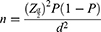Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Co-infections and Comorbidities of Multiple Parasites and Hepatitis B Virus Infections in the Lowland Area of Western Ethiopia: Implications for Integrated Approaches
Authors Assefa A , Erko B , Gundersen SG, Medhin G, Berhe N
Received 29 September 2021
Accepted for publication 24 November 2021
Published 8 December 2021 Volume 2021:14 Pages 3369—3383
DOI https://doi.org/10.2147/JMDH.S341100
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alemayehu Assefa,1,2 Berhanu Erko,2 Svein Gunnar Gundersen,3 Girmay Medhin,2 Nega Berhe2
1University of Assosa, College of Health Science, Assosa, Ethiopia; 2Akililu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Global Development and Planning, University of Agder, Agder, Norway
Correspondence: Alemayehu Assefa Email [email protected]
Background: In the current study area, the burden of morbidities associated with S. mansoni, soil-transmitted helminths (STHs), asymptomatic malaria, and hepatitis B virus (HBV) infections and co-infection has not been known for the last 20 years. This necessitated a systematic investigation of the status of these infections and their associated morbidities in the lowland areas of the Abbey and Didessa Valleys in Western Ethiopia.
Methods: We used a cross-sectional study design in three schistosomiasis endemic areas. Systematic random sampling and simple random sampling techniques were used to select households and one study participant from each household. Each selected and consented participants were give stool sample for S. mansoni and soil-transmitted helminths screening using duplicate kato-Katz technique; blood sample for screen of asymptomatic malaria using malaria rapid diagnostic test and microscopy and hepatitis B virus using hepatitis B surface antigen kit and anthropometric measurement to assess nutritional status and digital hemoglobin meter to measure hemoglobin and interviewed using structured questionnaire to assess factors associated with infections. A descriptive statistic to summarize the data and a chi-square test, Fisher’s exact test, and binary logistic regression models were used to see the associations.
Results: The overall prevalence of studied infections was 74.5%. It was highest for Schistosoma mansoni (53.9%), followed by asymptomatic Plasmodium falciparum infection (23.6%). The prevalence of Schistosoma mansoni co-infection with asymptomatic malaria was 8.6%, Schistosoma mansoni and soil-transmitted helminths co-infection was 6.2%, and the seroprevalence of hepatitis B virus was 2.9%. About half (49.9%) of the study participants were undernourished and about a quarter (24.4%) were anemic. Age group, the younger age group and infection status, those with multiple infections were more anemic and commonly undernourished.
Conclusion: There was a high prevalence of infections in the study area. Morbidities such as undernutrition and anemia were still prominent public health problems. There was a significant association between infection status and undernutrition and anemia.
Keywords: anemia, asymptomatic malaria, HBsAg, multiple infections, schistosomiasis, undernutrition, Western Ethiopia
Graphical Abstract:
Introduction
Poverty-related infectious diseases, including schistosomiasis, soil-transmitted helminthiasis (STHs), malaria and viral hepatitis are common infectious diseases that have adverse effect on human health. Despite ongoing control interventions in sub-Saharan Africa (SSA), schistosomiasis still accounts for more than 90% of the global burden of this parasite1.
Until recently, the conventional perception of parasitic infections was that “light worm burdens remain asymptomatic” implying that they do not provoke disease nor do they specifically require medical care.2 However, recent studies on the immunopathology of parasite infection and its chronic disease formation.3,4 It indicated that it is the presence, as well as the intensity of infection, that drives morbidity due to chronic parasites such as schistosomes, STHs and asymptomatic malaria. Under-recognized, subtle morbidities such as malnutrition, anemia, organomegaly, and poor school performance, and poor daily work capacity due to these morbidities are all significant impacts of these infections.5
Malaria is the top parasitic disease causing morbidity and mortality globally and particularly in SSA.6 Malaria transmission is seasonal, the dry season being characterized by limited transmission with almost no clinical cases of malaria and fewer mosquitoes.7 However, some Plasmodium parasites survive by establishing chronic, asymptomatic infections across the dry season.8 This infection has been shown to produce and transmit gametocytes.8 Besides their role in transmission of malaria, asymptomatic infections can cause chronic low-grade inflammation and activation of platelets and endothelial cells, which leads to a range of morbidities.4
Co-infection of schistosomiasis and Plasmodium falciparum is common, mainly due to the existence of favorable climatic conditions and water bodies as breeding grounds for intermediate hosts and vector of the two diseases respectively.9 This co-infection results in more severe and chronic debilitating morbidity.10 Ethiopia, particularly the Abbey and Didessa Valleys are where the two parasites are endemic.11,12
There are conflicting reports of co-infections of Plasmodium species and HBV and their impact on disease severity and morbidities. For example, a study in Brazil reported a negative relation between the two pathogens,13 while a study elsewhere showed that they exist independently as asymptomatic infections.14 We assumed that HBV co-infection has effect on prevalence and morbidity of schistosomiasis.
Worldwide, there is a strong overlapping between the distribution of extreme poverty and the distribution of infections.15 Due to limited access to safe water sources and poor environmental sanitations, the risk of infection is significantly higher for people with low socioeconomic status. In the setting of the impoverished rural tenant farmers, where the household, on average, must invest its entire human and physical capital each year simply to survive a minimal disability, morbidities due to these infections can have a great effect on the income, and food security of the households15 which further aggravates the morbidities due to these infections.
In the discussion of public health policy in endemic areas, the health and social burden of chronic infections is being re-emphasized.16 However, in the current study area the burden of morbidities associated with S. mansoni, STHs, asymptomatic malaria and HBV infections and co-infection have not been known for the last 20 years. This necessitated a systematic investigation of the status of these infections and their associated morbidities, at this time when the world is talking about elimination of these infections and controlling their associated morbidities. Hence, the present study evaluated the interactions and burden of morbidities associated with schistosomiasis, asymptomatic malaria, STHs and HBV infections and their co-infections in the lowland areas of the Abbey and Didessa Valleys of western Ethiopia. The findings would contribute to the integrated disease control plans and a widespread scaling up of existing interventions for each infection.
Materials and Methods
Study Area and Population
For map, see Figure 1. The study area is located in the lowlands of western Ethiopia with poor infrastructure. Benishangul Gumuz Region is one of the nine regional states in Ethiopia, which shares a border with Sudan in the west, Amhara Regional State in the east, and Oromia Regional State in the south. The Grand Ethiopian Renaissance Dam (GERD) is being constructed on the Nile River in this Regional State. The current study was conducted in three schistosomiasis and malaria-endemic areas of Kamashi zone, which is one of the three zones in Benishangul Gumuz Regional State at (700m–1200 m altitudes). The area is an extension of the Sudanese Savannah with a hot, dry climate and receives seasonal rains from May to October.
 |
Figure 1 Map of the study area. |
The 3 areas were: (a) Chessega village in the Sirba Abbey area of the Abbey Valley and (b) in the other two villages, Metti and Shimala in the adjacent Agallu Metti area, situated eastwards on the hilly slopes south of the junction between the Didessa and Abbey Rivers.
The study area is about 600 km west of Addis Ababa and is mostly inhabited by a Nilotic ethnic group known as Gumuz, except for some Oromos and Amharas, who moved to the lowland for farming. The total population of the three study areas at the last registration was 8375, of which 2660 are residents of Sirba Abbey (Chessega), 3800 are residents of Metti, and 1915 are residents of Shimala (Agallu Metti). All the inhabitants live under similar poor environmental sanitation and low socio-economic status. They earn their living in small-scale farming using the traditional farming methods. Maize and millet are the most commonly cultivated crops in the area.
Study Design and Period
A descriptive cross-sectional study was conducted in December 2020.
Sample Size Determination and Sampling of Study Participants
The sample size was calculated using a simple population proportion formula for each infection: Where n = sample size, z = z statistic for the level of confidence, P = expected prevalence and d = allowable error. We considered the expected prevalence of each dependent variable by using the following assumptions, Z = 1.96 (the value of the standard normal distribution curve corresponding to 95% confidence interval), d = 0.05 (the value of the standard normal distribution curve corresponding to 80% power), P = the proportion of each outcome variables.
Since the sample size calculated for schistosomiasis using 63% proportion from previous study in the same study area11 is larger than the other calculated sample sizes (358), so, we have used it as sample size for this particular study as the representative sample size. By adding of 5% non-response rate, our final sample size is 376 for all outcome variables.
After a systematic sampling to select households using the lists of households we obtained from the kebeles (the local administrative units), simple random sampling was applied to select one study participant from each household. The primary outcome variables are status of infection conditions (non-infected, single infection and/or co-infections or multiple infections). We also investigated the morbidities associated with the primary outcomes, through clinical examination, hemoglobin measuring and anthropometric measurement.
Inclusion Criteria
The following were inclusion criteria to participate in the study (i) 5 to 35 years of age for both sexes; (ii) parents or guardians who gave written informed consent; (iii) children who agreed and provided informed assent; and (iv) individuals who lived in the study area for more than a year.
Exclusion Criteria
Those who were (i) with history of anti-malarial/anti-helminthic medication within one month prior to registration; (ii) having fever history with in the last 48 hrs and/or treatment for malaria within the past week, or with clinical evidence of malaria (axillary temperature <37.5°C); and (iii) children less than 5 years and adults above 35 years, because children, adolescent and young adults are more at risk for those infections. In addition, asymptomatic malaria is not common in under-five children, but more common among the older (above 35 years old) due to immunity. Furthermore, morbidity among older (above 35 years) individuals could be due to different factors including chronic non-infectious diseases. We have excluded three individuals with fever (axillary temperature >37.5°C), and were replaced by another randomly selected household members.
Socio-Demographic and Other Data
Background data included: age, sex, educational status, occupational status, monthly income, and water contact habits; a history of deworming during the last deworming campaign for schistosomiasis earlier in the study; data on the availability of toilets for the household, and sources of drinking and bathing water for the household were collected. The availability and utility of insecticide treated nets (ITN), and indoor residual spraying (IRS) data were also collected. All data were obtained by interviewing parents and legal guardians and/or participants under the guidance of a structured questionnaire. Credibility of survey data was cross-checked by observing households, for availability and functionality of toilet and for availability and functionality of bed nets, the environment for open-air defecation, and waterbodies contact activities during field data collection.
Clinical Examination
Each individual was clinically examined by an experienced clinician. The abdominal palpation was performed as previously described.17 Using a tape measure, the following measurements were taken: the extension of the left-liver lobe beneath the sternum was measured in the mid-sternal line (MSL); the extension of the right-liver lobe beneath the rib cage was measured in the right mid-clavicular line (MCL); the extension of the spleen below the rib cage was measured both in the left MCL and left mid-axillary line (MAL). The liver tenderness and its consistency as well as the spleen consistency were assessed. The consistency of the organs and any signs of portal hypertension were assessed.
Stool Sample Collection and Parasitological Examination
The study participants were provided clean leak-proof plastic sheet and clean wooden applicators to bring sizable fresh, uncontaminated stool samples of their own. The stool containers were then collected and marked with an identification number and duplicate Kato-Katz smears were prepared from each sample as described by WHO18). The prepared Kato-Katz thick smears were examined within an hour not to miss the ova of hookworm. The stool samples were also checked for S. mansoni and for other intestinal helminth infections on the same day in the field and a week later in the parasitological laboratory at Aklilu Lemma Institute of Pathobiology, Addis Ababa University.
Quantification of eggs was done by counting the number of eggs on a smear of about 41.7 mg of stool using the Keto-Katz standard method used by WHO.18 The average number of eggs per Kato-Katz thick duplicate smears was obtained by multiplying the counts per slide by a factor of 24 to express the number of eggs per gram of feces (Epg). Classes of intensity were described as light, moderate, and heavy for parasitic infections including: S. mansoni, Ascaris lumbricoides, Trichuris trichiura and hookworms.18 The stool was also checked for infection of Taenia species and Hymenolepis nana.
Blood Sample Collection and Malaria Parasite Examination
About 5 mL of venous blood was collected using sterile disposable needles (21–23G) and Vacutainers (Becton Dickinson, Banbury, England), which were used for different investigations.
Malaria Rapid Diagnostic Test (RDT): RDT was performed using a kit containing monoclonal anti-P. falciparum histidine-rich protein II (HRP-II)-specific antibody and anti-Plasmodium vivax p-LDH-specific antibody to detect malaria parasite infection from venous blood. Microscopy: Thick and thin blood films were prepared from the venous blood, both stained with Giemsa, and examined by two microscopists to detect plasmodium parasites. Both procedures were performed immediately after the venous blood was collected. Parasite counts were performed by an experienced microscopist using a light microscope, were calculated by counting the number of parasites per 200 white blood cells (WBCs). Assuming a healthy WBC count of 8000/μL of blood, we have calculated parasitaemia and express it as the number of parasites per μL of blood. Parasite counts were used to classify the intensity of Plasmodium spp. infection into light, moderate, heavy, or very heavy infections respectively as followed: 1–499 parasites/µL, 500–1999 parasites/µL, 2000–9999 parasites/µL and ≥10,000 parasites/µL.18
Blood Collection and Screening for Hepatitis B Surface Antigen (HBsAg)
The blood samples were centrifuged, and separated serum was stored in screw cap vials with unique identification numbers and immediately placed in the refrigerator of the local health center during the field stay. The serum vials were kept frozen in a cold chain and transported to the virology laboratory of Aklilu Lemma Institute of Pathobiology (ALIPB), Addis Ababa University, where they were kept frozen at −20°C. Screening for HBsAg in serum was done using HBsAg rapid kits according to the manufacturer’s instructions (Atlas Medical UG, Mahlow Germany).
Determination of Hemoglobin (Hgb) Concentration
A portable Hb Hemoglobin Meter optical system (Mission, MDSS GmbH, and Hannover, Germany) was used to determine the hemoglobin concentration. The hemoglobin levels used to diagnose anemia were read from a digital counter in mg/dl and the anemia level was documented based on age, sex and pregnancy status as previously described by WHO.19
Anthropometric Measurements
The height was measured to the nearest 0.1 cm, and the weight was measured to the nearest 0.1 kg without shoes, and wearing the least amount of clothes using a standard weight scale from the local health institution, which also has a height pole. The body mass index (BMI)-for-age was calculated using the Anthroplus software version 10.4 online, which has been verified by reference to WHO charts and tables.20 According to the individual’s Z-scores, the children were categorized into groups of undernourished or well nourished. Children were classified as malnourished if BMI-for-age Z-score <−2 and well nourished otherwise. Adults aged 20 years and older were grouped as undernourished when their body mass index was <18.5 kg/m2. Undernutrition was also further classified as follows: severe, moderate, and mild undernutrition based on WHO reference charts and tables.20 The age of each study participant was obtained from the guardians and/or from the participants themselves.
Quality Assurance
The procedures were pretested using a sample of 19 individuals selected from a community that was not included in the current study. This step helped us to check whether the necessary information was correctly written and easily understood, and all steps of the field activities are well understood. During the sample collection process, strict supervision was done, especially on the amount of feces and soil contamination. Standard Operating Procedures (SOPs) were strictly followed during the course of sample collection, transportation, specimen processing, and laboratory works. All the Kato-Katz thick smears and malaria smears were examined by two experienced laboratory technologist and the discordant slides were re-checked by the third person at the Aklilu Lemma Institute of Pathobiology (ALIPB) Addis Ababa University and by WHO malaria expertise at Adama Center, Adama Ethiopia, respectively.
Data Analysis
Data were entered into the EpiData version 3.3 software and imported into IBM SPSS version 25 statistical software for statistical analysis. A descriptive statistic, a chi-square test, Fisher’s exact test, and binary logistic regression models were used to summarize the data descriptively, and to investigate bivariate associations. Logistic regression modelling was used to provide the association of pre-defined background variables with the outcome of interest. P-value of <0.05 was used as a cut-off in reporting statistical significance.
Results
Socio-Demographic Data
From the total planned sample size (376), results are reported for 373. An almost equal number of males and females with a mean age of 18.94 years (SD = 6.76) participated in the study. The mean weight was 45 kg (SD = 12.52) and the mean height was 156.83 cm (SD = 15.17). About 85% of the participants were from Gumuz ethnic group and the educational status of the majority (64.3%) was primary. Most of the study participants were students and farmers. About 4% of study participants were non-attending school-age children, and the data are published elsewhere.21
Utilization of Insecticide Treated Net (ITN) and Indoor Residual Spraying (IRS)
Only 49.3% (184/373) of the study participants had ITN, and 12% (22/184) said they have been using it regularly. Only 12.6% (47/373) of the respondents said that their home had been sprayed last year with an anti-mosquito chemical.
Organomegalies and Associated Factors
In the physical examination paleness was observed in 3.5% (13/373), splenomegaly in 3.2% (12/373), and only 4 study participants had clinically evident hepato-splenomegaly. Splenomegaly was significantly more common among individuals co-infected with S. mansoni and asymptomatic P. falciparum (P < 0.001).
Prevalence of Schistosoma mansoni Infection
Figure 2 summarizes the prevalence of infections and co-infection found in the study. The total prevalence of all studied infections was 74.5% (278/373). The prevalence was higher among females (78.3% (141/180)) than males (71% (137/193)), and it had a decreasing trends with increasing age.
 |
Figure 2 Prevalence of investigated infections and co-infections. |
The detailed schistosomiasis results are reported elsewhere.21 The overall prevalence of S. mansoni infection was 53.9% (201/373) with no significant difference between gender (50.8% among males and 57.2% among females) and decreasing trend with increasing age. In brief, from all the infections, 59% (119/201) were moderately infected, and 12.9% (12/201) were heavily infected. The mean EPG was 204 (min = 24 and max = 1464).
Prevalence of STHs and Other Intestinal Helminths Infection as Determined by Kato-Katz
The overall prevalence of STHs and other intestinal helminths (Enterobius vermicularis and Hymenolepis nana) infection was 12.9% (48/373) (Figure 2), with no significant gender difference (11.9% (23/193) among males and 13.9% (25/180) among females). The prevalence was 13% (10/77) among 5–12 years –old -age group, 8.6% (12/139) among 13–19 years-old -age group, and 16.6% (26/157) among 20–35 years-old-age group.
The prevalence of Ascaris infection was 4.3% (16/373), of which the majority (10/16) were lightly infected. The mean EPG of Ascaris infection was 3535.50 EPG (SD=1648.82 EPG). The prevalence of Trichuris trichiura was 4% (15/373), of which 73.3% (11/15) were moderately infected. The mean EPG of Trichuris trichiura infection was 2468.80 EPG (SD=1124.55 EPG). Only three persons were infected with hookworm and all are light infections. Prevalence of E. vermicularis was 2.1% (8/373), and prevalence of H. nana was 2.4% (9/373).
Prevalence and Intensity of Asymptomatic Plasmodium falciparum Infection
A total of 373 individuals who did not show any sign of clinical malaria were screened for malaria parasite by RDT and microscopy. By the RDT test 92 (24.7%) individuals tested positive for P. falciparum antigen. Out of 92 P. falciparum-positive individuals, as determined by RDT, a malaria parasite was detected by microscopy in 23.6% (88/373). Plasmodium falciparum parasite were detected in 88 people, and of them, 70 were positive for both the ring (asexual) form and gametocytes, 16 were positive for the ring form only and two were positive for gametocytes only. The average parasite load of the asexual form of P. falciparum was 3015/µL, with a range of 48/µL to 66,286/µL. An average parasite load for P. falciparum gametocyte was 232/µL, ranging from 16/µL to 2714/µL. No other species of Plasmodium were detected by either of the methods.
The majority of asymptomatic P. falciparum infections were light infections, accounting for 52.3% (46/88), while moderate infections accounted for 29.5% (26/88). Heavy and very heavy infections were accounted for 10.2% (9/88) and 8% (7/88), respectively.
HBV Seroprevalence
The seroprevalence of HBV among study participants was 2.9% (11/373). It was 3.6% (7/193) among males and 2.2% (4/180) among females. The prevalence was higher (4.3%) in the age range of 13 to 19 year-olds. All HBV seropositive individuals were co-infected with at least one of the investigated infections.
Schistosoma mansoni and Asymptomatic Plasmodium falciparum Co-Infection
The prevalence of S. mansoni and asymptomatic P. falciparum co-infection was 8.6% (32/373) regardless of other infections. Schistosoma mansoni infected individuals (15.4%) were less likely to be asymptomatic P. falciparum carrier than S. mansoni negative individuals (33.1%) (X2 = 16.34; P<0.001).
Prevalence of Undernutrition
The total prevalence of under-nutrition was 49.9% (186/373), of which the majority 51.6% (96/186) were moderately undernourished, and 21.5% (40/186) were severely undernourished. The mean BMI/BMI-for-age was 17.92 (SD = 2.90). As shown in Figure 3, the mean BMI/ BMI-for-age was lower among males and younger age group (5–12 years old age group).
 |
Figure 3 Mean BMI/BMI-for-age and mean Hgb level across sexes and age groups. |
Undernutrition and Degrees of Undernutrition and Their Associated Factors
The age group was significantly associated with nutritional status. Compared with participants in the 20–35 age group, study participants in the 5–12 and 13–19 age groups were 4.69 times and 6.75 times more likely to be malnourished, respectively (Table 1). Soil-transmitted helminths were significantly associated with nutritional status. STHs infected individuals were 0.229 times less likely to be undernourished than multiple infected individuals. None of the sociodemographic variables were significantly related to the degree of undernutrition.
 |
Table 1 Undernutrition and Associated Factors |
There was no significant association between S. mansoni intensity of infection and asymptomatic P. falciparum parasitemia and undernutrition (Table 2).
 |
Table 2 The Association Between Parasite Density and Nutritional Status |
Prevalence of Anemia
The overall prevalence of anemia was 24.4% (95% CI: 20.1–29.1) (91/373), of which 75.8% (69/91) were mild anemia and 24.2% (22/91) were moderate-to-severe anemia. The mean of Hgb level was 13.28 mg/dl (ranging from 7.50 mg/dl to 17.8 mg/dl). As indicated in Figure 3, the mean Hgb levels were lower among females compared to males and in younger age group (5 to 12 years old age group) compared to the older age groups.
Anemia/Hemoglobin Levels and Degrees of Anemia and Their Associated Factors
Anemia was significantly associated with study participant’s occupation (P = 0.036). Compared with day-to-day workers, salaried employees study participants were 0.186 times less likely to suffer from anemia. There was also a significant association between anemia and infection status. Compared with participants with multiple infections, study participants with only STHs infection, S. mansoni and asymptomatic malaria co-infection and other co-infections were 0.249 times, 0.358 times and 0.109 times less likely to develop anemia, respectively (Table 3). None of the sociodemographic variables were significantly related to the degree of anemia.
 |
Table 3 Anemia/Hemoglobin Level and Associated Factors |
As shown in Table 4, the association between S. mansoni infection intensity and asymptomatic P. falciparum parasite density classification with anemia was statistically significant, with P-values of 0.004 and 0.012, respectively. Anemia was more common among heavily infected individuals in both cases.
 |
Table 4 The Association Between Parasite Density and Hemoglobin Level/Anemia |
Discussion
In this study, the interactions and negative health impact of selected infections and/or co-infections were assessed. The investigated morbidities (such as malnutrition, anemia and organomegaly) were significant in the community, especially in the young age groups. About three-fourths of the population had at least one of the studied infections. The prevalence of the infections was highest for S. mansoni (53.9%), followed by asymptomatic P. falciparum (23.6%), and other selected intestinal helminths (12.9%). Only 2.9% of the study participants were hepatitis B seropositive. About half of the study participants were undernourished and about a quarter were anemic. The infection status had significant association with the assessed morbidities.
The finding of a common single parasite infection of S. mansoni followed by P. falciparum is similar to the report from Tanzania.22 On the other hand, the finding of combined parasitic infections is lower than the same report22 and another study in West Africa.23 These could be due to difference in study setting, study population and diagnostic methods used and difference in interventions in place.
The prevalence of asymptomatic malaria is similar to the pooled prevalence of asymptomatic malaria by all methods combined (microscopy/RDT and PCR), but higher than microscopy/RDT only report in Ethiopia.24 The P. falciparum gametocyte prevalence was 18.8%, which is higher than the prevalence reported from Kenya.25 This finding confirms that asymptomatic carriers are a possible source of malaria transmission.26 This high prevalence of chronic infection may be due to a neglected bed net use (only 12% reported routine use) and partial immunity to the parasite, because of the endemicity and a long season of transmission in the area.
The prevalence of S. mansoni co-infection with other helminths and S. mansoni co-infection with asymptomatic P. falciparum are similar to the research report from Democratic Republic of Congo27 and Tanzania,10 respectively. These prevalent co-infections may be due to environmental factors, infrastructural and behavioral problems such as poor sanitation, lack of toilet facilities, unsafe drinking water, and ineffective public health enlightenment programmes in the endemic area.28
The current study shows that about half of the study participants were undernourished and about one-tenth of them were severely undernourished. This prevalence is higher than most studies,29,30 but lower than a study in Tigray, northern Ethiopia.31 These differences may be due to socioeconomic differences between study areas and differences in the studied population, in which our study included different age groups, as most other studies focused only on children.
Undernutrition was more common and more severe among younger age groups. A study from Finchaa in western Ethiopia has also reported a similar relation.32 This may be due to the higher rate of multiple infections in this age group, as it is observed in this study. However, there are few studies that disagree.22,29 This difference could be due to difference in socioeconomic status and difference in infections intervention programmes in the areas.15 No other sociodemographic and related variables were found to be significantly associated with undernutrition. This could be due to the similar socioeconomic status of the community.
Soil-transmitted helminths (STHs)-infected individuals were less likely undernourished than those with multiple infections. This finding is in line with a research report from Angola29 and a review by Pullan and Brooker.33 Individuals who were concomitantly infected with S. mansoni and asymptomatic P. falciparum were more commonly undernourished than uninfected or mono-infected individuals. Similar conclusion is also reported by review of Pullan and Brooker.33 This may be due to parasite-induced gastro-intestinal pathophysiological change and reduced food intake.33,34
Approximately a quarter of the study participants were anemic, which puts the study area under the moderate public health problem classification category.19 No socio demographic and related variables were found to be significantly associated with undernutrition. This could be due to the similar socioeconomic status of the community. Only few anemic individuals were clinically indicated, probably because anemia is difficult to evaluate in dark skins. This shows the importance of hemoglobin measurements during such studies. Among the undernourished study participants, anemia was very common and severe. This may be due to the fact that the intake of certain nutrients is insufficient to meet the demands for the synthesis of hemoglobin and erythrocytes35 and the fact that underweight predisposes to iron depletion and increases the risk of anemia.36
There was a significant association between infection status and hemoglobin level/anemia. Anemia was more common and more serious among study participants with multiple infections. These findings are in line with studies from different endemic countries.22,29 This may be due to the pathologic synergistic effects of concomitant infections.28 This shows the impact of these infections on the normal daily performance of the individuals and affects food production of the households in such community where only traditional farming is practiced.5,15
Participants who were heavily infected with S. mansoni and those with high asymptomatic P. falciparum parasitemia had more anemia than those with light infections. This is due to chronic inflammation due to parasites and direct red blood cells destruction by plasmodium parasites.4,33 Other studies also reported this association,22,37 while a study in Uganda failed to report this co-relation.38 This difference could be due to difference in socioeconomic status and the number of co-infections or morbidities studied.
The strength of the study is that it examined the co-infections and comorbidities of many infections. Most other studies have a narrower spectrum of infections, examining concomitant infections are defined as the presence or absence of a pair of pathogens.22,27 In addition, and these studies ignore asymptomatic malaria and do not jointly study important pathogens, like HBV which has a major health impact on co-infection.22,27 Moreover, to understand the magnitude of infection within and among different groups and the correlation between infection and morbidity, it included a series of participants (school attending and non-attending school age children, adolescents, and young adults).
Despite these strengths, there are limitations: first: the study is cross-sectional and cannot imply causality relationships; second: few people were infected with HBV which may have limited the power and then statistical significance; third: routine microscopy with a detection limit of 50–100 parasites/μL may severely underestimate the reports of asymptomatic malaria infections,39 and the kato-taze method may underestimate the prevalence of intestinal parasites.
Conclusions
Different infectious diseases are widespread in the study area, which could be explained by the ecological environment and poor socio-economic conditions and poor health care system40 that promote the existence and spread of these diseases. The morbidities due to these infections are still common public health problems despite ongoing deworming interventions, especially among young people. It does not seem that the WHO control and elimination target for these parasitic infections will be achieved in the area.
It is recommended to scale up the ongoing schistosomiasis MDA and assess the drug efficacy surveillances in the area. It is also recommend that at least school-aged children in the study area to be treated for asymptomatic P. falciparum.41 These findings indicate the need for a comprehensive intervention plan that should combine ongoing treatment campaigns against schistosomiasis and STHs with other interventions to reduce the intensity of parasites, prevent the possibility of re-infection, and thereby reduce the morbidities.15 These highly prevalent infections and significant morbidities alert the concerned bodies to give urgent special attention to the area, to achieve the global control and elimination targets of these infections. It is recommended to study these infections using more sensitive diagnostic methods, such as PCR and MAD drug effectiveness survey in the area.
Abbreviations
BMI, Body mass index; CI, Confidence interval; EPG, Egg per gram; ERB, Ethical Review Board; Hgb, Hemoglobin; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; IRS, Indoor residual spraying; ITN, Insecticide treated nets; MDA, Mass drug administration; RDT, Rapid Diagnostic Test; SOP, Standard Operating Procedure; SPSS, Statistical package of social science; SSA, sub-Saharan Africa; STHs, Soil transmitted helminths; WBC, White blood cell; WHO, World Health Organization.
Data Sharing Statement
All data that support the findings of this study are available from the corresponding authors upon request.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Board of Aklilu Lemma Institute of Pathobiology, Addis Ababa University (Ref No: ALIPB/IRB/002/2017/18) and it was conducted in accordance with the Declaration of Helsinki. Prior to the conduct of the study, a letter of support was obtained from the district health offices and meetings were held with community leaders and community members to explain the aims of the study. The aim of the study was explained to the study participants, and an informed written and verbal consent were obtained from each participant and/or parents or guardians for younger children and an informed assent from children. Ethical approval and informed consent forms and paper copies of signed consents have been kept confidential. Those participants found positive for schistosomiasis and other intestinal parasites were treated using appropriate drugs. Those participants who were severely undernourished and/or anemic and who were found to be positive for HBsAg were referred to the local health facilities for treatment and care. Selected participants who were found to have symptomatic malaria cases were also linked to the local health facilities for possible treatment.
Acknowledgments
We thank Royal Society of Tropical Medicine and Hygiene, United Kingdom, London and the School of Graduate Studies, Addis Ababa University, Ethiopia, for their financial support. We would like to thank the study participants for voluntarily taking part in the study for their participation and local health officials and community leaders for their cooperation. We also thank Mr. Muluken Berhanu, Mr. Henok Tsehaye and Mr. Sisay Dessie, as well as Ms. Yirgalem Gebrehiwot for their technical assistance in the field and laboratory.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by a small grant obtained from the Royal Society of Tropical Medicine and Hygiene, United Kingdom, London and the School of Graduate Studies, Addis Ababa University, Ethiopia.
Disclosure
The authors declare that they have no competing interests to disclose that are relevant to this article.
References
1. World Health Organization (WHO). Summary of global update on preventive chemotherapy implementation in 2015. Wkly Epidemiol Rec. 2016;91(39):456–459. English, French. PMID: 27758092.
2. Gryseels B. The relevance of schistosomiasis for public health. Trop Med Parasitol. 1989;40(2):134–142.
3. Coutinho HM, Acosta LP, Wu HW, et al. Th2 Cytokines Are Associated with Persistent Hepatic Fibrosis in Human Schistosoma japonicum Infection. J Infect Dis. 2007;195(2):288–295. doi:10.1086/510313
4. De Mast Q, Brouwers J, Syafruddin D, et al. Is asymptomatic malaria really asymptomatic? Hematological, vascular and inflammatory effects of asymptomatic malaria parasitemia. J Infect. 2015;71(5):587–596. doi:10.1016/j.jinf.2015.08.005
5. Leenstra T, Acosta LP, Langdon GC, et al. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines 1. Am J Clin Nutr. 2006;83(2):371–379. doi:10.1093/ajcn/83.2.371
6. World Health Organization (WHO). Global malaria report 2020: global Malaria Programme. World Health Organization, Geneva; 2020. Available from: https://www.who.int/teams/global-malaria-programme/reports/.
7. Ceesay SJ, Casals-Pascual C, Erskine J, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372(9649):1545–1554. doi:10.1016/S0140-6736(08)61654-2
8. Dunyo S, Milligan P, Edwards T, Sutherland C, Targett G, Pinder M. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLoS Clin Trials. 2019;1(4):e20. doi:10.1371/journal.pctr.0010020
9. Booth M. The role of residential location in apparent helminth and malaria associations. Trends Parasitol. 2006;22(8):359–362. doi:10.1016/j.pt.2006.06.007
10. Mazigo RW. Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasites Vectors. 2010;44:3.
11. Gundersen SG, Birrie H, Torvik HP, Medhin G, Mengesha H. Delayed reinfection of Schistosoma mansoni in the Blue Nile Valley of western Ethiopia 10 years after mass chemotherapy. Acta Trop. 1998;70(1):35–42. doi:10.1016/S0001-706X(98)00006-0
12. Taffese HS, Hemming-Schroeder E, Koepfli C, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7(1):103. doi:10.1186/s40249-018-0487-3
13. Andrade BB, Santos CJ, Camargo LM, et al. Hepatitis B infection is associated with asymptomatic malaria in the Brazilian Amazon. PLoS One. 2011;6(5):e19841. doi:10.1371/journal.pone.0019841
14. Freimanis GL, Owusu-Ofori S, Allain JP. Hepatitis B virus infection does not significantly influence Plasmodium parasite density in asymptomatic infections in Ghanaian transfusion recipients. PLoS One. 2012;7(11):e49967. doi:10.1371/journal.pone.0049967
15. King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113(2):95–104. doi:10.1016/j.actatropica.2009.11.012
16. King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–1569. doi:10.1016/S0140-6736(05)66457-4
17. Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21(8):386–392. doi:10.1016/j.pt.2005.06.006
18. World Health Organization (WHO). Basic laboratory methods in medical parasitology. Geneva: World Health Organization; 1991. Available from: https://apps.who.int/iris/handle/10665/40793.
19. World Health Organization (WHO). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. Available from: https://apps.who.int/iris/handle/10665/85839.
20. World Health Organization (WHO). Growth reference standard for school-aged children and adolescents. BMI – for – age.2007; 2007. Available from: https://www.who.int/growthref/growthref_who_bull.pdf.
21. Assefa A, Erko B, Gundersen SG, Medhin G, Berhe N. Current status of Schistosoma mansoni infection among previously treated rural communities in the Abbey and Didessa Valleys. Western Ethiopia. 2021;16(2):e0247312.
22. Kinung’hi SM, Mazigo HD, Dunne DW, et al. Coinfection of intestinal schistosomiasis and malaria and association with haemoglobin levels and nutritional status in school children in Mara region, Northwestern Tanzania: a cross-sectional exploratory study. BMC Res Notes. 2017;10(1):583. doi:10.1186/s13104-017-2904-2
23. Hürlimann E, Yapi RB, Houngbedji CA, et al. The epidemiology of polyparasitism and implications for morbidity in two rural communities of Côte d’Ivoire. Parasit Vectors. 2014s;7(1):81. doi:10.1186/1756-3305-7-81
24. Hailemeskel E, Tebeje SK, Behaksra SW, et al. The epidemiology and detectability of asymptomatic plasmodium vivax and plasmodium falciparum infections in low, moderate and high transmission settings in Ethiopia. Malaria J. 2021;20(1):59. doi:10.1186/s12936-021-03587-4
25. Idris ZM, Chan CW, Kongere J, et al. High and Heterogeneous Prevalence of Asymptomatic and Sub-microscopic Malaria Infections on Islands in Lake Victoria, Kenya. Sci Rep. 2016;6:36958. doi:10.1038/srep36958
26. Schneider P, Bousema JT, Gouagna LC, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76(3):470–474. doi:10.4269/ajtmh.2007.76.470
27. Matangila JR, Doua JY, Linsuke S, et al. Malaria, schistosomiasis and soil transmitted helminth burden and their correlation with anemia in children attending primary schools in Kinshasa, Democratic Republic Of Congo. PLoS One. 2014;9(11):e110789. doi:10.1371/journal.pone.0110789
28. Afolabi MO, Ale BM, Dabira ED. Malaria and helminth co-infections in children living in endemic countries: a systematic review with meta-analysis. PLoS Neglected Tropical Diseases. 2021;15(2):e0009138. doi:10.1371/journal.pntd.0009138
29. Sousa-Figueiredo JC, Gamboa D, Pedro JM, et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS One. 2012;7(4):e33189. doi:10.1371/journal.pone.0033189
30. Berhe K, Gebremariam G. Magnitude and associated factors of undernutrition (underweight and stunting) among school adolescent girls in Hawzen Woreda (District), Tigray regional state, Northern Ethiopia: cross-sectional study. BMC Res Notes. 2020;13(1):59. doi:10.1186/s13104-020-4926-4
31. Gebremariam H, Seid O, Assefa H. Assessment of Nutritional Status and Associated Factors among School Going Adolescents of Mekelle City, Northern Ethiopia. Int J Nutr Food Sci. 2015;4:118. doi:10.11648/j.ijnfs.20150401.26
32. Mekonnen Z, Meka S, Zeynudin A, Suleman S. Schistosoma mansoni infection and undernutrition among school age children in Fincha’a sugar estate, rural part of West Ethiopia. BMC Res Notes. 2014;7:763. doi:10.1186/1756-0500-7-763
33. Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under-estimating the burden of parasitic diseases? Parasitology. 2008;135(7):783–794. doi:10.1017/S0031182008000346
34. Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. Am J Clin Nutr. 2004;80(6):1604–1610. doi:10.1093/ajcn/80.6.1604
35. Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi:10.1016/S0140-6736(10)62304-5
36. Sri Sumarmi M, Puspitasari N, Handajani R, Wirjatmadi B. Underweight as a Risk Factor for Iron Depletion and Iron-Deficient Erythropoiesis among Young Women in Rural Areas of East Java, Indonesia. Malays J Nutr. 2016;22:219–232.
37. Butler SM. Mechanism of Anemia in Schistosoma mansoni-Infected School Children in Western Kenya. Am J Trop Med Hyg. 2012;2:548.
38. Tukahebwa EM, Magnussen P, Madsen H, et al. A very high infection intensity of Schistosoma mansoni in a Ugandan Lake Victoria Fishing Community is required for association with highly prevalent organ related morbidity. PLoS Negl Trop Dis. 2013;7(7):e2268–e. doi:10.1371/journal.pntd.0002268
39. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–840. doi:10.1038/nrmicro3364
40. Assefa A, Erko B, Gundersen SG, Medhin G, Berhe N. Low awareness and common misconceptions about schistosomiasis in endemic lowland areas in Western Ethiopia: a mixed-methods study. BMC Public Health. 2021:2:1064.
41. Maiga H, Barger B, Sagara I, et al. Impact of Three-Year Intermittent Preventive Treatment Using Artemisinin-Based Combination Therapies on Malaria Morbidity in Malian Schoolchildren. Trop Med Infect Dis. 2020;5(3):458.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.