Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Co-occurrence of Behavioral Risk Factors of Non-communicable Diseases and Social Determinants among Adults in Urban Centers of Southwestern Ethiopia in 2020: A Community-Based Cross-Sectional Study
Authors Zenu S , Abebe E, Dessie Y, Debalke R , Berkessa T, Reshad M
Received 31 March 2021
Accepted for publication 26 May 2021
Published 23 June 2021 Volume 2021:14 Pages 1561—1570
DOI https://doi.org/10.2147/JMDH.S313741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sabit Zenu,1 Endegena Abebe,2 Yohannes Dessie,3 Rukiya Debalke,1 Tsegaye Berkessa,1 Mohammed Reshad1
1Department of Public Health, Mettu University, Mettu, Ethiopia; 2Department of Biomedical Science, Mettu University, Mettu, Ethiopia; 3Department of Nursing, Mettu University, Mettu, Ethiopia
Correspondence: Sabit Zenu
Department of Public Health, Mettu University, Mettu, Ethiopia
Email [email protected]
Background: Non-communicable diseases are priority global health problems. Smoking, harmful alcohol consumption, physical inactivity, and an unhealthy diet are four behavioral risk factors of these diseases. Studies in Ethiopia have focused on establishing associations between these factors and incommunicable diseases.
Objective: To assess the prevalence, co-occurrence, and social determinants of behavioral risk factors of non-communicable diseases among adults in urban centers of southwestern Ethiopia.
Methods: This study employed a cross-sectional design. Multistage sampling and the Kish method were used. The WHO’s STEPS instrument was used for data collection. Proportions and other descriptive measures are used to describe the data. Bivariate and multivariate logistic regression was run to assess associations. Associations between dependent and independent variables were determined using AORs, 95% CIs, and significance level of P=0.05.
Results: A total of 1,191 adults participated in the study for a 93.3% response rate. In sum, 4.8% of participants were smokers and 15.6% indulge in harmful drinking. Prevalence of physical inactivity was 60.45%, and 94.8% consumed insufficient fruit and vegetables. Regarding co-occurrence of these factors, 65.5% of participants had two or more behavioral risk factors. Female sex (AOR 3.1, 95% CI 1.8– 5.5), no formal employment (AOR 1.9, 95% CI 1.02– 3.7), greater wealth (AOR 2.44, 95% CI 1.1– 5.1), not having a friend who does physical exercise (AOR 2.7, 95% CI 1.5– 4.7), having friends who do not drink (AOR 0.20, 95% CI 0.09– 0.44), and participating in community activities (AOR 2.95, 95% CI 1.4– 6.0) were associated with co-occurrence of behavioral risk factors of non-communicable diseases.
Conclusion: The prevalence and co-occurrence of behavioral risk factors of non-communicable diseases in the study area are alarming. Several factors were associated with co-occurrence of these factors. Community-based interventions have to be implemented considering family settings. Special focus has to be given to physical inactivity and fruit and vegetable consumption.
Keywords: behavioral risk factors, non-communicable diseases, southwestern Ethiopia
Background
Non-communicable diseases (NCDs) are significant global health and development challenges. On top of their social and economic impact, they are leading causes of morbidity and mortality, resulting in 41.1 million deaths in 2019. Of special concern is global NCD-related premature death. Almost 75% of all ICD deaths and 82% of the 16 million people who died prematurely or before reaching 70 years of age occur in low- and middle-income countries (LMICs). Between 1990 and 2019, disability adjusted life years for NCDs increased by 13.1%.1–4
The major NCDs responsible for these deaths include cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes.5 The World Health Organization (WHO) has identified four behavioral risk factors associated with NCDs: harmful alcohol use, tobacco use, physical inactivity, and unhealthy diet. In addition, it has made action against these four risk factors one of the global objectives in the fight against NCDS.6
According to the global status report on alcohol and health in 2018, it is estimated that harmful alcohol use was the cause of some 3 million deaths worldwide and 132.6 million disability-adjusted life years. Mortality resulting from alcohol consumption is higher than that caused by such diseases as tuberculosis, HIV/AIDS, and diabetes.7
Tobacco use is one of the leading global risk factors of illness and death from NCD. In 2019, tobacco use resulted in 8.7 million deaths globally. Tobacco smoking accounted for 6.57 million deaths among men, accounting for >21.4% of all deaths among men. The prevalence of current tobacco use is 8.1% among men, 1.8% among women, 3.8% in urban areas, and 5.3% in rural areas. The overall prevalence of adult daily smoking is 3.2% (2.2 million).8
Physical inactivity has been identified as the fourth-leading cause of global mortality, causing 6% of global deaths. It is estimated to be the principal cause of approximately 21%–25% of breast and colon cancer, 27% of diabetes, and 30% of ischemic heart disease. Physical inactivity is also responsible for a great share of the rise in NCD morbidity and mortality. The WHO has put forth global recommendations for physical activity for health and urged its member states to prepare national physical activity action plans and draft policies to increase physical activity levels in their populations, with special emphasis on creating suitable and healthy transportation and suitable environments that promote physical activity.9
Fruit and vegetables are important components of a healthy diet, and sufficient daily consumption could help to prevent cardiovascular diseases and certain cancers. According to the WHO, low fruit-and-vegetable intake is estimated to cause about 31% of ischemic heart disease and 11% of strokes worldwide. It is estimated that up to 2.7 million lives could potentially be saved each year if fruit-and-vegetable consumption were sufficiently increased. Unhealthy diets and more importantly poor consumption of fruit and vegetables are also one of the main behavioral risk factors of NCDs. Despite this, the level of consumption of these food items is still far below that recommended.10
It is believed that these four behavioral risk factors of NCDs are preventable. In line with this, multiple efforts have been made both internationally and locally. Regarding tobacco, the World Health Assembly in 2003 adopted the World Health Organization Framework Convention on Tobacco Control, which entered into force in 2005. Ethiopia ratified the convention on January 21, 2014. Despite several shortcomings, Ethiopia has made progress in laying the groundwork to prevent the public-health impacts of smoking and excessive alcohol consumption.7
Several studies conducted over the last few decades concerning factors associated with NCDs have brought these factors to the level of established facts. It is a wastage of resource and time to conduct studies to assess the association of the four behavioral risk factors of NCDs. Rather than re-exploring the already-established facts, steps must be taken to assess prevalence and social determinants associated with these four behavioral factors. In addition, individuals with more risk factors are more likely to develop diseases and suffer the consequences, and no study has been done to assess the co-occurrence of these factors among individuals in Ethiopia. This study accordingly aimed to conduct a comprehensive assessment of the co-occurrence of behavioral risk factors of ICDs and their social determinants in the study area.
Methods
Study Area, Design, and Period
The study was conducted in urban centers of southwestern Ethiopia. These urban centers — Bedelle, Mettu, and Gambella — are administrative capitals of the zones and regions in Southwest Ethiopia. Despite their organizational differences, the developmental status of the three cities is comparable. This community-based cross-sectional study was conducted from January 15 to 30, 2020.
Sample-Size Determination and Sampling Procedure
Sample size was calculated for all objectives and the largest is used. This sample size was found using a single population–proportion formula with proportion of 41%,11 95% confidence level (ie, Z=1.96), design effect of 2, marginal error of 0.04 and nonresponse rate of 10%. The final sample size was 1,277.
The sampling approaches used in this study were a combination of multistage sampling, simple random sampling, the and Kish grid method.12 From the three urban centers, kebeles (lowest administrative units in Ethiopia) were randomly selected by lottery. Sample size was proportionally distributed to each kebele based on household numbers. At the household level, one individual among the eligible household members was selected using the Kish method.
Data-Collection Tools and Procedures
Data were collected using an interviewer-administered questionnaire adopted from the WHO’s STEPS instrument. This instrument included questions to assess tobacco smoking, harmful alcohol consumption, physical activity, and fruit-and-vegetable consumption. The wealth index was measured by using 27 locally validated questions to measure household assets. Social capital and social cohesion were assessed with an integrated questionnaire for the measurement of social capital validated for use in developing countries.13 Additional social determinants of health variables were measured by developing appropriate questions. Data were collected by trained public-health officers and nurses. Supervisors were senior public-health officers of the towns.
Data Processing and Analysis
Raw data were edited and coded on a daily basis. Edited and coded data were entered into EpiData 3.1 and exported to SPSS 20 for analysis. Presence or absence of the four behavioral risk factors was assessed for all participants. Participants who were current users of any tobacco product at the time of the study were classified as smokers. Harmful alcohol use was defined by assessing current use and comparing it with sex-specific threshold levels. Individuals who performed <150 minutes of moderate or <75 minutes of vigorous physical activity or an equivalent combination of the two in a week were categorized as physically inactive. Finally, participants who consumed fewer than five servings of fruit and vegetables per day in the week prior to the study were classified as poor consumers of fruits and vegetables. The occurrence and absence of each behavioral risk factor was coded as 0 if the risk factor is absent and one if present.
Co-occurrence of behavioral risk factors was computed by summing the results of the four risk factors. Participants with two or more behavioral risk factors at the time of the study were declared to have co-occurrence of behavioral risk factors of NCDs. Participants who had co-occurrence of two or more behavioral risk factors were coded as 1 and those with one or no risk factors coded as 0 in the software.
Proportions and other descriptive statistical analysis are used to describe the data. Bivariate logistic regression was run to screen variables for multivariate regression. Variables with P<0.25 on bivariate regression progressed to multivariate logistic regression to assess associations using backward stepwise logistic regression. On multivariate logistic regression, associations between co-occurrence of behavioral risk factors and independent variables were determined using AORs, 95% CIs, and significance level of P=0.05.
Data-Quality Management
Pretesting of the study instrument was conducted in Hurumu town on 5% of the total sample. The data-collection tool was translated into local languages and back-translated to English. Data collectors were trained on the objectives and general procedures of data collection prior to initiation of the study. Onsite supervision of data-collection procedures was done, and the data collected were carefully handled by assigned personnel.
Ethical Considerations
The research was conducted in full compliance with the ethical principles of the Declaration of Helsinki. The protocol of the research was reviewed and approved by the Research Ethics Review Committee of Mettu University College of Health Sciences. Participants were provided complete information concerning the nature, objectives, procedures, risks, and benefits of the study. The right of the study participants to refuse to participate or to stop at any time in the process was clearly communicated and assured. After the provision of comprehensive information, participants provided written informed consent. All information provided by the participants was kept confidential and not transferred to any third party. In addition, any information that may have led to identification of study participants was not included in the data-collection tool.
Results
Sociodemographic Characteristics of Participants
A total of 1,191 participants participated in the study, yielding a 93.3% response rate. Of original sample, 54 participants (4.2%) refused to participate, despite repeated attempts to convince them. The remaining 32 (2.5%) were absent after three home visits on workdays and at weekends. Median age of participants was 36 years, with an IQR of 82(22–100). Well over half (654, 54.9%) were female. The Oromo were the predominant ethnic group (857, 71.9%), 693 (58.8%) participants had achieved secondary education and beyond, and 150 were Orthodox (37.8%), 126 Protestant (31.7%), and 114 Muslim (28.7%). Married participants comprised around four in ten participants (152, 38.3%), while cohabiting participants numbered 120 (30.2%). Concerning occupation the biggest group was self-employed (106, 26.7%) (Table 1).
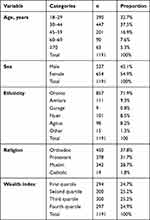 |
Table 1 Sociodemographic characteristics of participants |
Prevalence of Behavioral Risk Factors
A total of 57 (4.8%, 95% CI 2.8%–6.8%) of participants were smokers and 186 (15.6%, 95% CI 12.3%–19.4%) were harmful alcohol consumers. In addition, 720 (60.45%, 95% CI 56.4%–66.0%) were physically inactive, while 1,119 (94.8%, 95% CI 92.7%–96.9%) were currently consuming fewer than five servings of fruit and vegetables a day (Figure 1).
 |
Figure 1 Prevalence of behavioral risk factors of ICDs in three urban centers of south western Ethiopia. |
Co-occurrence of Behavioral Risk Factors
In sum, 780 (65.5%, 95% CI 60.7%–70.0%) of participants had co-occurrence of at least two of the four behavioral risk factors of NCDs: 663 (56.7%) had two, 105 (8.8%) had three factors, and 12 (1%) had all four. The median number of risk factors per participant was two, with an IQR of 2 (1–3).
Almost half the males (273, 50.8%) and 507 (77.5%) females had co-occurrence of at least two behavioral risk factors (Table 2).
 |
Table 2 Cross tabulation of sex and co-occurrence of behavioral risk factors among study participants |
Of those aged 18–29 years, 231 (59.2%) had at least two behavioral risk factors, This was 77.6% for those aged 45–59 years, 61.1% for those aged 30–44 years, 70% for those aged 60–69 years, and 90.5% for those aged >70 years (Figure 2).
 |
Figure 2 Age distribution of the co-occurrence of behavioral risk factors among study participants. south western |
Social Factors Associated with Co-Occurrence of Behavioral Risk Factors
On the final model, female sex, no formal employment, greater wealth, having a friend who drinks alcohol, not having a friend who do physical activities, and participating in community activities were significantly associated with co-occurrence of at least two behavioral risk factors of NCDs at a time (Table 3).
 |
Table 3 Bivariate and multivariate logistic regression of co-occurrence of behavioral risk factors and social determinants among participants |
Female participants were three times as likely to have co-occurrence of behavioral risk factors as male participants (AOR 3.1, 95% CI 1.8–5.5). Occupation was also associated with co-occurrence of behavioral risk factors. Those with no formal employment were nearly twice as likely to have co-occurrence of behavioral risk factors as employed participants (AOR 1.9, 95% CI 1.02–3.65).
Wealth was another variable significantly associated with co-occurrence of behavioral risk factors of . Accordingly, individual participants in the second quartile were more than twice as likely to have co-occurrence as participants in the lowest quartile (AOR 2.44, 95% CI 1.15–5.15). Similarly, individuals whose wealth fell in the third quartile were also more than twice as likely to have co-occurrence of behavioral risk factors as those in the lowest quartile (AOR 2.3 CI, 95% 1.09–4.89).
Not having a friend who drinks alcohol was also associated with lower probability of co-occurrence of behavioral risk factors. Participants whose friends do not drink alcohol have an 80% reduction in probability of co-occurrence compared to those whose friends drank alcohol (AOR 0.2, 95% CI 0.09–0.44). Participants who do not have physically active friends had nearly triple the likelihood of experiencing co-occurrence of behavioral risk factors of NCDs as those whose friends were physically active (AOR 2.7, 95% CI 1.5–4.7).
Participating in community activities was also significantly associated with lower probability of co-occurrence of behavioral risk factors. Individuals who did not participate in community activities in the last year preceding the study were almost three times as likely to have co-occurrence as those who engaged in community activities (AOR 2.95, 95% CI 1.42–6.09).
Discussion
Less than 5% of participants were smokers, while 15.6% were alcohol consumers. Concerning physical activity, six in ten fell below WHO recommendations. Inadequate consumption of fruit and vegetables was another important behavioral risk factor of NCDs: 92.7% of participants consumed less than the recommended servings of fruits and vegetables per day.
The prevalence of tobacco smoking and alcohol consumption was low compared to another study outside Ethiopia: up to 69.8% for tobacco and 40.7% for alcohol consumption in West Bengal,India.14 The level of smoking in the study area was also lower than a report from Nepal, where prevalence was 19%.15 This difference may be due to sociocultural difference between the study areas. In Ethiopia, cultural and religious conservatism prevails.16 Religious banning of alcohol and tobacco smoking among Muslims and Protestants (>60% of the participants) may have played a role in reducing the prevalence of these two risk factors.
The high level of physical inactivity and poor consumption of fruit and vegetables in the study area was comparable with findings from studies conducted in West Bengal, where 96.5%14 consumed an unhealthy diet, and Nepal, where 95.3% consumed less than the recommended level of fruit and vegetables.17 It is also similar to a report from Kenya, where 99.8% of participants did not stick to fruit and vegetable–consumption recommendations.18 This similarity may be due to similar income status of the countries in which the studies were conducted (all LMICs), which may make it financially difficult to access the required fruit and vegetables.
Almost two-thirds of participants in the current study had co-occurrence of two or more behavioral risk factors. This is high, and needs proportional focus to avert future problems.
The current findings on co-occurrence (65.5%, 95% CI 60.7%–70.0%) are line with a report from a study conducted in Nepal among women, which reported prevalence of co-occurrence of two or more behavioral risk factors as 66%.19
Prevalence of co-occurrence in the current study was higher than a report from Uganda, where 56.4% of participants had co-occurrence.20 In addition, it was higher than a report from Florianópolis, where 43% of males and 36% of females had co-occurrence.21 The relatively lower prevalencein these areas may be due to variations in study settings. Our study was conducted among residents of urban centers, where people tend to adopt Western-style living conditions, while the studies in Uganda and Florianópolis involved a mix of urban and rural residents.
Contrarily, the prevalence in co-occurrence in our study was lower than a report from Nepal, which showed up to 83% co-occurrence. The relatively higher proportion of participants with co-occurrence in the Nepal study may be due to the type of population involved in the study. The study in Nepal involved adolescents, while ours enrolled those aged ≥18 years.17
Median risk factors per participant in this study was tw, and 65.5% of participants had two.Median factors per participant in this study was lower than reports from Bhutan (three),22 2.5 among Indonesian adolescents,23 and 3 (three among south African adults.24 The relatively lower number of risk factors per person in the current study when compared with these may be due to differences in the number of risk factors analyzed. While our studyy focused only on four risk factors (smoking, alcohol, physical inactivity and fruit-and-vegetable consumption), the otherss included physical measurements like BMI and laboratory measures.
Several factors were associated with the likelihood of having two or more risk factors. Female participants were more than three times as likely to have co-occurrence. Other research in Africa has reported a similar finding.25 This may be due to working types and conditions, income, and educational status of females in in urban centers of LMICs that predispose them to stay home, consume less fruit and vegetables and have multiple behavioral risk factors.26
Higher wealth was also positively associated with co-occurrence. In the current study, participants in the second wealth quartile were twice as likely to have co-occurrence. Similarly, participants in the third wealth quartile were more than twice as likely to have co-occurrence asthose in the lowest wealth quartile. This finding is contrary to the trend of NCDs in recent years, where the premature death is more common among poorer nations.27 Other studies also reported significantly higher prevalence of behavioral risk factors among socioeconomically disadvantaged populations.28,29 The importance of poor socioeconomic conditions as predisposing factors for unhealthy behavior is well documented in developed countries. This issue needs further investigation in developing countries like Ethiopia.
Social factors also determine the probability of having behavioral risk factors of NCDs. In this study, participants whose friends did not drink had up to an 80% reduction in likelihood of co-occurrence. In addition, participants whose friends did not exercise were roughly three times as likely to have co-occurrence of sas their counterparts. This may be due to the social impact and pressure that friends and family members exert on their friends and housemates .30
Participating in community activities helps to share health information and adapt to best practices in the community. This truth is supported by our study: participants who did not take part in community activities prior to the study were up to three times as likely to have co-occurrence asthose who did.31
Limitations
We used the WHO STEPS instrument to assess the behavioral risk factors, including physical inactivity, rather than usage of objective measures. This may have resulted in variations when compared with objective measurements of these risk factors.
Conclusion
Almost all participants had one or more of the four behavioral risk factors of NCDs studied.The highest prevalence was recorded for level of fruit and vegetable consumption. The next highest was for physical inactivity, alcohol consumption, and smoking. More than six in ten participants had co-occurrence of two or more behavioral risk factors of NCDs. This can be considered high.
Several factors were also associated with co-occurrence of behavioral risk factors. Community-based interventions to lower the prevalence and co-occurrence of these factors has to be implemented to protect the health of the community from the looming danger these risk factors pose.
Interventions to tackle these behavioral risk factors have to target the entire community. Family-based changes have to be fostered to reduce behavioral risk factors, as many family factors are found to be associated with co-occurrence of these risk factors. Strengthening of social capital and social cohesion also helps to tackle the problem by fostering collective action and societal changes.
Abbreviations
BRFs, behavioral risk factors; LMICs, low and middle income countries; NCDS, Non-communicable diseases; WHO, World Health Organization.
Data Sharing Statement
All data for this research article is available and can be accessed from the corresponding author at any time.
Ethics and Consent to Participate
This research was approved by the Mettu University Ethical Review Committee. Informed written consent was obtained from participants ahead of data collection. All information provided by the participants was not transferred to any third party. In addition, any information leading to identification of study participants was not included in the data-collection instrument.
Acknowledgment
We would like to thank all those who helped us in the realization of this work. Our organization, Mettu University, also deserves gratitude for preparing the staff research program to assess community-health problems. Last but not least, we appreciate the efforts of our families for taking complete responsibility for family issues during busy times of this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
The entire cost of this work was covered by Mettu University. The university provides funding for its staff to conduct research and utilizes this for approved proposals. The funder had no role in design, conduct, analysis, or interpretation of the study.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Roth G. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980 – 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788.
2. UNDP. Discussion Paper Addressing the Social Determinants of Noncommunicable Diseases.
3. Juma K, Juma PA, Mohamed SF, et al. First Africa non-communicable disease research conference 2017: sharing evidence and identifying research priorities. J Glob Health. 2019;9(1):1–13.
4. Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
5. World Health Organization. Non Communicable Disease Country Profiles.
6. WHO. Global Action Plan for the Prevention and Controlof Non Communicable Diseases 2013–2020.
7. World Health Organization. Global Status Report on Alcohol and Health 2018.
8. Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249.
9. WHO. Global Recommendations on Physical Activity for Health for Age 18–64 Years Old. 2011:01
10. World Health Organization. Fruit and Vegetable Promotion Iitiative- Report of the Meeting, Geneva, 25–27 August 2003.
11. Ethiopian Public Health Institute. Ethiopia STEPS Report on Risk Factors for Non- Communicable Diseases and Prevalence of Selected NCDs in Ethiopia.
12. Lewis-Beck M, Bryman AE, Liao TF. The SAGE Encyclopedia of Social Science Research Methods. Sage Publications, Inc; 2012.
13. Grootaert C, Narayan D, Jones VN, Woolcock M. Measuring Social Capital an Integrated Questionnaire.
14. Bhar D, Bhattacherjee S, Das DK. Behavioral and biological risk factors of noncommunicable diseases among tribal adults of rural siliguri in Darjeeling District, West Bengal: a Cross - Sectional Study. Indian J Public Health. 2019;63(2):119–127. doi:10.4103/ijph.IJPH_326_18
15. Aryal KK, Meheta S, Neupane S, et al. The burden and determinants of non communicable diseases risk factors in Nepal: findings from a nationwide STEPS survey. PLoS One. 2015;1–18.
16. Central Statistical Authority. 2007 Population and Housing Census of Ethiopia Adminstrative Report Addis Ababa.
17. Dgungana RR, Bista B, Pandey AR, Cpurten MD. Prevalence, clustering and sociodemographic distributions of non- communicable disease risk factors in Nepalese adolescents: secondary analysis of a nationwide school survey. BMJ Open. 2019;1–9. doi:10.1136/bmjopen-2019-030833
18. Wekessah FM, Nyanjau L, Kibachio J, et al. Individual and household level factors associated with presence of multiple non- communicable disease risk factors in Kenyan adults. BMC Public Health. 2018;18(Suppl 3):41–53.
19. Paudel R, Lee K, Singh JK, Yoo S, Acharya D, Kadel R. Prevalence of behavioral risk factors of cardiovascular diseases and associated socio-economic factors among pregnant women in a rural area in Southern Nepal. BMC Pregnancy Childbirth. 2018;18(484):1–9. doi:10.1186/s12884-018-2122-5
20. Wesonga R, Guwatudde D, Bahenderka SK, Mutungi G, Nabugoomu F. Burden of cumulative risk factors associated with non-communicable diseases among adults in Uganda: evidence from a national baseline survey. Int J Equity Health. 2016;15(195):1–10. doi:10.1186/s12939-016-0486-6
21. Costa FF, Benedet J, Leal DB, Assis MA. Clustering of risk factors for non communicable diseases in adults from Florianopolis, SC Agregação de fatores de risco não transmissíveis em adultos de. Rev Bras Epidemiol. 2013;16(2):398–408. doi:10.1590/S1415-790X2013000200015
22. Pelzom D, Isaakidis P, Oo MM, Gurung MS, Yangchen P. Alarming prevalence and clustering of modifiable noncommunicable disease risk factors among adults in Bhutan: a nationwide cross-sectional community survey. BMC Public Health. 2017;17:1–11. doi:10.1186/s12889-017-4989-x
23. Peltzer K, Pengpid S. Prevalence and correlates of behavioral non-communicable diseases risk factors among adolescents in the seychelles: results of a National School Survey in 2015. Int J Environ Res Public Health. 2019;16:1–11.
24. Phaswana-Mafuya K, Peltzer K, Chirinda W, Musekiwa A. Sociodemographic predictors of multiple non- communicable disease risk factors among older adults in South Africa. Glob Health Action. 2013;6:1–8.
25. Yaya S, Uthman OA, Ekholuenetale M, Bishwajit G. Socioeconomic inequalities in the risk factors of noncommunicable diseases among women of reproductive age in sub-Saharan Africa: a multi-country analysis of survey data. Front Public Health. 2018;6(OCT):1–11.
26. Lailulo YA, Susuman AS, Blignaut R. Correlates of gender characteristics, health and empowerment of women in Ethiopia. BMC Womens Health. 2015;15(1):1–9. doi:10.1186/s12905-015-0273-3
27. Bollyky TJ, Templin T, Andridge C, Dieleman JL. Understanding the relationships between noncommunicable diseases, unhealthy lifestyles, and country wealth. Health Aff. 2015;34(9):1464–1471. doi:10.1377/hlthaff.2015.0343
28. Allen L, Williams J, Townsend N, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017;5(3):e277–e289. doi:10.1016/S2214-109X(17)30058-X
29. Hosseinpoor AR, Bergen N, Kunst A, et al. Socioeconomic inequalities in risk factors for non communicable diseases in low-income and middle-income countries: results from the World Health Survey. BMC Public Health. 2012;12(1):1.
30. Morrissey JL, Janz KF, Letuchy EM, Francis SL, Levy SM. The effect of family and friend support on physical activity through adolescence: a longitudinal study. Int J Behav Nutr Phys Act. 2015;12(1):1–9. doi:10.1186/s12966-015-0265-6
31. Santini ZI, Jose PE, Koyanagi A, Meilstrup C, Nielsen L. Formal social participation protects physical health through enhanced mental health: a longitudinal mediation analysis using three consecutive waves of the Survey of Health, Ageing and Retirement in Europe (SHARE). Soc Sci Med. 2020;251(August2019):112906. doi:10.1016/j.socscimed.2020.112906
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
