRAC2, TSC2, and DDR2 have been identified, mutations in CTNNB1 demonstrated the highest frequency in L-FLAC. In the current case, although AIS was co-existed with L-FLAC in the same pulmonary nodule, these two lesions harbored different mutations, indicating that both tumors were primary lesions, instead of intrapulmonary metastasis. We speculated that AIS within the same nodule of L-FLAC can be interpreted as intra-tumor spatial heterogeneity owing to different driver genes. If the lesion was not identified and resected during the early stages, the AIS region may progress to invasive adenocarcinoma. Therefore, this case might elucidate an early event of the development of H-FLAC, in which the fetal component presents with a transition to conventional invasive adenocarcinoma.
 |
Table 1 Clinical Review of 6 Reports of L-FLAC with Genetic Analysis |
In conclusion, we report an extremely rare case of a 39-year old woman with L-FLAC and AIS within the same pulmonary nodule. The two lesions demonstrated distinct genetic mutations. This is the second reported case of L-FLAC bearing DICER1 mutations. Currently, the patient remains asymptomatic and disease-free following a 17-months follow-up period.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Ethical Approval and Consent to Participate
Ethical approval for this study was obtained from the institutional ethic review boards of the First Affiliated Hospital of China Medical University. Writing consent to participate was provided by the patients for the present research.
Patient Consent for Publication
The informed consent was obtained from the patient for the publication of her case and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgments
The authors thank the technical assistance in genetic analysis from Dr. Dadong Ding and Dr. Peng Yang from Nanjing Geneseeq Technology Inc.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Kradin RL, Young RH, Dickersin GR, Kirkham SE, Mark EJ. Pulmonary blastoma with argyrophil cells and lacking sarcomatous features (pulmonary endodermal tumor resembling fetal lung). Am J Surg Pathol. 1982;6(2):165–172. doi:10.1097/00000478-198203000-00009
2. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–1260. doi:10.1097/JTO.0000000000000630
3. Zhang J, Sun J, Liang XL, Lu JL, Luo YF, Liang ZY. Differences between low and high grade fetal adenocarcinoma of the lung: a clinicopathological and molecular study. J Thorac Dis. 2017;9(7):2071–2078. doi:10.21037/jtd.2017.07.14
4. Nakatani Y, Masudo K, Miyagi Y, et al. Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma of fetal lung type: up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol. 2002;15(6):617–624. doi:10.1038/modpathol.3880575
5. Ricaurte LM, Arrieta O, Zatarain-Barron ZL, Cardona AF. Comprehensive review of fetal adenocarcinoma of the lung. Lung Cancer. 2018;9:57–63. doi:10.2147/LCTT.S137410
6. Patnayak R, Jena A, Rukmangadha N, Lakshmi AY, Chandra A. Well-differentiated fetal adenocarcinoma of the lung in an adult male: report of an unusual tumor with a brief review of literature. J Cancer Res Ther. 2014;10(2):419–421. doi:10.4103/0973-1482.136677
7. Morita S, Yoshida A, Goto A, et al. High-grade lung adenocarcinoma with fetal lung-like morphology: clinicopathologic, immunohistochemical, and molecular analyses of 17 cases. Am J Surg Pathol. 2013;37(6):924–932. doi:10.1097/PAS.0b013e31827e1e83
8. Suzuki M, Nakatani Y, Ito H, et al. Pulmonary adenocarcinoma with high-grade fetal adenocarcinoma component has a poor prognosis, comparable to that of micropapillary adenocarcinoma. Mod Pathol. 2018;31(9):1404–1417. doi:10.1038/s41379-018-0057-z
9. Nakatani Y, Miyagi Y, Takemura T, et al. Aberrant nuclear/cytoplasmic localization and gene mutation of beta-catenin in classic pulmonary blastoma: beta-catenin immunostaining is useful for distinguishing between classic pulmonary blastoma and a blastomatoid variant of carcinosarcoma. Am J Surg Pathol. 2004;28(7):921–927. doi:10.1097/00000478-200407000-00012
10. Agaimy A, Witkowski L, Stoehr R, et al. Malignant teratoid tumor of the thyroid gland: an aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term “thyroblastoma” more appropriate? Virchows Arch. 2020. doi:10.1007/s00428-020-02853-1
11. McCluggage WG, Apellaniz-Ruiz M, Chong AL, et al. Embryonal Rhabdomyosarcoma of the Ovary and Fallopian Tube: rare Neoplasms Associated With Germline and Somatic DICER1 Mutations. Am J Surg Pathol. 2020;44(6):738–747. doi:10.1097/PAS.0000000000001442
12. Wu Y, Chen D, Li Y, Bian L, Ma T, Xie M. DICER1 mutations in a patient with an ovarian Sertoli-Leydig tumor, well-differentiated fetal adenocarcinoma of the lung, and familial multinodular goiter. Eur J Med Genet. 2014;57(11–12):621–625. doi:10.1016/j.ejmg.2014.09.008
13. de Kock L, Bah I, Wu Y, Xie M, Priest JR, Foulkes WD. Germline and Somatic DICER1 Mutations in a Well-Differentiated Fetal Adenocarcinoma of the Lung. J Thorac Oncol. 2016;11(3):e31–e33. doi:10.1016/j.jtho.2015.09.012
14. de Kock L, Bah I, Brunet J, et al. Somatic DICER1 mutations in adult-onset pulmonary blastoma. Eur Respir J. 2016;47(6):1879–1882. doi:10.1183/13993003.00172-2016
15. Sekine S, Shibata T, Matsuno Y, et al. Beta-catenin mutations in pulmonary blastomas: association with morule formation. J Pathol. 2003;200(2):214–221. doi:10.1002/path.1352
16. Fu Y, Wu Q, Su F, et al. Novel gene mutations in well-differentiated fetal adenocarcinoma of the lung in the next generation sequencing era. Lung Cancer. 2018;124:1–5. doi:10.1016/j.lungcan.2018.07.016
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.