Back to Journals » Infection and Drug Resistance » Volume 12
Coexistence of blaNDM-1 and rmtC on a Transferrable Plasmid of a Novel ST192 Klebsiella aerogenes Clinical Isolate
Authors Shen X, Liu L, Yu J, Cao X, Zhan Q, Guo Y, Wang L, Yu F
Received 22 August 2019
Accepted for publication 2 December 2019
Published 13 December 2019 Volume 2019:12 Pages 3883—3891
DOI https://doi.org/10.2147/IDR.S228130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Xiaofei Shen,1 Li Liu,2 Jingyi Yu,2 Xingwei Cao,3 Qing Zhan,4 Yinjuan Guo,5,6 Liangxing Wang,1 Fangyou Yu5,6
1Department of Respiratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China; 2Department of Laboratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China; 3Jiangxi Provincial Key Laboratory of Medicine, Clinical Laboratory of the Second Affiliated Hospital of Nanchang University, Nanchang 330006, People’s Republic of China; 4Jiangxi Provincial Key Laboratory of Preventive Medicine, Nanchang University, Nanchang 330006, People’s Republic of China; 5Department of Clinical Laboratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200082, People’s Republic of China; 6Shanghai Key Laboratory of Tuberculosis, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200082, People’s Republic of China
Correspondence: Liangxing Wang
Department of Respiratory Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, People’s Republic of China
Email [email protected]
Fangyou Yu
Department of Clinical Laboratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200082, People’s Republic of China
Email [email protected]
Introduction: The occurrence and development of antibiotic resistance are mainly caused by the spread of large plasmids carrying multiple antibiotic resistance genes. Recently, the association between 16S rRNA methyltransferase genes and β-lactamase genes carried by the same plasmid is of concern.
Methods: The Klebsiella aerogenes 1564 was isolated from the catheter tip of a patient in a tertiary hospital, Shanghai, China. The presence of the blaNDM-1 and rmtC genes were assessed by PCR. Complete sequence of plasmid p1564 was determined. The K. aerogenes 1564 was characterized by antimicrobial susceptibility testing, Carbapenemase phenotype confirmation testing, conjugation experiment, S1-PFGE and multilocus sequence typing (MLST).
Results: Herein, we found that a New Delhi Metallo-β-lactamase-1 gene (blaNDM-1) and a 16S rRNA methyltransferase gene (rmtC) coexisted on a transferrable plasmid of a carbapenem-resistant K. aerogenes clinical isolate. The K. aerogenes clinical isolate was found to belong to a novel sequence type 192 (ST192) determined by MLST. The sequencing results of the plasmid p1564 carrying blaNDM-1 gene and rmtC gene showed that the size and guanine-cytosine content of the plasmid were 136, 902 bp and 51.8%, with 164 putative ORFs and two multidrug resistance gene islands. In addition to blaNDM-1and rmtC, the plasmid contained bleomycin resistance gene (bleMBL), CMY-6β-lactamase gene (blaCMY-6), quaternary ammonium compound resistance gene (sugE), truncated quaternary ammonium compound resistance gene (qacEΔ1), aminoglycoside resistance gene (aacA4) and sulfonamide resistance gene (sul1). By comparison, p1564 has high homology with pHS36-NDM from Salmonella enterica subsp. enterica serovar Stanley reported in China, with similar size and both belonging to plasmid incompatibility group A/C.
Conclusion: The present study demonstrated for the first time the co-existence of rmtC and blaNDM-1 in a novel ST192 K. aerogenes. The spread of plasmids harboring both blaNDM-1 and rmtC may occur among Enterobacteriaceae in China.
Keywords: Klebsiella aerogenes, plasmid, blaNDM-1, rmtC
Introduction
Klebsiella aerogenes is a Gram-negative bacterium which is widely found in human gastrointestinal tract and various other in vivo environments, but is generally not pathogenic to healthy humans.1 This organism was previously known as Enterobacter aerogenes. Since the early 1990s, K. aerogenes has become an important opportunistic pathogen, often leading to hospital-acquired infections such as pneumonia, urinary tract infections, bacteremia, intracranial infections and surgical wound infections.2–6 It has been considered an important new multidrug resistance (MDR) pathogen in the past two decades.7
With the increasing use of carbapenems in clinical practice, infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a significant threat to human health.8 The emergence and spread of antibiotic resistance have caused widespread concern around the world. New Delhi Metallo-β-lactamase-1 (NDM-1) was first identified in a clinical isolate of Klebsiella pneumoniae from a Swedish patient from India in 2009.9 The blaNDM-1 gene has the ability to spread widely in bacterial species through horizontal gene transfer.10 In recent years, plasmid-mediated NDM-1 has spread rapidly among Enterobacteriaceae, mainly Klebsiella pneumoniae and Escherichia coli throughout the world.11 It has recently been reported that 16S rRNA methyltransferase genes are associated with New Delhi Metallo-β-lactamase-1 (NDM-1) in Enterobacteriaceae.12 The combination of beta-lactam and aminoglycoside plays an important role in antimicrobial therapy in severe infections. Gram-negative pathogens co-producing NDMs and 16S rRNA methylases have high levels of resistance to clinically important carbapenems and aminoglycosides, which may result in aminoglycoside and beta-lactam antibiotic combinations lose its clinical therapeutic significance. This study aimed to describe the first time that co-existence of rmtC and blaNDM-1 genes in a novel ST192 K. aerogenesin China.
Materials and Methods
Bacterial Isolates
The Klebsiella aerogenes 1564 was isolated from the catheter tip of a patient in a tertiary hospital in Shanghai, China, and identified by Matrix-Assisted Laser Desorption/Ionisation - Time Of Flight (MALDI-TOF MS) according to the manufacturer’s instructions. Escherichia coli ATCC25922, Staphylococcus aureus ATCC25923 and Pseudomonas aeruginosa ATCC87253 were used as control strains for the identification of the species.
Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) of antimicrobial agents for the bacteria tested were determined using the broth microdilution method and interpreted according to CLSI standards.13 A total of 17 antimicrobial agents were tested, including carbapenems (imipenem and meropenem), β-lactam/β-lactamase inhibitor complexes (piperacillin-tazobactam and ceftazidime-avibactam), monocyclic β-lactam (aztreonam), cephalosporin (cefoxitin, cefotaxime, cefepime and ceftazidime), aminoglycosides (gentamicin and amikacin), fluoroquinolones (ciprofloxacin), folate metabolic pathway inhibitors (sulfamethoxazole), tetracyclines (tetracycline, minocycline and tigecycline) and polymyxin B. E. coli ATCC25922 was used as a control strain for the antimicrobial susceptibility test.
Carbapenemase Phenotype Confirmation Testing
A modified carbapenem inactivation test (mCIM) was performed to detect carbapenemases according to CLSI 2018 standards.13 The tested strains were incubated with meropenem disk (10 μg) in 2 mL TSB at 37°C for 4 hrs. Escherichia coli ATCC25922 was used as an indicator bacteria and its suspension was adjusted to 0.5 McFarland using sterile physiological saline solution. The E. coli ATCC25922 suspension was evenly coated on an MH agar plate. After the plate is dried for 3–10 mins, the meropenem disk (10 μg) was placed on the surface of the agar plate. The treated plate was incubated at 37°C for 18–24 hrs.
Detection of Resistance Genes
The carbapenemase genes responsible for carbapenem resistance (blaKPC, blaVIM, blaGES, blaIMP, blaSPM, blaOXA-23, blaOXA-48, blaSME, blaSIM and blaNDM) and 16S rRNA methyltransferase genes (rmtA, rmtB, rmtC, rmtD, armA and nmpA) were detected using PCR as described previously.14–16 Following PCR, the DNA fragments were analyzed using gel electrophoresis on 1% agarose gels and were sequenced on both strands.
Conjugation Experiment
In order to determine whether the aminoglycoside antibiotic resistance gene rmtC and blaNDM-1 carried by the plasmid on K. aerogenes 1564 can be transferred horizontally, conjugation experiment was carried out in LB broth medium using rifampicin-resistant E. coli EC600 (E. coli EC600Rif-R) as recipient. Cultures of donor and recipient cells in logarithmic phase (200 μL, 100 μL, respectively) were added to 4 mL of fresh LB broth and incubated overnight at 37°C without shaking. To screen for transconjugants, serial dilutions of mixed cultures were plated onto MH agar plates containing amikacin (128 mg/L) and rifampicin (600 mg/L). The donor cells alone and recipient cells alone were used as controls to ensure the effectiveness of the selective plates used. The transconjugant colonies were selected from the selective plates and cultivated onto the selective plates again for purification of transconjugant strains. All transconjugants were confirmed by PCR for the presence of rmtC and blaNDM-1 genes. The antibiotic susceptibilities were also investigated as mentioned above.
Pulsed-Field Gel Electrophoresis (PFGE)
S1-PFGE was performed to obtain plasmid profiles in donor strains, recipient strains and transconjugants, as described previously.17 The Salmonella enterica serotype Braenderup strain H9812 was used as a control standard strain and molecular size marker.
Multilocus Sequence Typing (MLST)
Multilocus Sequence Typing (MLST) was performed on K. aerogenes 1564 by amplifying internal fragments of the seven standard housekeeping loci, includingdnaA, fusA, gyrB, leuS, pryG, rplB and rpoB. Sequence types (STs) were determined according to the Klebsiella aerogenes MLST Databases (https://pubmlst.org/kaerogenes/).
Plasmid Extraction and Sequencing
Plasmid DNA from E. coli EC600Rif-R transconjugants was extracted using Qiagen Plasmid Midi Kit (Qiagen, Valencia, CA, United States of America) according to the manufacturer’s protocol. A library of different inserts was constructed using the Whole Genome Shotgun (WGS) strategy, and these libraries were Paired-end (PE) sequenced on the Illumina MiSeq sequencing platform. The sequencing reads were de novo assembled using the SPAdes v3.9.0.18 The plasmid splicing results were co-linearly analyzed using mummer v3.1 software to determine the positional relationship between contigs. Gaps between contigs were closed through PCR and Sanger sequencing. The results were corrected using Pilon v1.18 software to obtain the final plasmid DNA sequence.19 The circular representation of p1564 was generated with CGview (http://stothard.afns.ualberta.ca/cgview_server/).20 Mauve 2.3.1 was used to perform comparative genome alignment for related plasmids.21
Result
Antimicrobial Susceptibility Testing
Antimicrobial sensitivity results are shown in Table 1. K.aerogenes 1564 was resistant to all beta-lactam antibiotics (cephalosporins, carbapenems, penicillins and monocyclic β-lactams) and aminoglycosides tested, but susceptible to ciprofloxacin, sulfamethoxazole, polymyxin B, tetracycline, minocycline and tigecycline.
 |
Table 1 Antimicrobials MIC Values of for K. Aerogenes, Its Transconjugant and the Recipient Strain |
Detection of Carbapenemases and Resistance Genes
K. aerogenes 1564 was positive for the mCIM assay, indicating that the isolate produced carbapenemases. Consequently, a carbapenemase gene (blaNDM-1) was found among K. aerogenes strain 1564, which was determined by PCR and DNA sequencing. As the K. aerogenes strain 1564 was resistant to gentamicin and amikacin, 16S rRNA methyltransferase genes were detected by PCR and DNA sequencing and rmtC was identified.
Conjugation and S1-PFGE
The conjugation experiments result showed that the rmtC and blaNDM-1 genes were successfully transferred from K. aerogenes 1564 to the E. coli EC600Rif-R recipient, clearly demonstrates the potential for horizontal transfer of the rmtC and blaNDM-1 genes. The drug susceptibility spectrum of the transconjugant is consistent with its donor strain K. aerogenes 1564 (Table 1). The MIC of amikacin for transconjugant was >64 mg/L. In addition, the MIC value of imipenem for transconjugant is at least 8-fold higher than that of E. coli EC600Rif-R. S1-PFGE result showed that only one plasmid of approximately 136 Kb was found (Figure 1). It was confirmed that the plasmid harboring rmtC and blaNDM-1 genes were successfully transferred into recipient E. coli EC600Rif-R by conjugation experiment.
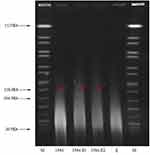 |
Figure 1 S1-nuclease pulsed-field gel electrophoresis profiles. M, Salmonella enterica serotype Braenderup strain H9812; E, E. coli EC600; 1564-E1, transconjugant1; 1564-E2, transconjugant2. |
Multilocus Sequence Typing (MLST)
MLST result showed that K. aerogenes 1564 belonged to a novel ST (ST192). This new ST was arranged by the MLST database (Bacterial Isolate Genome Sequence Database) for ST number assignment. (https://pubmlst.org/kaerogenes/).
Analysis of Genetic Characteristics of p1564
p1564 is a circular molecule 136, 902 bp in length with an average G+C content of 51.8%, harbored 164 predicted ORFs and belonged to plasmid incompatibility group A/C (Figure 2). Plasmid p1564 carries genes for plasmid replication (IncA/C repA), antibiotic resistance (blaNDM-1, rmtC, aacA4, bleMBL, blaCMY-6 and sul1) and conjugation (tra clusters) (Figure 3). Two multidrug resistance gene islands were found on the plasmid p1564. The first was the ISEcp1-blaCMY-6 transposable unit containing the CMY-6β-lactamase gene (blaCMY-6) and quaternary ammonium compound resistance gene (sugE). The other is the intI1-ISCR21 module containing six resistance genes including two aminoglycoside resistance genes (rmtC and aacA4), truncated quaternary ammonium compound resistance genes (qacEΔ1), sulfonamide resistance gene (sul1), carbapenem resistance gene (blaNDM-1) and bleomycin resistance gene (bleMBL). Interestingly, the truncated fragments of the insertion sequences ISAba125 and ISEcp1 were found in the surrounding environment ofblaNDM-1 and rmtC genes, respectively, which promoted the expression and transposition of blaNDM-1 and rmtC (Figure 3). The molecular chaperones groEL and groES genes and rhs gene were identified. The phage integrase-rhs regions were considered to be a hot spot for the integration of accessory genes in the IncA/C plasmid.22 The Class 1 integron of p1564 is composed of integral gene intl1 and the antibiotic resistance markers aacA4, qacEΔ1 and sul1. A class 1 integron with a different gene cassettes arrangement was also found in pHS36-NDM from Salmonella enterica subsp. enterica serovar Stanley in China,23 which is composed of intl1, dfrA12, aadA2, qacE∆1 and sul1. In addition, p1564 is highly similar to pHS36-NDM, and blaNDM-1 and rmtC genes were identified in both plasmids.
Full plasmid sequence BLAST search against the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) showed an overall 99% query coverage with 100% sequence similarity to plasmid pNDM-PstGN576 from Providencia stuartiiisolate GN576and 99% query coverage with 99. 98% sequence similarity to plasmid pNDM-US from Klebsiella pneumoniae strain ATCCBAA-2146.24,25 Furthermore, pNDM-PstGN576 and pNDM-US were used as references for annotating p1564.
Discussion
Recently, 16S rRNA methyltransferases have emerged as an acquired high-level resistance mechanism to clinically relevant aminoglycosides such as gentamicin, tobramycin and amikacin.26 Since 2003, ten 16S rRNA methyltransferase genes, including armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA, have been identified in clinical isolates of Gram-negative bacilli from multiple geographic locations.27 The genes encoding these enzymes are usually borne by mobile genetic elements and have been associated with other important mechanisms such as carbapenemases, extended-spectrum β-lactamases (ESBLs).12 Despite having low prevalence in different types of bacteria, the 16S RMTase-encoding genes are globally spreading because of the plasmids that disseminate carbapenemase and ESBL genes among Gram-negative bacilli.28 The recent appearance of multidrug-resistant bacteria with the 16S rRNA methyltransferases and carbapenemases such as NDM-1 is becoming an increasing clinical and public health threat.
This study revealed the complete nucleotide sequence of plasmid p1564 in K. aerogenes, harboring the blaNDM-1 and rmtC genes, conferring high levels of resistance to carbapenems and aminoglycosides. Although it has been reported that coexistence of blaNDM-1 and rmtC in Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli and Salmonella enterica subsp. enterica serovar Stanley, there has been no report in K. aerogenes.10,29–31 In China, the rmtC gene has only been reported in Salmonella enterica subsp. enterica serovar Stanley.23 To the best of our knowledge, this is the first report of co-existence in K. aerogenes isolate. Moreover, the MLST result showed that K. aerogenes 1564 belongs to a novel ST192 not reported before. High similarity of p1564 compared to blaNDM-harboring plasmids pHS36-NDM from Salmonella enterica subsp. enterica serovar Stanley (KU726616), pMS6198A from E. coli (CP015835.1), pB577-NDM from Enterobacter cloacae (KX786648.1), pNDM-PstGN576 from Providencia stuartii (KJ802405.1) and pNDM-US from Klebsiella pneumoniae (CP006661.1) suggest lateral transfer of this plasmid among different members of the Enterobacteriaceae. The conserved type IV secretion system and the stbA gene were identified in p1564, indicating that this plasmid has the potential for horizontal transfer and stable passage, confirming the speculation obtained above. Previous studies have shown that multiple resistance transfer of plasmids can result from rare gene capture events mediated by different mobile genetic elements, clustering and combinatorial evolution of resistance genes and related mobile elements.32 Upstream of the blaNDM-1 gene, a truncated insertion sequence, ISAba125, was identified, which provides a promoter for the expression of blaNDM-1,22 and indicates that the blaNDM-1 gene may originally be derived from Acinetobacter baumannii.33,34 Simultaneously, a truncated fragment of ISEcp1 was found in the downstream of rmtC gene, which promoted the expression and transposition of rmtC.35 The two above mentioned insertion elements (ISAba125 and ISEcp1) may play acritical role in the widespread transmission of blaNDM-1 and rmtC genes in Enterobacteriaceae.
K. aerogenes 1564 was resistant to all beta-lactam and aminoglycoside antibiotics tested, but susceptible to ciprofloxacin, polymyxin B, tetracycline, minocycline and tigecycline. These antibiotic susceptibility results were consistent with the resistance genes carried in the two multidrug resistance gene islands ISEcp1-blaCMY-6 and intI1-ISCR21 identified in the plasmid. Comparing p1564 with other blaNDM-1-bearing plasmids reported in China, it was found to be highly homologous to pHS36-NDM from Salmonella enterica subsp. enterica serovar Stanley strains, but the class 1 integron structures identified were not identical. Collectively, the variable region of these two plasmids was found to be composed of similar multiple antibiotic resistance markers and IS elements, suggesting that there may be some unknown association between the two plasmids. These results indicate that p1564 may be derived from the horizontal transfer of the harboring blaNDM-1 plasmid in Enterobacteriaceae in China, followed by a series of homologous recombination evolution events.
Conclusion
In conclusion, the present study demonstrated for the first time that co-existence of rmtC and blaNDM-1 in a novel ST192 K. aerogenes. The spread of plasmids harboring both blaNDM-1 and rmtC may occur among Enterobacteriaceae in China. Therefore, a prudent surveillance and therapeutic strategy should be implemented to restrict the widespread spread of plasmids containing blaNDM-1 and rmtC.
Nucleotide Sequence Accession Number
The complete nucleotide sequences of plasmid p1564 have been deposited in GenBank under accession no.MN603981.
Ethics Statement
As the Klebsiella aerogenes clinical isolate in this study was part of the routine hospital laboratory procedure, the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine Academy of Sciences exempted this research for review.
Acknowledgments
The authors thank the excellent technical assistance provided by Liang Chen.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Chen Z, Li H, Feng J, et al. NDM-1 encoded by a pNDM-BJ01-like plasmid p3SP-NDM in clinical Enterobacter aerogenes. Front Microbiol. 2015;6:294. doi:10.3389/fmicb.2015.00294
2. Georghiou PR, Hamill RJ, Wright CE, et al. Molecular epidemiology of infections due to Enterobacter aerogenes: identification of hospital outbreak-associated strains by molecular techniques. Clin Infect Dis. 1995;20(1):84–94. doi:10.1093/clinids/20.1.84
3. Davin-Regli A, Saux P, Bollet C, Gouin F, De Micco P. Investigation of outbreaks of Enterobacter aerogenes colonisation and infection in intensive care units by random amplification of polymorphic DNA. J Med Microbiol. 1996;44(2):89–98. doi:10.1099/00222615-44-2-89
4. De Gheldre Y, Maes N, Rost F, et al. Molecular epidemiology of an outbreak of multidrug-resistant Enterobacter aerogenes infections and in vivo emergence of imipenem resistance. J Clin Microbiol. 1997;35(1):152–160.
5. Jalaluddin S, Devaster JM, Scheen R, Gerard M, Butzler JP. Molecular epidemiological study of nosocomial Enterobacter aerogenes isolates in a Belgian hospital. J Clin Microbiol. 1998;36(7):1846–1852.
6. Ronveaux O, Gheldre Y, Glupczynski Y, Struelens M, Mol P. Emergence of Enterobacter aerogenes as a major antibiotic-resistant nosocomial pathogen in Belgian hospitals. Clin Microbiol Infect. 1999;5(10):622–627. doi:10.1111/j.1469-0691.1999.tb00419.x
7. Chevalier J, Mulfinger C, Garnotel E, Nicolas P, Davin-Regli A, Pages JM. Identification and evolution of drug efflux pump in clinical Enterobacter aerogenes strains isolated in 1995 and 2003. PLoS ONE. 2008;3(9):e3203. doi:10.1371/journal.pone.0003203
8. Sheu CC, Chang YT, Lin SY, Chen YH, Hsueh PR. Infections caused by carbapenem-resistant enterobacteriaceae: an update on therapeutic options. Front Microbiol. 2019;10:80. doi:10.3389/fmicb.2019.00080
9. Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53(12):5046–5054. doi:10.1128/AAC.00774-09
10. Tafaj S, Gona F, Kapisyzi P, et al. Isolation of the first New Delhi metallo-ss-lactamase-1 (NDM-1)-producing and colistin-resistant Klebsiella pneumoniae sequence type ST15 from a digestive carrier in Albania, May 2018. J Global Antimicrob Resist. 2019;17:142–144. doi:10.1016/j.jgar.2018.12.002
11. Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol. 2011;19(12):588–595. doi:10.1016/j.tim.2011.09.005
12. Hidalgo L, Hopkins KL, Gutierrez B, et al. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J Antimicrob Chemother. 2013;68(7):1543–1550. doi:10.1093/jac/dkt078
13. CLSI. 2018CLSI.Performance Standards for Antimicrobial Susceptibility Testing.
14. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi:10.1128/CMR.00001-07
15. Nordmann P, Poirel L, Carrer A, Toleman MA, Walsh TR. How to detect NDM-1 producers. J Clin Microbiol. 2011;49(2):718–721. doi:10.1128/JCM.01773-10
16. Yeganeh Sefidan F, Mohammadzadeh-Asl Y, Ghotaslou R. High-level resistance to aminoglycosides due to 16S rRNA methylation in enterobacteriaceae isolates. Microb Drug Resist. 2019;25:1261–1265. doi:10.1089/mdr.2018.0171
17. Chen Y, Zhou Z, Jiang Y, Yu Y. Emergence of NDM-1-producing Acinetobacter baumannii in China. J Antimicrob Chemother. 2011;66(6):1255–1259. doi:10.1093/jac/dkr082
18. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
19. Walker BJ, Abeel T, Shea T, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9(11):e112963. doi:10.1371/journal.pone.0112963
20. Grant JR, Stothard P. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res. 2008;36(WebServer issue):W181–184. doi:10.1093/nar/gkn179
21. Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE. 2010;5(6):e11147. doi:10.1371/journal.pone.0011147
22. Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. Evolution of IncA/C blaCMY-(2)-carrying plasmids by acquisition of the blaNDM-(1) carbapenemase gene. Antimicrob Agents Chemother. 2012;56(2):783–786. doi:10.1128/AAC.05116-11
23. Huang J, Wang M, Ding H, et al. New Delhi metallo-beta-lactamase-1 in carbapenem-resistant Salmonella strain, China. Emerg Infect Dis. 2013;19(12):2049–2051. doi:10.3201/eid1912.130051
24. Tijet N, Richardson D, MacMullin G, Patel SN, Melano RG. Characterization of multiple NDM-1-producing Enterobacteriaceae isolates from the same patient. Antimicrob Agents Chemother. 2015;59(6):3648–3651. doi:10.1128/AAC.04862-14
25. Hudson CM, Bent ZW, Meagher RJ, Williams KP. Resistance determinants and mobile genetic elements of an NDM-1-encoding Klebsiella pneumoniae strain. PLoS ONE. 2014;9(6):e99209. doi:10.1371/journal.pone.0099209
26. Galimand M, Courvalin P, Lambert T. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother. 2003;47(8):2565–2571. doi:10.1128/AAC.47.8.2565-2571.2003
27. Doi Y, Wachino JI, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am. 2016;30(2):523–537. doi:10.1016/j.idc.2016.02.011
28. Guven Gokmen T, Nagiyev T, Meral M, Onlen C, Heydari F, Koksal F. NDM-1 and rmtC-producing klebsiella pneumoniae isolates in Turkey. Jundishapur J Microbiol. 2016;9(10):e33990. doi:10.5812/jjm
29. Rahman M, Prasad KN, Pathak A, et al. RmtC and RmtF 16S rRNA methyltransferase in NDM-1-producing pseudomonas aeruginosa. Emerg Infect Dis. 2015;21(11):2059–2062. doi:10.3201/eid2111.150271
30. Kapmaz M, Erdem F, Abulaila A, Yeniaras E, Oncul O, Aktas Z. First detection of NDM-1 with CTX-M-9, TEM, SHV and rmtC in Escherichia coli ST471 carrying IncI2, A/C and Y plasmids from clinical isolates in Turkey. J Global Antimicrob Resist. 2016;7:152–153. doi:10.1016/j.jgar.2016.10.001
31. Huang J, Deng S, Ren J, Tu J, Ye M, Wang M. Characterization of a blaNDM1harboring plasmid from a Salmonella enterica clinical isolate in China. Mol Med Rep. 2017;16(2):1087–1092. doi:10.3892/mmr.2017.6733
32. Partridge SR. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiol Rev. 2011;35(5):820–855. doi:10.1111/j.1574-6976.2011.00277.x
33. Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(2):1087–1089. doi:10.1128/AAC.05620-11
34. Toleman MA, Spencer J, Jones L, Walsh TR. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(5):2773–2776. doi:10.1128/AAC.06297-11
35. Hopkins KL, Escudero JA, Hidalgo L, Gonzalez-Zorn B. 16S rRNA methyltransferase RmtC in Salmonella enterica serovar Virchow. Emerg Infect Dis. 2010;16(4):712–715. doi:10.3201/eid1604.090736
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


