Back to Journals » Cancer Management and Research » Volume 12
Coactosin-Like Protein (COTL1) Promotes Glioblastoma (GBM) Growth in vitro and in vivo
Authors Shao S, Fan Y, Zhong C, Zhu X, Zhu J
Received 27 February 2020
Accepted for publication 23 September 2020
Published 30 October 2020 Volume 2020:12 Pages 10909—10917
DOI https://doi.org/10.2147/CMAR.S246030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Shike Shao, Yongjun Fan, Chongpei Zhong, Xianlong Zhu, Jiaqiu Zhu
Department of Neurosurgery, The Second People’s Hospital of Lianyungang City, Jiangsu, People’s Republic of China
Correspondence: Jiaqiu Zhu Email [email protected]
Objective: To assess the expression levels of COTL1 in human GBM tissues and evaluate the potential involvement of COTL1 in cancer progression.
Methods: Bioinformation analysis was performed to evaluate COTL1 mRNA levels in GBM tissues and normal tissues, according to the TCGA database, and explore the effects on prognosis. Immunohistochemical (IHC) assays were performed to evaluate COTL1 expression in human GBM tissues and the clinical pathological analysis was performed. Colony formation and MTT assays were performed to evaluate the effects of COTL1 on GBM cell proliferation. Immunoblot assays were performed to detect the expression level of COTL1, Ki67, and PCNA. A xenograft model was developed in mice to assess the effects of COTL1 on tumor growth in vivo.
Results: We found COTL1 had an obvious high expression in human GBM tissues. The expression of COTL1 was related to recurrence (P=0.006**) and prognosis of patients with GBM. Our data further demonstrated COTL1 promoted cell proliferation in vitro and contributed to tumor growth of GBM cells in mice.
Conclusion: We therefore identified a novel and promising therapeutic target for the treatment of GBM.
Keywords: coactosin-like protein, COTL1, glioblastoma), GBM, proliferation, recurrence, prognosis
Introduction
Glioblastoma (GBM), as the most common form of malignant brain cancer, are genetically unstable, highly infiltrative, angiogenic, and resistant to chemotherapy.1,2 In decades, the median survival of GBM is approximately 7–15 months from the time of diagnosis.3–5 Surgical resection combined with radiotherapy and chemotherapy are the conventional therapy for GBM.6,7 However, these therapies are efficacy limited due to high rates of recurrence, drug resistance, and devastating neurological deterioration.8,9 Despite the advances in diagnosis and treatment of GBM, the patient prognosis has not been obviously improved. Targeted therapy showed a great prospect in the treatment of GBM. To combat this disease, novel therapeutic targets and approaches for the treatment of GBM are still needed.
Coactosin-like protein (COTL1), which belongs to the ADF/cofilin family, is an actin binding protein that could bind to actin filaments to mediate the actin cytoskeleton.10,11 Additionally, COTL1 could suppress cofilin-induced actin depolymerization.12 A previous study also demonstrated that COTL1 affected the migration of mouse neocortical neurons and was a potential regulator of T-cell activation.13 COTL1 could compete with cofilin to promote lamellipodial protrusion.12 In proinflammatory leukotriene biosynthesis, COTL1 increased 5-LO activity through interacting with 5-lipoxygenase (5-LO or ALOX5).14 CLP/COTL1 was also reported to be involved in TGFβ signaling via TGFβ receptor 1 associated protein 1 (TGFBRAP1).
Notably, recent studies indicated the involvement of COTL1 in cancer progression. In myeloid cells, high COTL1 expression was observed.15 Similarly, positively stained COTL1 was identified in most NSCLC tissues.16 Additionally, COTL1 overexpression promoted cell proliferation in HCC827 cells suggesting COTL1 could act as a potential oncogene.17,18 In breast cancer, COTL1 suppressed cancer growth by activating IL-24/PERP and inhibiting non-canonical TGFβ signaling.19 Though there are wide effects of COTL1 in cancer progression, its possible role in GBM remains unclear.
Herein, we found COTL1 had an obvious high expression in human GBM tissues. The expression of COTL1 was related to clinical pathological features such as recurrence. Our data further suggested COTL1 promoted cell proliferation in vitro and contributed to tumor growth of GBM cells in mice. We therefore identified a novel and promising therapeutic target for the treatment of GBM.
Materials and Methods
Antibodies, Primers, and shRNA Plasmids
Anti-COTL1 (1:100 dilution for IHC, and 1:1,000 for Immunoblot, ab235833, Abcam, Cambridge, UK), anti-β-actin (1:5,000 dilution, 60008-1-Ig, Proteintech, Chicago, IL), anti-Ki67 (1:1,000, ab16667, Abcam, Cambridge, UK), anti-PCNA (1:1,000, SAB2108448, Sigma-Aldrich, St. Louis, MO).
The quantitative PCR primers were listed as follows: COTL1: Forward, 5ʹ- CCAAGATCGACAAAGAGGCTT-3ʹ and Reverse, 5ʹ-CGATGGTGGAGCCGTCATATTT-3ʹ; GAPDH Forward: 5ʹ-CGACCACTTTGTCAAGCT-3ʹ, Reverse, 5ʹ-GGTTGAGCACAGGGTACTTTATT-3ʹ. Pre-designed shRNA constructs targeting COTL1 were bought from Origene Co (Beijing, China). COTL1 shRNA sequence were as follows: sense, 5ʹ-CCGTCATCTGGGTGACTTTTAAA-3ʹ.
Human Tissue Samples and Analysis
A total of 66 human GBM tissue and adjacent non-tumor tissues were collected from patients who underwent surgical resection in our hospital. Formal consent was obtained from all patients. Tissue specimens were frozen until use. Our study was approved by the Ethics Committee of the Second People’s Hospital of Lianyungang City. The clinicopathological characteristics of 66 patients with GBM were respectively obtained and analyzed.
To explore the COTL1 expression in human GBM tissues, immunohistochemical (IHC) assays were first conducted. Fixed tissues in 4% PFA at room temperature, embedded in paraffin blocks, were then cut into 5-μm sections. Sections were antigen retrieved, blocked, and incubated with primary antibody targeting COTL1 for 2 hours and secondary antibody for 1 hour at room temperature. Slides were stained with DAB. Images were taken using a microscope (Olympus BX43).
The expression levels of COTL1 were scored as follows. The positive staining cells percentage was quantified as 0 for negative staining cells; 1 meant 10–50% positive staining, and 2 for more than 50% positive staining cells. The staining intensity was considered as 0 (negative staining), 1 (low level staining), and 2 (high level staining). The expression levels were quantified according to staining intensity score plus positive tumor cell staining score. Staining scores 0–2 were low expression, while 3–4 were considered high COTL1 expression.
Cell Culture and Transfection
Human GBM cell line U251 and U87 cells were obtained from ATCC and maintained in ATCC-formulated Eagle’s Minimum Essential Medium (Catalog No. 30–2003) supplied with 10% of fetal bovine serum (FBS, Gibco, Grand Island, CA) at 37°C in a 5% CO2 incubator.
The transfection of COTL1 shRNA plasmids were conducted by Lipofectamine 3000 (Thermo Fisher Scientific, Inc.). COTL1 stable ablation was screened through puromycin successive addition.
Quantitative PCR Assay
U251 and U87 cells were lysed for total RNAs extraction by Trizol reagent (Invitrogen, Carlsbad, CA). Subsequently, M-MLV reverse transcriptase were utilized to reverse transcribe RNA to cDNA. SYBR mixture (Takara, Kusatsu, Japan) was used to amplify genes. And targeted gene levels were normalized to GAPDH.
Immunoblot Assays
Protein were extracted from cells or tumor tissues by lysis buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 20% glycerol, 0.25% bromophenol blue, 1.25% 2-mercaptoethanol, and protease inhibitor cocktail). Protein were isolated by SDS-PAGE, subsequently transferred onto PVDF membrane (Millipore, IPSN07852, USA). Then blocked with 5% BSA in TBST buffer and incubated with the indicated primary antibodies. After washing, it was incubated with the secondary antibodies. Each blot was analyzed with an ECL kit (GE Healthcare, RPN 2109, USA).
Colony Formation Assay
Approximately 1,000 cells were placed into 6-well plates and maintained for 2 weeks and subsequently stained with 0.2% crystal violet and washed with PBS. Colony numbers in each well were analyzed.
MTT Assay
Cells were plated into 96-well plates. After adherence, MTT was added to the cells. The stained cells were treated with 150 μL DMSO. The OD value was measured at a 570 nm wavelength with a microplate reader.
Tumor Growth Assays
Animal assay procedures involved in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the Second People’s Hospital of Lianyungang City. Male nude BalB/c mice were bought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). To conduct tumor growth assay, 1×106 U251 cells with or without stable COTL1 depletion were subcutaneously injected into nude mice. After 14 days, the tumor volume was measured every 3 days. According to the tumor volume, the tumor growth curve was displayed. After 29 days, the tumors were excised and photographed.
Statistics
Statistical analysis was performed by Graph Pad software. Mean±standard deviation (SD) was displayed in the results. The correlation analysis between COTL1 level and clinical pathological features were obtained by χ2 analysis. Students’ t-test was used for statistical difference. * Indicates P<0.05, and P<0.05 was considered a significant difference.
Results
Bioinformation Analysis Revealed COTL1 Expression Was Upregulated in Human GBM Tissues and Correlated with the Prognosis
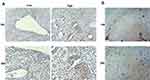
Since the wide involvement of COTL1 in cancer progression, we assessed the expression of COTL1 in human GBM tissues. Though bioinformatic analysis, we found enhanced expression levels of COTL1 in human GBM patients in the TCGA database. The COTL1 mRNA levels in tumor tissues (n=163) were significantly higher than non-tumor tissues (n=207) (Figure 1A). Furthermore, we observed that COTL1 mRNA levels were correlated with the disease-free survival rate and overall survival rate (P=0.03 and P=0.029, respectively, Figure 1B). Patients with high COTL1 expression in tumors had relatively lower disease-free survival rates and overall survival rates, suggesting the poor prognosis in the high COTL1 expression group. We therefore indicated that COTL1 was increased in human GBM tissues and correlated with the poor prognosis of GBM patients.
COTL1 Was Upregulated in Human GBM Tissues and Associated with the Clinical Pathological Features of Patients with GBM
To further assess the expression levels of COTL1 in GBM patients, we performed IHC assays. As shown in Figure 2A and B, COTL1 staining intensity was obviously stronger in GBM tissues than adjacent non-tumor tissues (photograph shown in 100× or 200× magnification). Our results demonstrated high expression levels of COTL1 in human GBM tissues.
Then we further analyzed the relationship between COTL1 expression level and clinicopathological characteristics in 66 patients with GBM patients. According to the staining results, patients were divided into COTL1 high and low groups. Twenty-two patients exhibited low COTL1 expression and the remaining (n=44) showed high expression of COTL1 (Table 1). We noticed that COTL1 expression was obviously correlated with recurrence (P=0.006**, Table 1), whereas there was no obvious correlation between COTL1 expression and clinical features such as patient age (P=0.212), gender (P=0.278), tumor lateralization (P=0.460), and IDH1 mutations (P=0.712, Table 1). Therefore, we revealed that COTL1 expression was correlated with the recurrence of GBM patients.
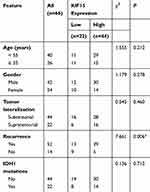 |
Table 1 Relationships of COTL1 and Clinicopathological Characteristics in 66 Patients with Glioblastoma |
COTL1 is Effectively Downregulated in GBM Cells Transfected with COTL1 shRNA Plasmids
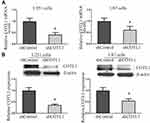
Since the abnormal expression of COTL1 was detected in GBM patients, we explored whether COTL1 was involved in GBM progression. Human GBM cell line U251 and U87 cells were selected as the cell models of in vitro study on GBM. First, control or COTL1 shRNA plasmids were transfected into U251 and U87 cells to deplete the expression of COTL1. Through quantitative PCR assays, we found the COTL1 mRNA level decreased obviously after the transfection of its shRNA plasmids (Figure 3A). Similarly, COTL1 protein levels were also decreased, as shown in Immunoblot assays following the transfection of its shRNA plasmids (Figure 3B). Our data therefore confirmed COTL1 was effectively downregulated by the transfection of its shRNA plasmids.
COTL1 Ablation Inhibited Cell Proliferation in vitro
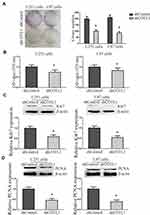
To further explore the function of COTL1 in GBM cells, colony formation assays were then conducted. Both U251 and U87 cells transfected with control or COTL1 shRNA plasmids were seeded and maintained for 14 days to form colonies in a 6-well plate. We found COTL1 depletion resulted in a sharp reduction in colony numbers in both U251 and U87 cells (Figure 4A). In addition, we observed a decreased OD value at 570 nm wavelength through MTT assays in U251 and U87 cells transfected with COTL1 shRNA plasmids, which further proved the decreased proliferation ability (Figure 4B). Ki67 and PCNA were proliferate cell biomarkers reflecting the proliferation ability, and the expression of Ki67 and PCNA was also detected in U251 and U87 cells after transfection of control or COTL1 shRNA plasmids. Consistently, reduction of Ki67 and PCNA expression level in COTL1-depleted U251 and U87 cells were observed (Figure 4C and D), suggesting the depletion of COTL1 inhibited the proliferation of GBM cells.
COTL1 Ablation Suppressed Tumor Growth of GBM Cells in vivo
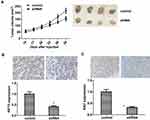
As we found COTL1 contributed to cell proliferation in vitro, we therefore assessed that COTL1 might induce tumor formation in vivo. To confirm our assumption, we detected the tumor growth degree through a xenograft model. U251 cells were stably transfected with control or COTL1 shRNA lentivirus to alter COTL1 expression, and subsequently injected the control or COTL1 depleted U251 cells into BALB/c nude mice subcutaneously for 2 weeks, we monitored the tumor growth every 3 days until 29 days (n=6). Interestingly, the tumor volume in the COTL1 depletion group was significantly smaller than the control group (Figure 5A). Representative images of tumors in each group are displayed in Figure 5A. We then detected the expression levels of COTL1 and Ki67 in isolated tumors from control and COTL1 depletion mice through IHC assays. We found the expression of COTL1 and Ki67 in COTL1 depletion groups was obviously lower than that in the control group (Figure 5B and C), consistent with our in vitro data. Taken together, we confirmed COTL1 depletion tumor proliferation of GBM cells growth in vivo.
Discussion
GBM is the most aggressive malignant tumor in the brain, with poor prognosis.20 Neurological symptoms of GBM vary differently.21 In decades, patient prognosis is still poor due to the limited therapies and high occurrence.22 Median survival upon this treatment was 14 months (only four in the absence of treatment).23,24 Therefore, revealing the molecular mechanisms related to GBM progression and founding promising therapeutic targets is still needed. In this study, we observed obvious upregulation of COTL1, an actin binding protein, in human GBM tissues. We also noticed COTL1 expression level was associated with the clinical features such as recurrence of GBM patients through the clinical pathological analysis. Therefore, we thought COTL1 as a potential therapeutic target for the treatment of GBM.
Interestingly, a previous study identified COTL1 was up-regulated in small cell lung cancer (SCLC) tissues which was validated by Immunoblot analysis, Immunohistochemistry, and quantitative PCR assays.15 In addition, most SCLC tissues (93%; 28/30) were COTL1-positive in immunohistochemistry, therefore COTL1 could be a potential biomarker in SCLC.15 COTL1 was also overexpressed in pancreatic cancer tissues, compared to normal pancreatic tissues.25 CLP/COTL1 is highly expressed in a rat epithelial breast cancer cell line (FE1.3) compared with its mesenchymal counterpart (FE1.2).19 Knockdown of COTL1 in FE1.3 cells promoted cell proliferation, whereas its overexpression in FE1.2 cells inhibited proliferation and reduced tumor growth in xenograft assays.19 Therefore, we speculated that COTL1 might be important in GBM progression. By conducting colony formation and MTT assays, we observed COTL1 inhibited GBM cell proliferation. COTL1 knockdown also reduced Ki67 and PCNA expression level in vitro and in mice. Our data therefore indicated COTL1 inhibited GBM cell proliferation.
COTL1, as an actin-binding protein, competes with cofilin for binding to F-actin. COTL1 protects F-actin from cofilin-mediated depolymerization.26 CLP/COTL1 potentiated the growth suppressive effect of transforming growth factor-β1 (TGFβ1), leading to downregulation of TGFβ-responsive genes vascular growth factor A/B (VEGFA/VEGFB), hypoxia inducing factor 1α (HIF-1α), and thrombospondin 1 (TSP1), which mediate various hallmarks of cancer progression including angiogenesis, invasion, and metastasis.19 In this study, we noticed COTL1 promoted the proliferation of GBM cells both in vitro and in mice, and whether COTL1 inhibited cell proliferation via affecting actin dynamics or TGFβ1 requires further investigation.
COTL1 is involved in the regulation of neuronal migration and morphogenesis.17,18,27 COTL1 overexpression suppressed the migration of neurons during mouse corticogenesis, and delayed neuronal migration in late-born neurons.11,27 Its expression also disturbed neuron morphology.27 These studies suggested that COTL1 could affect the migration and invasion of cells, particularly cancer cells. We next should assess whether COTL1 affects the migration and invasion of GBM cells.
In conclusion, we here found the abnormal high expression of COTL1 in human GBM tissues. The expression of COTL1 was correlated with clinical pathological features and the prognosis of patients with GBM. Through the in vitro and animal assays, we found COTL1 promoted the proliferation of GBM cells. We therefore provide a novel and promising therapeutic target for the treatment of GBM.
Ethics Statement
Our study was approved by the Ethics Committee of the Second People’s Hospital of Lianyungang City. The clinicopathological characteristics of 66 patients with GBM were respectively obtained and analyzed. All of the patients’ consent was informed consent, and all of them were conducted in accordance with the Declaration of Helsinki. Animal assay procedures involved in this study were approved by Institutional Animal Care and Use Committee (IACUC) of the Second People’s Hospital of Lianyungang City. Written confirmation has been given that all experiments were performed following the ethical guidelines of Second People’s Hospital of Lianyungang City.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alcaide-Leon P, Luks TL, Lafontaine M, et al. Treatment-induced lesions in newly diagnosed glioblastoma patients undergoing chemoradiotherapy and heat-shock protein vaccine therapy. J Neurooncol. 2019.
2. Amoozgar Z, Jain RK, Duda DG. Role of apelin in glioblastoma vascularization and invasion after anti-VEGF therapy: what is the impact on the immune system? Cancer Res. 2019;79(9):2104–2106. doi:10.1158/0008-5472.CAN-19-0749
3. Amsbaugh MJ, Yusuf M, Gaskins J, et al. The impact of timing of adjuvant therapy on survival for patients with glioblastoma: an analysis of the National Cancer Database. J Clin Neurosci. 2019;66:92–99. doi:10.1016/j.jocn.2019.05.013
4. Bagley SJ, Desai AS, Linette GP, June CH, O’Rourke DM CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–1438.
5. Bailly C, Vidal A, Bonnemaire C, et al. Potential for nuclear medicine therapy for glioblastoma treatment. Front Pharmacol. 2019;10:772. doi:10.3389/fphar.2019.00772
6. Balana C, Estival A, Pineda E. Bevacizumab rechallenge in glioblastoma patients with initial response to bevacizumab who later progress off therapy. J Neurooncol. 2018;139(3):779–780. doi:10.1007/s11060-018-2902-9
7. Banasavadi-Siddegowda YK, Welker AM, An M, et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro Oncol. 2018;20(6):753–763. doi:10.1093/neuonc/nox206
8. Abdel Gaber SA, Müller P, Zimmermann W, et al. ABCG2-mediated suppression of chlorin e6 accumulation and photodynamic therapy efficiency in glioblastoma cell lines can be reversed by KO143. J Photochem Photobiol B. 2018;178:182–191. doi:10.1016/j.jphotobiol.2017.10.035
9. Angara K, Borin TF, Rashid MH, et al. CXCR2-expressing tumor cells drive vascular mimicry in antiangiogenic therapy–resistant glioblastoma. Neoplasia. 2018;20(10):1070–1082. doi:10.1016/j.neo.2018.08.011
10. Provost P, Doucet J, Stock A, et al. Coactosin-like protein, a human F-actin-binding protein: critical role of lysine-75. Biochem J. 2001;359(2):255–263. doi:10.1042/bj3590255
11. Liu L, Wang Y, Zhang P, et al. Expression, purification and preliminary crystallographic studies of human coactosin-like protein. Acta Crystallogr D Biol Crystallogr. 2004;60(9):1651–1653. doi:10.1107/S0907444904016701
12. Dai H, Huang W, Xu J, et al. Binding model of human coactosin-like protein with filament actin revealed by mutagenesis. Biochim Biophys Acta. 2006;1764(11):1688–1700. doi:10.1016/j.bbapap.2006.06.017
13. Li G, Yin Y, Chen J, et al. Coactosin-like protein 1 inhibits neuronal migration during mouse corticogenesis. J Vet Sci. 2018;19(1):21–26. doi:10.4142/jvs.2018.19.1.21
14. Basavarajappa D, Wan M, Lukic A, et al. Roles of coactosin-like protein (CLP) and 5-lipoxygenase-activating protein (FLAP) in cellular leukotriene biosynthesis. Proc Natl Acad Sci U S A. 2014;111(31):11371–11376. doi:10.1073/pnas.1410983111
15. Jeong H-C, Kim G-I, Cho S-H, et al. Proteomic analysis of human small cell lung cancer tissues: up-regulation of coactosin-like protein-1. J Proteome Res. 2011;10(1):269–276. doi:10.1021/pr100714b
16. Sun W, Guo C, Meng X, et al. Differential expression of PAI-RBP1, C1orf142, and COTL1 in non-small cell lung cancer cell lines with different tumor metastatic potential. J Investig Med. 2012;60(4):689–694. doi:10.2310/JIM.0b013e31824963b6
17. Guo S, Yang P, Jiang X, et al. Genetic and epigenetic silencing of mircoRNA-506-3p enhances COTL1 oncogene expression to foster non-small lung cancer progression. Oncotarget. 2017;8(1):644–657.
18. Jin E-H, Shim S-C, Kim H-G, et al. Polymorphisms of COTL1 gene identified by proteomic approach and their association with autoimmune disorders. Exp Mol Med. 2009;41(5):354–361. doi:10.3858/emm.2009.41.5.040
19. Xia L, Xiao X, Liu WL, et al. Coactosin-like protein CLP/Cotl1 suppresses breast cancer growth through activation of IL-24/PERP and inhibition of non-canonical TGFβ signaling. Oncogene. 2018;37(3):323–331. doi:10.1038/onc.2017.342
20. Zhang Y, Li A, He J, Wang M. A NOVEL MKL method for gbm prognosis prediction by integrating histopathological image and multi-omics data. IEEE J Biomed Health Inform. 2019;24(1):171–179.
21. Arivazhagan A, Kumar DM, Sagar V, et al. Higher topoisomerase 2 alpha gene transcript levels predict better prognosis in GBM patients receiving temozolomide chemotherapy: identification of temozolomide as a TOP2A inhibitor. J Neurooncol. 2012;107(2):289–297. doi:10.1007/s11060-011-0758-3
22. Holmes D. Glomerular disease: MN linked to improved prognosis in anti-GBM disease. Nat Rev Nephrol. 2013;9(11):626. doi:10.1038/nrneph.2013.190
23. Rios Velazquez E, Meier R, Dunn WD
24. Wang C, Cao S, Yan Y, et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer. 2010;10(1):415. doi:10.1186/1471-2407-10-415
25. Nakatsura T, Senju S, Ito M, et al. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32(3):826–836. doi:10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y
26. Kim J, Shapiro MJ, Bamidele AO, et al. Coactosin-like 1 antagonizes cofilin to promote lamellipodial protrusion at the immune synapse. PLoS One. 2014;9(1):e85090. doi:10.1371/journal.pone.0085090
27. Liu M, Li G, Wang M, et al. Further studies about Coactosin-like protein-1 affecting the migration of mouse neocortical neurons. J Mol Histol. 2018;49(5):519–530. doi:10.1007/s10735-018-9790-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.