Back to Journals » Drug Design, Development and Therapy » Volume 13
Codelivery of GRP78 siRNA and docetaxel via RGD-PEG-DSPE/DOPA/CaP nanoparticles for the treatment of castration-resistant prostate cancer
Authors Zhang X , He Z, Xiang L, Li L, Zhang H, Lin F, Cao H
Received 16 December 2018
Accepted for publication 5 March 2019
Published 29 April 2019 Volume 2019:13 Pages 1357—1372
DOI https://doi.org/10.2147/DDDT.S198400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Xiangyu Zhang,1,* Zelai He,2,* Longquan Xiang,1 Liang Li,1 Haiyan Zhang,1 Fanzhong Lin,1 Hongying Cao1
1Department of Pathology, Jining First People’s Hospital, Jining Medical University, Jining 272000, People’s Republic of China; 2Department of Radiation Oncology, The First Affiliated Hospital of Bengbu Medical University & Tumor Hospital Affiliated to Bengbu Medical University, Bengbu 233004, People’s Republic of China
*These authors contributed equally to this work
Background: Castration-resistant prostate cancer (CRPC) accounts for the majority of prostate cancer deaths, and patients with CRPC are prone to developing drug resistance. Therefore, there is a need to develop effective therapeutics to treat CRPC, especially drug-resistant CRPC. Although various nanoparticles have been developed for drug or gene delivery and control release, approaches to reproducibly formulate the optimal treatment with nanoparticles that could effectively target CRPC and bone metastasis remain suboptimal. Recently, codelivery of a chemotherapeutic agent and a small interfering RNA (siRNA) has become a promising strategy for the treatment of drug-resistant prostate cancer.
Methods: In a previous study, we prepared a novel RGD-PEG-DSPE/CaP nanoparticle as an effective and biocompatible drug and gene delivery system. In this study, we further modify the nanoparticle to obtain the LCP-RGD nanoparticle, which contains a calcium phosphate (CaP) core, dioleoyl phosphatidic acid (DOPA) and RGD modified poly(ethylene glycol)-conjugated distearoyl phosphatidylethanolamine (RGD-PEG-DSPE). This drug delivery system was used for codelivery of GRP78 siRNA and docetaxel (DTXL) for the treatment of the PC-3 CRPC.
Results: The nanoparticles contain the CaP core, which can effectively compress the negatively charged siRNA, while the DOPA and RGD-PEG-DSPE component can effectively carry DTXL. The arginine-glycine-aspartic acid (RGD) segment can target the prostate cancer site, as the cancer site is neovascularized. This novel nanoparticle has good stability, excellent biocompatibility, high drug and siRNA loading capacity, and an in vitro sustainable release profile.
Conclusion: Codelivery of DTXL and GRP78 siRNA has enhanced in vitro and in vivo anti-prostate cancer effects which are much greater than using free DTXL and free GRP78 siRNA together. Our study also indicated that codelivery of DTXL and GRP78 siRNA have an in vitro and in vivo combinational anti-prostate cancer effect and also could effectively sensitize the cell-killing effect of DTXL; this method may be especially suitable for drug-resistant CRPC treatment.
Keywords: codelivery, docetaxel, RANK, siRNA, nanoparticles
Introduction
Prostate cancer is the second most commonly diagnosed cancer in men worldwide, and its incidence continues to grow in China.1,2 In spite of the great advances made in both radical and palliative treatment for prostate cancer, the development of castration-resistant prostate cancer (CRPC) and chemotherapy resistance remains to be two great challenges, which are closely related to poor chemosensitivity.3 Recently, endoplasmic reticulum (ER) stress has been studied as an important mechanism for chemotherapy resistance.4 In fact, castration treatment and/or taxane-based chemotherapy induce cancer cell ER stress and then activate the unfolded protein response signaling pathway. GRP78, as an important ER molecular chaperone, involved in various cellular activities, such as protein folding and assembly, protein quality control, ER Ca2+ binding, activation of transmembrane ER stress sensors.5 In addition, GRP78 has been found to be overexpressed in some tumor cells compared with normal cells. Accordingly, GRP78 has been recently considered as a therapeutic target in many tumors.6–9 Some studies have shown that GRP78 plays important roles in both castration-resistance and drug resistance processes of prostate cancer, and GRP78 is an important downstream effector protein of the androgen receptor (AR).10–14 Therefore, strategies aimed at interfering with GRP78 expression may shed some light on drug resistance mechanisms in prostate cancer. Actually, some studies reported that the silencing of GRP78 using siRNA effectively inhibited prostate cancer cell proliferation, metastasis and sensitized prostate cancer cells to castration therapy or chemotherapy.15–17 Therefore, the combined use of a chemotherapeutic agent and the silencing of GRP78 may be a promising strategy to treat CRPC, and might even be effective in the treatment of metastatic CRPC.
Gene therapy gains more and more interest as an option to treat refractory cancers, and RNA interference technology is the most widely used approach. It is well-established that siRNA is an effective tool to knockdown some specific gene expression; the advantages of siRNA include its reduced toxicity and high specificity.18 However, due to its poor in vivo stability and poor cellular uptake, this approach requires an effective gene delivery vehicle to deliver the siRNA to target cancer cells.19 Over the past few years, various nanoparticle delivery vectors have been designed to effectively deliver chemotherapeutic agent and siRNA.20–23 Among them, calcium phosphate (CaP) nanoparticles have attracted much attention with regard to its use in siRNA delivery.24,25 The positively charged Ca2+ ion can effectively condense the negatively charged siRNA to form a complex, which interacts with phosphate ion and finally forms the CaP/siRNA cores. In order to increase the nanoparticle stability, the CaP/siRNA core was coated with lipids, namely dioleoyl-sn-glycerol-3-phosphoethanolamine, dipalmitoyl-glycerol-3-phosphocholine, and poly(ethylene glycol)-conjugated distearoyl phosphatidylethanolamine (DSPE-PEG).25 The CaP nanoparticle could easily be internalized by cells, then the CaP/siRNA can be resolved in the lysosomes and the siRNA efficiently escaped from cell lysosomes by proton sponge effect, which enables the siRNA encapsulated in the nanoparticle to exert a much more durable gene silencing effect.25
After prostate cancer patients reach the CRPC stage, androgen-deprivation therapy does not inhibit the disease progression into metastasis. Docetaxel (DTXL) is the only approved therapy that has been shown to prolong survival of metastatic CRPC patients, conferring a median survival of 2–3 months, while mitoxantrone and prednisone do not have such advantages.26 Continued signaling from the AR, activation of oncogenic survival pathway, and crosstalks between various signaling pathways contributed to DTXL resistance and CRPC progression.27 However, DTXL has so many side effects, especially hematological toxicity, that sometimes the standard dose of DTXL must be modified to decrease its toxicity reaction. Recently, many novel drug delivery systems have been developed to decrease the toxic side-effects of DTXL and enhance its stability in blood circulation. For instance, one group used a PLA-PEG nanoparticle to deliver DTXL, and effectively decreased the tumor volume whereas the same dose of free DTXL had almost no tumor growth inhibitory effect, this may be due to the sustained in vivo release of DTXL from drug-loaded nanoparticles.28
In this study, we prepared a novel LCP-RGD drug delivery system, which can effectively compress siRNA into its core and load DTXL into its lipid layer (DOPA/RGD-PEG-DSPE), while the PEG polymer can effectively protect the nanoparticle from endocytosis by phagocytes and is beneficial to the stability of the nanoparticle in long blood circulation. The arginine-glycine-aspartic acid (RGD) segment is very important for this drug delivery system as it can target the prostate cancer site because the tumor site is characterized by neovascularization. This LCP-RGD codelivery system for GRP78 siRNA and DTXL may act synergistically to address the PC-3 cell drug resistance.
Materials and methods
Materials
PEG (Mn=2000), RGD, DSPE, and DOPA were purchased from Sigma–Aldrich Co., Ltd. (Shanghai, China). N-(3-Dimethylaminopropyl)-N0-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from GL Biochem Co., Ltd. (Shanghai, China). GRP78 siRNA and scrambled siRNA (negative control, NC) were designed and synthesized by Ruibo Biotechnology Inc. (Guangzhou, China) (Table 1), rabbit anti-human GRP78 polyclonal antibody and anti-GAPDH monoclonal antibody were purchased from Abcam. Docetaxel (Adriamycin, ADR) was purchased from Beijing Huafenglianbo Technology Co., Ltd (Beijing, China). TRIzol® reagent was purchased from Invitrogen (Gibco, Paisley, Renfrewshire, UK), and 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) was purchased from Qianchen Biotechnology Inc. (Shanghai, China).
 | Table 1 Primers and GRP78 siRNA used in this study |
PC-3 cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and cells were grown in DMEM medium (Paisley, UK) containing 10% fetal bovine serum at 37°C in a humidified environment containing 5% CO2.
Nude male Balb/c mice (4–6 weeks old; body weight: 14−21 g) were purchased from the Jining Medical University (Jining, China). All animal procedures were performed according to the research protocol approved by the Animal Care and Use Committee of Jining No.1 People’s Hospital, and this study was approved by this committee.
Synthesis and characterization of the copolymer
DSPE-PEG2000-RGD copolymers were synthesized according to our previous study.29 Briefly, the following synthesis procedures were performed: 1) DSPE-PEG2000-NHS was synthesized by copolymerizing the DSPE and NHS-PEG2000-NHS; 2) DSPE-PEG2000-RGD was prepared using NHS and DIC to graft RGD to the DSPE-PEG2000-NHS. Then, the composition of the RGD-PD copolymers was determined by 1H-NMR spectroscopy using a 400 MHz WB Solid-state NMR Spectrometer (Avance III, Bruker, Germany).
Preparation and physicochemical characterization of the nanoparticles
The LCP-RGD nanoparticle was prepared with reference to previous studies.24,29,30 Briefly, two water in oil microemulsions was prepared: 1) 100 μL of 500 mM CaCl2 and 16 μL of 2 mg/mL siRNA were emulsified in 8 mL cyclohexane oil phase using the JY92-II ultrasonic cell disruptor from Ningbo Scientz Biotechnology Co. Ltd., (Ningbo, China). 2) 100 μL of 100 mM pH 9.0 Na2HPO4 in 8 mL cyclohexane oil phase with 320 μL of 20 mM DOPA added as the inner leaflet lipid. After mixing the two microemulsions for 45 mins, 30 mL of absolute ethanol was added and the mixture was centrifuged at 12,500 g for 15 mins to precipitate the CaP cores. To create the outer leaflet lipid coating, 100 μL of 20 mM of DSPE-PEG2000-RGD micelle (DTXL: 0.1 mg/mL, 10 μg dissolved in 100 μL of chloroform) was mixed with the cores, and suspended in a small volume of ethanol and then dispersed in water.
Nanoparticle stability evaluation
A total of 2 mL of LCP-RGD nanoparticles was stored in 5 mL of phosphate-buffered saline (PBS) (0.1 M, pH 7.4) or 5% BSA with continuous stirring at 128 rpm, at 4°C or 37°C for 6 days. Then, the size of the nanoparticles was measured at 0, 1, 2, 3, 4, 5, and 6 days with a Zetasizer IV analyzer (Malvern Zetasizer Nano ZS90, UK). And the corresponding polydispersity index (PDI) of nanoparticles was also evaluated.
Analysis of physical and chemical properties of nanoparticles
The size and surface potential of blank nanoparticles were measured with Zetasizer IV analyzer (Malvern Zetasizer Nano ZS90, UK). The morphology and size of blank nanoparticles were evaluated using a transmission electron microscope (H-800; Hitachi, Japan).
The DTXL or siRNA encapsulated in nanoparticles was assessed using UV spectrophotometry. Briefly, 4 mL of DTXL NPs or siRNA NPs was centrifuged (15,000 rpm × 30 mins) at 4°C, and DTXL or siRNA concentration in the supernatant was determined by UV spectrophotometry. The drug encapsulation efficiency (EE%) was expressed as the percentage of the amount of DTXL or siRNA encapsulated in the nanoparticles in relation to the total amount of DTXL or siRNA initially added. The drug loading (DL%) was expressed as the percentage of the amount of DTXL or siRNA encapsulated in the nanoparticles with regard to the total amount of nanoparticles initially added.
In vitro release of DTXL and GRP78 siRNA
The release profile of nanoparticles in PBS or Tris-EDTA (TE) buffer solution was evaluated using a dialysis method. Briefly, 2 mL of NPs loaded with DTXL or siRNA was added into in a dialysis bag (Greenbird Inc, Shanghai, China) and gently shaken at 37°C. Aliquots 2 mL in volume were drawn and replaced with an equal volume of fresh media. The concentration of DTXL or siRNA was measured using UV spectrophotometry at a wavelength of 480 or 260 nm, respectively.
Cell viability analysis
The cytotoxicity of LCP-RGD against PC-3 cells was evaluated with the MTT assay. The PC-3 cells were seeded into 96-well plates at a concentration of 5×103 cells/well and incubated for 24 hrs, and then the cells were incubated for 24, 48, and 72 hrs with different concentrations of blank LCP-RGD NPs. Then, an MTT-water solution (100 μL, 5 mg/mL) was added into each well and incubated for 4 hrs. After incubation, the culture medium was completely replaced with 100 μL of DMSO and incubated for 10 mins. The absorbance was then measured at 490 nm with a microplate reader from Bio-Rad Laboratories (CA, USA). We also tested the cytotoxicity of free DTXL, free siRNA, free DTXL + siRNA, NPs (DTXL + siRNA) against PC-3 cells (DTXL 1 μg/mL, siRNA 50 nM) using the same method.
Additionally, we used Calcein AM staining to evaluate the cell viability of PC-3 cell; green fluorescence of the cytosol indicated viable cells. PC-3 cells were incubated with different formulations of DTXL and siRNA in 12-well plates for 24 hrs; after 24 hrs incubation, the media was replaced with 1 mL PBS containing 1 μL of Calcein AM stock solution (1 mg/mL in DMSO) for 30 mins. Subsequently, each well was washed twice with PBS and observed using an IX-51 fluorescence microscope from Olympus Optical Company, Ltd (Tokyo, Japan).
Blood compatibility assays
Calculation of whole blood clotting time (CT)
The CT was determined by the Lee-White method. Initially, NPs were placed in the silicified glass tubes. Blood was rapidly taken from the healthy New Zealand White rabbits. As soon as the blood began to enter into the syringe, timing was started. Then, 1 mL of blood was quickly injected into the tubes containing NPs, at a final concentration of 1 mg/mL. After 30 s, the tubes were tilted 30° at each interval of a predetermined time until they were tilted to 90° and the blood was no longer able to flow. Then, at this timepoint, the CT was recorded. The blank group and polyethyleneimine group were used as negative and positive control, respectively. Every experiment was performed at a temperature of 37 °C (n=3).
Plasma recalcification time (PRT), PT, and APTT
Exposing NPs to blood has the potential to produce a coagulation cascade. To determine the effect of the prepared NPs on the blood coagulation cascade, a few coagulation assays were performed. PRT was used to determine the CT in which the platelet-poor plasma (PPP) is coagulated by a substrate after activating prothrombin (factor І) in the presence of Ca2+. PPP was obtained by centrifuging anticoagulated blood (blood/3.8% citrate acid solution =9:1), which was collected from healthy New Zealand White rabbits, at 3,000 rpm for 10 mins. PT is primarily used in order to evaluate the extrinsic coagulation pathways. These extrinsic pathways can reflect the effects of NPs for coagulation factors І, II, V, VII, X signaling pathway. APTT is primarily incorporated in order to evaluate the intrinsic coagulation pathways. These intrinsic pathways can reflect the effects of NPs on coagulation factors VIII, IX, XI, XII signaling pathway.
PRT: The NPs samples (1 mg/mL) were prepared by directly dissolving the NPs in 0.025 M CaCl2. Next, 100 μL of the NPs sample was added into a silicified glass tube. A volume of 100 μL of PPP was preheated to a temperature of 37°C and added into the NPs samples. After a time period of 50–100 s, the glass tubes were tilted every 1–2 s in order to evaluate the CT. The glass tube and the siliconized glass tube were used as a positive and negative control, respectively.
PT and APTT: 0.9 mL of PPP were incubated with 0.1 mL of NPs solution (10 mg/mL) and incubated for a time period of 30 mins at 37°C. Then, 0.5 mL of PT Hemostasis reagent (Labtest®) or APTT partial thromboplastin reagent (Labtest®) were added in conjunction with 0.5 mL of CaCl2 (0.025 mol/L) to the plasma samples. The PT and APTT were determined on a coagulation analyzer. Normal saline treated-PPP was used as negative control (n=3).
Complement activation
Serum was obtained from the blood of healthy rabbits that was collected into glass vacutainers in the absence of anticoagulants. Blood was allowed to coagulate for 1 hr at 37°C. At that point, the blood was centrifuged at 1,000 g for 20 mins, and then the serum was collected. The NPs were added into the serum in order to form a solution with the NPs at a concentration of 1 mg/mL. The solution was incubated for 1 hr at 37°C. The turbidimetric method was used to assess the concentration of the complement protein C3 in the serum following the kit manufacturer protocol.
Gene silencing efficiency assay
PC-3 prostate cancer cells were seeded in 12-well plates at a density of 2×105 cells/well and cultured for 24 hrs (37°C, 5% CO2). After 24 hrs, the culture medium was changed to RPMI-1640 (free of fetal serum). Free GRP78 siRNA, scramble siRNA and GRP78 siRNA NPs (0.4 µg siRNA per well) were added to the wells. After incubation for 48 hrs, the gene silencing effect of siRNA was quantitated using real-time PCR. The primers used in this study are shown in Table 1. Data analysis was performed using the ΔΔCt method, with the Ct value of GAPDH as the standard, ΔCt=the Ct value of GRP78 gene – Ct value of GAPDH. The expression of GRP78 gene was reported as 2-ΔΔCt.
Western blotting analysis
PC-3 cells were plated in six-well plate at a concentration of 5×106 cells/well, and incubated for 24 hrs. Then, cells were lysed with RIPA lysis buffer supplemented with protease inhibitor phenylmethylsulfonyl fluoride (1 mM). Bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA, USA) was used to determine protein concentration. Protein was separated by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Millipore, MA, USA). The membrane was incubated with GAPDH primary antibody (Abcam), GRP78 antibody (Abcam), LC3 antibody (Abcam). This was followed by incubation with HRP-conjugated secondary goat anti-rabbit or anti-mouse antibody at 37°C for 1 hr, and the images were captured in a Bio-Rad imaging system (Shanghai, China).
Evaluation of cell cycle and apoptosis
The PC-3 cells were plated, separately, into six-well culture plates at 5×105 cells/well, and incubated for 24 hrs at 37°C. The cells were further incubated for a period of 24 hrs with free DTXL, free GRP78 siRNA, free DTXL + free siRNA, or NPs (DTXL + siRNA) at an equivalent concentration of DTXL (0.2 μg/mL) or siRNA (100 nM) for 24 hrs. Subsequently, cells were harvested at the predetermined time points and fixed in 1 mL cold 70% ethanol for 24 hrs. Afterwards, the cells were centrifuged, washed, and incubated with 0.1% RNase A for 1 hr at 37°C. Finally, the cells were stained with propidium iodide (PI) for 30 mins at 4°C, and analyzed by a FACScan flow cytometer from Becton Dickinson (New York, NY, USA). Cells without any treatment were considered to be negative control.
Cell apoptosis was assessed using Annexin V-PI (Beyotime Co., Ltd, Haimen, China). Briefly, the PC-3 cells were seeded into a six-well plate at a concentration of 4×105 cells/well and incubated for 24 hrs. Then, PC-3 cells were incubated with free DTXL, free GRP78 siRNA, free DTXL + free siRNA, NPs (DTXL + siRNA) at an equivalent concentration of DTXL (0.2 μg/mL) or siRNA (100 nM) for 24 hrs. Then, cells were collected and centrifuged (1,500 rpm ×5 mins), resuspended in PBS and incubated with 195 μL of binding solution. The cells were incubated with 5 μL of Annexin V-FITC and 10 μL of PI solution. After incubation for 15 mins at room temperature, the cells were analyzed with FACScan flow cytometry from Becton Dickinson. Cells that were not treated with Annexin V-PI were used as a control.
Therapeutic effects
The PC-3 prostate cancer-bearing cells were established in nude female BALB/c mice by subcutaneous inoculation of a suspension of PC-3 cells (2×106 cells/100 μL) into the armpit. Animal experiments were not carried out until the volume of the tumor grew to approximately 50 mm3. Then, the PC-3 cancer-bearing mice described earlier were randomly divided into five groups (five mice per group), and treated with different treatments: (1) PBS, (2) free DTXL, (3) free siRNA, (4) free DTXL + free siRNA, or (5) NPs (DTXL + siRNA). The nude mice were treated every 3 days for 24 days. During the period of prostate cancer therapy, the surviving mice were monitored throughout the experiment. siRNA were administered at an equivalent dose of 1.3 mg/kg in the aforementioned group, DTXL were administered at an equivalent dose of 2 mg/kg. The tumor size was measured by a Vernier caliper, and its volume (V) was calculated as V=d2*D/2, where d and D corresponded to the shortest and the longest diameter of the tumor in mm, respectively. After the period of treatment, the mice were sacrificed to obtain the tumor, heart, liver, spleen, lung, and kidney, and the morphology of these major organs was observed.
Statistical analysis
SPSS 13.0 statistical software was utilized to analyze the relevant data. All data experiements were performed in triplicate. The two-tailed Student’s t-test was used to compare the means between two independent samples; One-way ANOVA was used to measure the differences between the means of multiple samples; comparison among groups was performed by the SNK method. Log-rank analyses were used to analyze significance in Kaplan–Meier survival curves. and P<0.05 indicated that the differences were statistically significant.
Results
Characterization of NPs
The scheme for the preparation of LCP-RGD preparation is shown in Figure 1A, and the surface of nanoparticles used to codeliver DTXL and siRNA (Figure 1B) was smooth and no aggregation was observed. The size and zeta potential of different nanoparticles are shown in Table 1; the size and zeta potential of DTXL and siRNA-loaded nanoparticles were 39.7 nm and −24.2 mV, respectively (Table 2). The PDI was 0.142. The results revealed that these LCP-RGD nanoparticles can effectively encapsulate DTXL and siRNA, and the drug encapsulation efficiency of DTXL and siRNA was 83.8% and 82.4%, respectively.
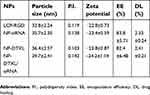 | Table 2 The characterization of drug loaded RGD-LCP nanoparticles |
Drug-loaded nanoparticles stability and release profile
Nanoparticles stability is very important for nanoparticle storage, optimal therapeutic effect, and in vivo targeting effect. It was found that LCP-RGD nanoparticles were stable in PBS at 4°C for 48 hrs, as the size of the nanoparticles remained at less than 40 nm with no noticeable changes, and the PDI values were less than 0.2 during the storage period of 48 hrs. However, when the nanoparticles were stored in 5% BSA, the nanoparticle size changed from 42 to 45 nm between day 0 and day 6, and the PDI was less than 0.20. These results indicated that the LCP-RGD nanoparticles had good stability at 4°C or in 5% BSA (Figure 2A and B).
Nanoparticle drug delivery had a distinct advantage that drug or siRNA could be released from nanoparticle in a controlled manner, and can exert long-term effects toward tumor cells. We found that the siRNA or DTXL could be released from drug-loaded nanoparticles in a sustainable manner. As for DTXL, the free DTXL released 71.2%, 74.3%, and 80.4% at 24, 48, and 72 hrs, respectively, while the DTXL-siRNA codelivered nanoparticles released 44.7, 53.3, and 61.3 during 24, 48, and 72 hrs, respectively. We also found that DTXL loaded by nanoparticles released much more slowly than the free DTXL (Figure 2C). As for the siRNA, free siRNA released 62.4%, 73.5%, and 81.4% at 24, 48, and 72 hrs, respectively, while the DTXL-siRNA codelivered nanoparticles released 46.4, 59.7%, and 64.6% for 24, 48, and 72 hrs, respectively, and after siRNA being loaded by nanoparticles, siRNA released much more slowly than the free siRNA (Figure 2D).
GRP78 expression after treatment with different formulations of the siRNA-loaded nanoparticle
The real-time PCR analysis and Western blotting analysis results showed that GRP78 was robustly expressed in PC-3 cells at the mRNA and protein level (Figure 3A and B). The negative control siRNA (scrambled siRNA) had almost no gene silencing effects. Compared with the free siRNA group (gene expression level was 0.83), the siRNA-NPs showed more efficient gene silencing effect and the GRP78 gene expression level was only 0.34 compared with the control group; even at a concentration of less 5 nM the siRNA effectively silenced gene expression. This may be due to the effective encapsulation of the siRNA by LCP-RGD nanoparticles, which prevent free siRNA from degradation. The other reason is that the CaP core may induce the siRNA escape from the endosome, and then siRNA could bind with the target mRNA in the cytoplasm.
Changes of the siRNA-transfected PC-3 cells sensitivity to DTXL
The MTT assay was used to calculate the IC50 values of PC-3 cells after treatment with different formulations of DTXL and/or siRNA. The IC50 values of PC-3 cells treated with different formulations are shown in Table 3, demonstrating that 0.32 μg/mL of free DTXL induced a 50% reduction in PC-3 cell proliferation. However, for free DTXL + free siRNA, the concentration required for 50% inhibition of cell proliferation was 0.27 μg/mL. As expected, the exposure of PC-3 cells to nanoparticles codelivering DTXL and siRNA significantly decreased the IC50 value of PC-3 cells treated with DTXL and GRP78 siRNA (IC50 values of 0.15 μg/mL for siRNA and DTXL codelivered NPs).
 | Table 3 IC50 values of DTXL in PC-3 cells after transfection with different DTXL and siRNA formulations |
PC-3 cells viability treated with different nanoparticles formulations
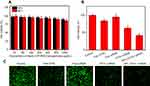
First, we evaluated the cytotoxicity effects of blank LCP-RGD; different concentrations (from 10 to 1,000 μg/mL) of blank nanoparticles had almost no cytotoxicity toward PC-3 cells, since after incubation with nanoparticles for 24 and 48 hrs, cell viability was still above 80% (Figure 4A). Then, we evaluated the cell-killing effect of DTXL and/or siRNA-loaded nanoparticles on PC-3 cells after incubation for 24 hrs; the viability of cells in the control group, free DTXL group, free siRNA group, free DTXL + free siRNA group, NPs (DTXL + siRNA) group was 100%, 84%, 95%, 63%, and 42%, respectively. Codelivery of DTXL and siRNA via nanoparticles had the most potent antitumor effects, that is much better than simultaneous usage of free DTXL and free siRNA (Figure 4B), which was consistent with the Calcein AM staining results (Figure 4C).
Cell cycle arrest and cell apoptosis effects of drug-loaded nanoparticles
In order to determine whether the PC-3 cell growth inhibitory was associated with cell cycle arrest and apoptosis, we evaluated the effect of exposure to the drug and/or siRNA-loaded nanoparticles for 24 hrs on cell cycle and apoptosis in PC-3 cells. The proportion of G2/M phase cells of the control group, free DTXL group, free siRNA group, free DTXL + free siRNA group, and NPs (DTXL + siRNA) was 18.0, 23.2, 19.7, 24.1%, and 30.4%, respectively. Meanwhile, the total apoptosis rate of cells in the control group, free DTXL group, free siRNA group, free DTXL + free siRNA group, and NPs (DTXL + siRNA) group was 1.9%, 17.7%, 2.5%, 37.1%, and 66.4%, respectively. Therefore, the NPs (DTXL + siRNA) had the strongest inducing effect on the G2/M cell cycle arrest and cell apoptosis including total apoptosis and late apoptosis, compared with cells in the other three groups (p<0.05). Thus, we conclude that codelivery of DTXL and siRNA via nanoparticles has a synergistic effect on cell cycle arrest and apoptosis induction, which is much stronger than that of simultaneous treatment with free DTXL and free siRNA (Figure 5).
Blood compatibility analysis
Blood compatibility is very important for nanoparticle usage in the clinic. Therefore, we evaluated blood compatibility with free DTXL group, free siRNA group, free DTXL + free siRNA group, and NPs (DTXL + siRNA). The CT, APPT, PT, and PRT values of NPs (DTXL + siRNA) did not change significantly when compared with control group; this finding indicated that the drug/siRNA loaded NPs possessed excellent blood compatibility (Figure 6A–C). Then, we evaluated complement C3 activation, and the results indicated that C3 was not activated in the NPs (DTXL + siRNA) group (Figure 6D).
Cell cycle arrest mechanism and cell autophagy analysis
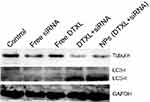
To investigate the cell cycle arrest mechanism, we first evaluated changes in α-tubulin protein after PC-3 cells were treated with different formulations of DTXL and/or siRNA. The results revealed that the α-tubulin protein expression was much more effectively decreased in the NPs (DTXL + siRNA) group than in the other groups; this finding was consistent with the cell cycle analysis result. After the decrease of the α-tubulin protein level, the cell cycle was arrested at the G2/M phase (Figure 7). In order to determine whether the PC-3 cell growth inhibition was associated with cell autophagy, we evaluated the LC3 protein change; we found that the LC3I protein change to LC3II was most noticeable in cells of the NPs (DTXL + siRNA) group, this indicated that codelivery of DTXL and GRP78 siRNA could induce cell autophagy (Figure 7).
In vivo tumor growth inhibition
PC-3 tumor xenografts were established to study the in vivo tumor therapeutic efficiency of the codelivery of DTXL and GRP78 siRNA via LCP-RGD nanoparticles. Nude mice bearing PC-3 tumor xenografts were subjected to various formulations at 1.3 mg/kg GRP78 siRNA and 0.5 mg/kg DTXL every 3 days. As shown in Figure 8A and B, there was little tumor growth inhibition in either the free DTXL or free siRNA group when compared with the control group (Table S1). The tumor growth was much more efficiently inhibited in the free DTXL + free siRNA group, this indicated that there were some synergistic effects between DTXL and GRP78 siRNA. However, the tumor growth was almost completely inhibited in the NPs (DTXL + siRNA) group, suggesting that codelivery of DTXL and GRP78 siRNA via nanoparticles can be passively targeted to the tumor and then exert the most effective therapeutic effects (Figure 8A). And the NPs (DTXL + siRNA) group possessed the longest survival time compared with free DTXL + free siRNA group (Figure 8C). These excellent therapeutic effects appear to be related to the downregulation of the GRP78 gene in the tumor (Figure 8D).
Systemic toxicity evaluation
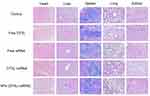
Biosafety of drug and biomaterials is the basis for their wide use in medicine. Accordingly, we adopted histological analysis to evaluate the in vivo toxicity of free DTXL, free siRNA, free DTXL + free siRNA, and NPs (DTXL + siRNA). The physiological states of mice were recorded daily during the whole duration of the treatment. At the end of the treatment, all mice were sacrificed and the major organs were dissected and examined. All animals showed adequate activity. There was some damage in the heart in free DTXL group, and there was no obvious damage in heart, liver, spleen, lung, or kidney in all other groups. This indicated that DTXL and siRNA loaded LCP-RGD nanoparticles had no systemic toxicity (Figure 9). Thus, it is possible that the codelivery of DTXL and siRNA using LCP-RGD nanoparticles can be further developed for use in clinical practice in the future.
Discussion
In this study, we prepared a novel LCP-RGD nanoparticle for codelivery of DTXL and GRP78 siRNA for CRPC therapy. CRPC is a progression phenomenon that often occurred in prostate cancer after a period of castration treatment, and often leads to patient death. And GRP78 overexpression is considered to be involved in the development of castration resistance.12,31 Additionally, some studies have shown that GRP78 overexpression is correlated with chemotherapy resistance in breast cancer, ovarian cancer, pancreatic cancer, etc.32–35 Therefore inhibiting the function of GRP78 or knockdown GRP78 expression may reverse castration resistance and chemotherapy resistance. The advantages of using siRNA to downregulate the expression of GRP78 include high specificity and low toxicity to normal cells, but an efficient siRNA delivery vector is in urgent need as free siRNA can be easily degraded in blood and be hardly internalization by cells.36 Nanoparticles have been used as carriers for siRNA delivery for quite some time, and they can protect the siRNA from degradation during the delivery process.20,37 CaP-based nanoparticles are especially widely used for siRNA delivery, due to their good biocompatibility and high delivery efficiency. Since neovascular vessels are abundant in tumors, and αvβ3 integrin is highly expressed in cancer, we used the RGD peptide as a target moiety in our study.23,38 In this study, we prepared a novel LCP-RGD targeted nanoparticle, which can effectively encapsulate and then slowly release the siRNA and DTXL. We found that LCP-RGD nanoparticles can effectively load the GRP78 siRNA and silence the expression of the GRP78 gene in PC-3 cells, by more than 50% at the mRNA and protein expression level. After knocking down the GRP78 gene, the drug resistance ability of prostate cancer cells was dramatically reversed and the sensitivity of PC-3 cells to DTXL was increased. This may be due to the induction of cell cycle arrest, apoptosis and cell autophagy in PC-3 cells.
Cancer combinatorial therapy via codelivery of drug and gene is gaining more and more attention, and some studies have shown that treatment with drug and gene can have a synergistic effect.39–41 Some carriers developed to codeliver drug and gene are not very effective in practice; these carriers often have low drug-loading ability and poor stability. However, the LCP-RGD nanoparticles showed good loading ability for both DTXL and siRNA, and also excellent sustained release of DTXL and siRNA, which lay a great foundation for its combined antitumor effects. DTXL can be easily encapsulated in the bilayer lipid of DOPA and DSPE-PEG, and siRNA can be easily encapsulated into the CaP core. Therefore, the DTXL was firstly released from the nanoparticle and then the siRNA was released from the nanoparticle. This property of LCP-RGD is useful for DTXL to kill cancer cells. Moreover, previous studies have also reported that knocking down GRP78 expression could enhance the chemotherapeutic agent efficiency.42–44
Additionally, in vitro cell viability experiments and in vivo prostate xenograft cancer experiments indicated that codelivery of DTXL and siRNA had the most effective therapeutic effects. There are at least three explanations for this synergism. First, more DTXL and siRNA may be taken up by PC-3 cells after encapsulation into the LCP-RGD nanoparticles. Second, the drug and siRNA can be more gradually released into the cytoplasm of PC-3 cells, compared with the bustle release of free DTXL and free siRNA, which is very important for cancer cell killing effects. Third, after knockdown of GRP78 expression by GRP78 siRNA, the DTXL could effectively induce cell cycle arrest, apoptosis, and autophagy. The core of the LCP-RGD nanoparticles contains the cationic Ca2+, and thus could bind the negative siRNA via electrostatic adsorption. Another possible reason may be attributed to the RGD group, as the RGD moiety bind to integrin αvβ3 and made nanoparticle concentrate in the tumor site via affinity to integrin αvβ3 integrin, thus increasing their chances of being taken up by cancer cells and delivering more drug.
Biostability is a very important problem concerning nanoparticles. After evaluating the LCP-RGD nanoparticles stability in 4°C and BSA, we determined that the size and PDI of nanoparticles in both surroundings are relatively stable. These findings indicated that LCP-RGD nanoparticles have good stability during physiological circulation after entering into the bloodstream. In addition, we also found that LCP-RGD has a low hemolysis rate, good anticoagulation property, optimal cell, and immune safety. Biosafety of biomaterials is a very important consideration for their future use in clinical practice. Accordingly, we evaluated in vivo biosafety of LCP-RGD in PC-3 tumor-bearing mice. The heart in the free DTXL group showed a little damage, however, when DTXL was encapsulated into the nanoparticles, almost no damage was observed. This suggested that DTXL and siRNA loaded nanoparticles had no obvious side effects on the tumor-bearing mice.
In conclusion, we developed a novel LCP-RGD nanoparticle for codelivery of DTXL and siRNA to treat CRPC, which has a good combinatorial therapeutic effect. We established that DOPA and DSPE-PEG polymer could encapsulate the chemotherapeutic agent DTXL, while the CaP core of the nanoparticle could effectively compress the negatively charged siRNA. The possible synergistic antitumor effects observed between DTXL and GRP78 siRNA may be related to increased cell cycle, apoptosis and autophagy after silencing GRP78, ultimately leading to a greatly enhanced cell killing effect of DTXL. Our study may have great potential for clinical applications.
Acknowledgments
This research was supported through grants from the Natural Science Foundation of Shandong Province (No. ZR2017QH005) and the National Natural Science Foundation of China (No. 81803097 and 81602727).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev. 2017;57:16–27. doi:10.1016/j.ctrv.2017.04.008
4. Avril T, Vauléon E, Chevet E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis. 2017;6(8):e373. doi:10.1038/oncsis.2017.72
5. Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi:10.1016/S0968-0004(01)01908-9
6. Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434(2):181–188. doi:10.1042/BJ20101569
7. Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nat Rev Cancer. 2014;14(4):263–276. doi:10.1038/nrc3701
8. Luo B, Lee AS. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32(7):805–818. doi:10.1038/onc.2012.130
9. Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529(7586):326–335. doi:10.1038/nature17041
10. Delie F, Petignat P, Cohen M. GRP78-targeted nanotherapy against castrate-resistant prostate cancer cells expressing membrane GRP78. Target Oncol. 2013;8(4):225–230. doi:10.1007/s11523-012-0234-9
11. Mandelin J, Cardó-Vila M, Driessen WH, et al. Selection and identification of ligand peptides targeting a model of castrate-resistant osteogenic prostate cancer and their receptors. Proc Natl Acad Sci USA. 2015;112(12):3776–3781. doi:10.1073/pnas.1500128112
12. Tan SS, Ahmad I, Bennett HL, et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. J Pathol. 2011;223(1):81–87. doi:10.1002/path.2795
13. Pootrakul L, Datar RH, Shi SR, et al. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12(20 Pt 1):5987–5993. doi:10.1158/1078-0432.CCR-06-0133
14. Bennett HL, Fleming JT, O’Prey J, Ryan KM, Leung HY. Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells. Cell Death Dis. 2010;1:e72. doi:10.1038/cddis.2010.50
15. Ferrara F, Staquicini DI, Driessen WHP, et al. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proc Natl Acad Sci USA. 2016;113(45):12786–12791. doi:10.1073/pnas.1615400113
16. Lu T, Yang W, Wang Z, et al. Knockdown of glucose-regulated protein 78/binding immunoglobulin heavy chain protein expression by asymmetric small interfering RNA induces apoptosis in prostate cancer cells and attenuates migratory capability. Mol Med Rep. 2015;11(1):249–256. doi:10.3892/mmr.2014.2737
17. Tanimoto R, Sakaguchi M, Abarzua F, et al. Down-regulation of BiP/GRP78 sensitizes resistant prostate cancer cells to gene-therapeutic overexpression of REIC/Dkk-3. Int J Cancer. 2010;126(7):1562–1569. doi:10.1002/ijc.24764
18. Tuschl T, Borkhardt A. Small interfering RNAs: a revolutionary tool for the analysis of gene function and gene therapy. Mol Interv. 2002;2(3):158–167. doi:10.1124/mi.2.3.158
19. Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi:10.1038/nature03121
20. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi:10.1038/nrg3763
21. Wang Z, Liu G, Zheng H, Chen X. Rigid nanoparticle-based delivery of anti-cancer siRNA: challenges and opportunities. Biotechnol Adv. 2014;32(4):831–843. doi:10.1016/j.biotechadv.2013.08.020
22. Majumder P, Bhunia S, Bhattacharyya J, Chaudhuri A. Inhibiting tumor growth by targeting liposomally encapsulated CDC20siRNA to tumor vasculature: therapeutic RNA interference. J Control Release. 2014;180:100–108. doi:10.1016/j.jconrel.2014.02.012
23. Chen CW, Yeh MK, Shiau CY, Chiang CH, Lu DW. Efficient downregulation of VEGF in retinal pigment epithelial cells by integrin ligand-labeled liposome-mediated siRNA delivery. Int J Nanomedicine. 2013;8:2613–2627. doi:10.2147/IJN.S39622
24. Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. doi:10.1016/j.jconrel.2011.10.020
25. Xu X, Li Z, Zhao X, Keen L, Kong X. Calcium phosphate nanoparticles-based systems for siRNA delivery. Regen Biomater. 2016;3(3):187–195. doi:10.1093/rb/rbw010
26. Tanimoto T, Hori A, Kami M. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(20):1966. doi:10.1056/NEJMoa1011205
27. Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8(1):12–23. doi:10.1038/nrclinonc.2010.136
28. Hrkach J, Von Hoff D, Mukkaram Ali M, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39. doi:10.1126/scitranslmed.3003651
29. Huang J, Zhang X, Wu Z, et al. Preparation and biocompatibility evaluation of PEG-PLL/RGD-PEG-DSPE/Phospholipid/CaP nanoparticles. J Biomed Nanotechnol. 2018;14(1):98–113. doi:10.1166/jbn.2018.2460
30. Liu Y, Hu Y, Huang L. Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles. Biomaterials. 2014;35(9):3027–3034. doi:10.1016/j.biomaterials.2013.12.022
31. Azad AA, Zoubeidi A, Gleave ME, Kn C. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat Rev Urol. 2015;12(1):26–36. doi:10.1038/nrurol.2014.320
32. Roller C, Maddalo D. The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol. 2013;4:10. doi:10.3389/fphar.2013.00010
33. Cook KL, Clarke R. Role of GRP78 in promoting therapeutic-resistant breast cancer. Future Med Chem. 2015;7(12):1529–1534. doi:10.4155/FMC.15.80
34. Deying W, Feng G, Shumei L, Hui Z, Ming L, Hongqing W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci Rep. 2017;37(2):
35. Gifford JB, Huang W, Zeleniak AE, et al. Expression of GRP78, master regulator of the unfolded protein response, increases chemoresistance in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2016;15(5):1043–1052. doi:10.1158/1535-7163.MCT-15-0774
36. Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59(2–3):75–86. doi:10.1016/j.addr.2007.03.005
37. Dahlman JE, Kauffman KJ, Langer R, Anderson DG. Nanotechnology for in vivo targeted siRNA delivery. Adv Genet. 2014;88:37–69. doi:10.1016/B978-0-12-800148-6.00003-1
38. Masood F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C Mater Biol Appl. 2016;60:569–578. doi:10.1016/j.msec.2015.11.067
39. Miao L, Guo S, Lin CM, Liu Q, Huang L. Nanoformulations for combination or cascade anticancer therapy. Adv Drug Deliv Rev. 2017;115:3–22. doi:10.1016/j.addr.2017.06.003
40. Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv. 2016. Epub 2016 Jul 6.
41. Chen CK, Law WC, Aalinkeel R, et al. Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug–gene co-delivery to cancer cells. Nanoscale. 2014;6:1567–1572. doi:10.1039/c3nr04804g
42. Zhang CG, Yang SD, Zhu WJ, et al. Distinctive polymer micelle designed for siRNA delivery and reversal of GRP78 gene-dependent multidrug resistance. J Biomed Mater Res B Appl Biomater. 2016. doi:10.1002/jbm.b.33748
43. Yang X, Iyer AK, Singh A, et al. GRP78 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci Rep. 2015;5:8509. doi:10.1038/srep08509
44. Yang H, Ding R, Tong Z, et al. siRNA targeting of GRP78 reverses multidrug resistance in a nude mouse model of dtxlorubicin-resistant human hepatocellular carcinoma. Anticancer Res. 2016;36(6):2675–2682.
Supplementary material
 | Table S1 The PC-3 cancer volume during the treatment period |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.