Back to Journals » International Journal of Nanomedicine » Volume 12
Co-delivery of polyinosinic:polycytidylic acid and flagellin by poly(lactic-co-glycolic acid) MPs synergistically enhances immune response elicited by intranasally delivered hepatitis B surface antigen
Authors Dai X, He J , Zhang R, Wu G, Xiong F, Zhao B
Received 21 July 2017
Accepted for publication 12 August 2017
Published 7 September 2017 Volume 2017:12 Pages 6617—6632
DOI https://doi.org/10.2147/IJN.S146912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dongwoo Khang
Xiaojing Dai, Jintian He, Ruxia Zhang, Guanghao Wu, Fangfang Xiong, Baohua Zhao
College of Life Science, Hebei Normal University, Shijiazhuang City, Hebei Province, People’s Republic of China
Abstract: The aim of the present work was to investigate the synergistic effect between toll-like receptor (TLR) 3 ligand polyinosinic:polycytidylic acid (pI:C) and TLR5 ligand flagellin (FLN) on immune responses induced by nasally delivered hepatitis B virus surface antigen (HBsAg). Mannan and chitosan oligosaccharide-modified, pH-responsive poly(lactic-co-glycolic acid) (MC-PLGA) microparticles (MPs) containing HBsAg, FLN, pI:C or both ligands were prepared with a double-emulsion method. In vitro uptake experiments show that cellular uptake of MC-PLGA MPs by macrophages was through energy-dependent, receptor-mediated endocytosis mechanism. After uptake of MPs by macrophages, MC-PLGA MPs existed both in the endosome and in the cytoplasm. FLN and pI:C in solution or MP formulation could synergize to activate macrophages and induce higher pro-inflammatory cytokines interleukin (IL)-6, IL-12, interferon-γ and anti-inflammatory cytokines IL-10 compared to single TLR ligand (P<0.05). In vivo immunogenicity studies indicated that co-delivery of FLN and pI:C within MC-PLGA MPs synergistically induced higher serum anti-HBsAg IgG levels and Th1 cytokine levels compared with MC-PLGA MPs encapsulated single TLR ligand plus MPs encapsulated HBsAg (P<0.05). These results suggest that synergic TLR3 and TLR5 stimulation might be a promising novel tool for nasally delivered HBsAg.
Keywords: toll-like receptor, polyinosinic:polycytidylic acid, flagellin, hepatitis B surface antigen, PLGA MPs, intranasal vaccination
Introduction
The needle-free mucosal vaccine has been identified as one of the most important goals toward increasing global health. Mucosal vaccination can not only stimulate antigen-specific systemic humoral and cell-mediated immune response but also simultaneously elicit mucosal immunity.1,2 Most importantly, mucosal vaccines do not require needles and syringes. Being needle-free, mucosal vaccinations can generate many advances in vaccine delivery, such as better safety, improved compliance with immunization schedules, avoided vaccination-related pain and reduced cost.2,3
Mucosal immunization through the nasal cavity is an interesting route that has been explored over many years. The nasal immunization possesses many advantages for vaccine delivery than other routes of delivery, such as avoiding first-pass metabolism by the liver, enzymatic degradation in the gastrointestinal tract, slow absorption and low bioavailability.4,5 In addition, there are many M cells and dendritic cells (DCs) in the nasal cavity that facilitate inducing strong systemic and local immune responses.6,7 However, there are many factors that restrict the potential of nasal vaccine delivery, which include low permeability of antigens through the epithelium, mucociliary clearance and potential enzymatic degradation. As a result, intranasal delivery of antigens, especially subunit antigens, often elicits poor antigen-specific immune responses.2,8
To increase delivery efficiency, poly(lactic-co-glycolic acid) (PLGA) nano-/microparticles (NPs/MPs) have been extensively explored for intranasal vaccination with numerous antigens, such as epitope peptides, antigenic proteins, lipoproteins and plasmid DNA.4,7,9 Similar to antigen delivery system, PLGA micro-/nanoparticulate formulations have many advantages over soluble formulations. PLGA NPs/MPs can limit antigen degradation by protease, targeting antigens to antigen presenting cells (APCs) and control release rates of antigen.10,11 On the other hand, mucosal adjuvants, such as toll-like receptor (TLR) ligands, can enhance the efficacy of weak antigens and have proved to be key components in mucosal vaccines.12,13 Synergistic activation of APCs by combination of multiple TLR ligands might play critical roles in eliciting strong immune response and long-term immune memory.12,14 It has been demonstrated that combination of TLR ligands, polyinosinic:polycytidylic acid (pI:C, TLR3 ligand) and R848 (TLR7 ligand), monophosphoryl lipid A (TLR4 ligand) and R837 (TLR7 ligand), oligodeoxynucleotides (TLR9) and monophosphoryl lipid A (TLR4), can synergistically enhance vaccine potency.13,14
TLR3 ligand pI:C and TLR5 ligand flagellin (FLN) have been shown to be effective adjuvants.15,16 pI:C, a mimic of viral dsRNA, is a TLR3 ligand, which is recognized primarily by endosomal TLR3 and activates multiple kinds of transcription factors such as IFN-regulatory factor 3 (IRF3) and NF-κB, resulting in the expression of type II interferons and proinflammatory cytokines including interleukin (IL)-12 and IL-6, respectively. The pI:C has been shown to be a potential adjuvant for live-attenuated influenza, HIV-1 CN54gp140 (gp140) and tetanus toxoid (TT) in mice.15,17 FLN is a structural component of bacterial flagellar filament and is the only reported TLR5 ligand.16,18 FLN binds to TLR5 located on the cell surface and nucleotide-binding ligomerization domain-like receptor (NLR) protein NLRC4 in the cytoplasm of APCs, activating nuclear factor-κB (NF-κB) and NLRC4 inflammasome signaling, respectively. As a result, FLN induces secretion of IL-6, IL-12 and IL-23, and then promotes Th1, Th2, and Th17 cell-mediated immune response. The adjuvant effect of FLN has been proved for many antigens such as ovalubumin, influenza M2e, Escherichia coli heat-stable toxin, circumsporozoite protein of Plasmodium falciparum, and TT.18 However, FLN is a bacterial toxin, while pI:C has been identified as a trigger toward autoimmunity.
When delivered via the mucosal routes, FLN and pI:C are prone to be degraded by enzymes secreted by the mucosal epithelial cells. Thus, encapsulating them into NPs/MPs should be an alternative strategy.10,15 Until now, although adjuvant effects of pI:C and FLN have been studied in detail, synergistic effects between pI:C and FLN are not sufficiently investigated, and the synergistic effects between pI:C and FLN might be important for the development of mucosal vaccine delivery system.
In the previous study, mannan and chitosan-modified, pH-responsive PLGA-based MPs were successfully used to encapsulate hepatitis B virus surface antigen (HBsAg) for nasal delivery.19 Mannose receptor natural ligand mannan and mucoadhesive polymer chitosan was used to modify surface of PLGA microspheres in order to improve potency of PLGA microspheres as a nasal vaccine delivery vehicle.20 In the present work, HBsAg, pI:C, FLN or both TLR ligands were encapsulated into mannan and chitosan oligosaccharide (COS)-modified, pH-responsive PLGA (MC-PLGA) MPs by a double-emulsion method. Then, the uptake mechanism of MC-PLGA MPs by macrophages was investigated systematically. Moreover, the effects of FLN and pI:C in MP formulation on activation of macrophages were compared with that in solution formulation. The synergistical effects of FLN and pI:C within MC-PLGA MPs on activation of macrophages and HBsAg-specific immune response were further investigated systematically.
Materials and methods
Reagents and animals
PLGA with a 75:25 LA/GA ratio and an average molecular weight of 13 kDa was obtained from Jinan Daigang Biomaterial Co., Ltd. (Jinan, People’s Republic of China). COS (MW <2×103 Da, degree of deacetylation ~95%) was obtained from Qingdao BZ-Oligo Co., Ltd (Qingdao, People’s Republic of China; medicine grade). Recombinant HBsAg and an aluminum-containing adjuvant Hepatitis B vaccine (alum-HBsAg vaccine) were obtained from NCPC Gene Tech Biotechnology Development Co., Ltd. (Shijiazhuang, People’s Republic of China). The pI:C LMW was purchased from InvivoGen (San Diego, USA). FLN, poly(vinyl) alcohol (PVA; MW 13–23 kDa, 87%–89% hydrolyzed), mannan (MW, 35–60 kDa) and fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). SYBR Green I and OliGreen were purchased from Thermo Fisher Scientific (Waltham, MA, USA). LysoTracker-Red DND-99 was purchased from Molecular probes (Thermo Fisher Scientific). Bicinchoninic acid (BCA) protein assay kit and human serum albumin (HSA) were obtained from Sangon Biotech (Shanghai) Co., Ltd (Shanghai, People’s Republic of China). EtEraser Endotoxin Removal Kit was obtained from Chinese Horseshoe Crab Reagent Manufactory, CO., Ltd (Xiamen, People’s Republic of China).
Sprague Dawley rats (Grade II, Certificate No 06057) were purchased from the Experimental Animal Center of Hebei Province. All animal studies were performed at Hebei Normal University and were approved by the Animal Ethics Committee of Hebei Normal University. These rats were treated in accordance with the Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation.
Preparation of MC-PLGA MPs
The MC-PLGA MPs encapsulating HBsAg, pI:C, FLN or both TLR ligands were prepared with a modified W1/O/W2 technique.19 In brief, the primary emulsion (W1/O) was formed by emulsifying 2 mL of aqueous solution containing HBsAg (0.5 mg mL−1), HSA (5 mg mL−1), PVA (10 mg mL−1), NaHCO3 (5 mg mL−1) or pI:C (2.5 mg mL−1), or FLN (0.5 mg mL−1), or both TLR ligands into 4 mL of dichloromethane (DCM) containing 240 mg PLGA. As an effective stabilizer of HBsAg, HSA was added into the inner aqueous phase together with HBsAg as previously reported.21 The W1/O emulsion was prepared with an ULTRA-TURRAX stirrer (IKA-WERKE, Germany) at 20,000 rpm for 5 min. Then the W1/O emulsion was added dropwise to 15 mL of 2% (w/v) PVA solution containing NaCl (50 mg mL−1), COS (20 mg mL−1) and mannan (20 μg mL−1). The mixture was then emulsified for 15 min with an ULTRA-TURRAX stirrer at 15,000 rpm to prepare the W1/O/W2 double emulsion. CH2Cl2 were evaporated by continuous stirring for 8 h with a magnetic stirrer at 300 rpm. MC-PLGA MPs were collected by centrifugation (8,000 rpm, 20 min) at 4°C. After washing three times with sterile deionized water, MPs were collected, lyophilized overnight and finally stored at −20°C until usage.
Entrapment efficiency and drug loading
A BCA protein assay kit was used to determine the concentration of FLN and HBsAg in solution. The protein content in the MC-PLGA MPs was measured with a sodium hydroxide (NaOH)/sodium dodecyl sulfate (SDS) method.21 In brief, 10 mg of MC-PLGA MPs were digested in 2 mL of 0.05 mol/L NaOH containing 0.5% (w/v) SDS and gently agitated overnight at 37°C. After centrifugation, the protein content in the supernatant was determined using a BCA protein assay kit. The pI:C content in the microspheres was determined by an extraction method. Briefly, MPs were dispersed in 500 μL Tris-EDTA buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.4) and mixed with 1 mL of DCM in an Eppendorf tube. The suspension was vortexed for 1 min, placed in a ZWY-2102 rocking incubator (Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd, Shanghai, People’s Republic of China), and shaken at 150 rpm for 1 h. After centrifugation, 200 μL of the pI:C solution from each sample was removed and placed in a 96-well plate. SYBR Green I or Oli-Green nucleic acid dye was used to quantify pI:C.22 The drug loading (DL) was calculated from the amount of encapsulated antigen or TLR ligand within the MPs to the weight of MPs:
|
The encapsulation efficiency (EE) of antigen or TLR ligand in the MPs was calculated using the following equation:
|
Microparticle morphology, average particle size and surface charge
MC-PLGA MP morphology was studied with a Hitachi S-520 scanning electron microscopy (SEM; Hitachi, Ltd., Japan). To observe the surface morphology, MC-PLGA MPs were first coated with a thin gold layer under vacuum and then analyzed on SEM. The surface charge (zeta potential) and average particle diameter of MC-PLGA MPs were determined with a ZetaSizer nano ZS apparatus (Malvern Instruments Ltd., Malvern, UK). MC-PLGA MPs were dispersed in 0.01 M phosphate buffer at different pH values of 5, 6, 7.4.
In vitro release studies
Thirty milligrams of MC-PLGA MPs were dispersed in 2 mL of 0.02 M phosphate buffered saline (PBS) solution with different pH values to form a suspension. Then test tubes containing the suspension were shaken in a ZHWY-103B rotary shaker (Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd) at 100 rpm at 37°C. At each sampling time, tubes were centrifuged at 8,000 rpm for 20 min at 4°C. Then, the samples were removed and the same volume of fresh PBS buffer was added to keep a constant volume. The amount of released TLR ligands was measured as described above. In vitro antigenicity of HBsAg was measured with a commercial HBsAg ELISA kit from Shanghai Kehua Bio-Engineering Co., Ltd (Shanghai, People’s Republic of China).
In vitro uptake of MPs by macrophages
In this study, FITC-labeled HSA (FITC-HSA) and Rhodamine B were used as fluorescence probes to investigate in vitro uptake of MPs by macrophages. For preparation of Rhodamine B-loaded MC-PLGA MPs containing HBsAg, FLN or/and pI:C, 0.5 mg/mL Rhodamine B was added into the organic phase during MP preparation. To prepare FITC-HSA-loaded MC-PLGA MPs, 1 mg/mL FITC-HSA was added to the internal water phase taking the place of some HSA during the preparation process.
Peritoneal macrophages were extracted from female Sprague Dawley rats by peritoneal cavity lavage as previously reported by Li et al.19 The macrophages (5×105 cells/well) were seeded into each well of 12-well tissue culture plate and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum at 37°C under an atmosphere containing 5% CO2 overnight. Then culture medium was changed with DMEM supplemented with 10% fetal calf serum containing 250 μg/mL FITC-HSA-loaded MPs. After 12 h of co-culture, non-ingested MPs were washed off with PBS. Subsequently, MP uptake by macrophages was detected both quantitatively and qualitatively. For qualitative analysis of MP uptake by macrophages, cells growing on the glass slides were fixed with 4% paraformaldehyde for 20 min and then cell nuclei were stained using DAPI for 5 min. Finally, cells were observed with an Olympus IX71-F22FL/PH inverted fluorescent microscope (Olympus Corporation, Tokyo, Japan).
To quantitatively determine Rhodamine B-loaded MC-PLGA MPs taken up by macrophages, the cells were lysed in 500 μL of 0.01 M PBS solution containing 1% Triton X-100 and 2% sodium dodecyl sulfate on ice for 30 min.19,23 The fluorescence intensity of lysate (λex 554 nm, λem 570 nm) was detected with a fluorescence spectrometer (model LS55; Perkin Elmer, Boston, USA).
To evaluate the specific mechanisms involved in MPs uptake, macrophages were incubated with MC-PLGA MPs at 4°C for 2 h.23 Besides, macrophages were pre-incubated with the metabolic inhibitor 10 mM sodium azide and 50 mM deoxyglucose, the endocytosis inhibitor 40 mM ammonium chloride, 0.45 M D(+)-Sucrose or 10 μM chlorpromazine, for 30 min prior to MC-PLGA MPs application.24,25 Controls were prepared without the inhibitor pre-incubation. For saturation of mannose receptor, macrophages were pre-incubated with 50 mM mannose for 30 min.26
Intracellular localization of MPs
For observing intracellular localization of MPs within macrophages, 5×105 cells/well were seeded into 12-well plates and cultured at 37°C and 5% CO2 in DMEM supplemented with 10% fetal calf serum overnight. Then culture medium was changed with DMEM supplemented with 10% fetal calf serum containing 250 μg/mL FITC-HSA-loaded MPs. After washing three times with sterile PBS (10 mM, pH 7.4), cells were incubated with media containing Lyso Tracker Red DND-99 for 1 h. Then, the cells were fixed with formaldehyde for 1 h. Images were acquired by a Leica TCS SP5 II confocal laser scanning microscope (CLSM; Leica Microsystems CMS Gmbh, Mannheim, Germany). A representative macrophage was selected at random and fluorescent images were merged to determine the relative localization of MC-PLGA MPs within macrophages.
In vitro macrophage activation
Macrophages were seeded into 12-well tissue culture plates at a density of 5×105 cells/well and cultured at 37°C and 5% CO2 in DMEM supplemented with 10% fetal calf serum overnight. Then culture medium was changed with DMEM containing HBsAg, FLN, pI:C or MC-PLGA(FLN), MC-PLGA(pI:C), MC-PLGA(FLN+pI:C) MPs and then incubated at 37°C and 5% CO2. Culture supernatants were harvested after 4 days culture and IL-6, IL-10, IL-12, and IFN-γ content in supernatant were determined with ELISA kits (Shanghai Qiaodu Biotechnology Co., Ltd., Shanghai, People’s Republic of China) according to the manufacturer’s instructions. EtEraser Endotoxin Removal Kit was used to remove the endotoxin in the samples for cell test.
Animal immunization protocol and sample collection
Female Sprague Dawley rats, weighting 180–210 g, were used for immunization studies. The animals were divided into six groups and each group consisted of six rats. Group 1 rats were inoculated subcutaneously with alum-HBsAg vaccine. Group 2–5 rats were intranasally inoculated with MC-PLGA(HBsAg) alone, or MC-PLGA(HBsAg) together with MC-PLGA(pI:C), MC-PLGA(FLN) or MC-PLGA(FLN+pI:C), respectively. Group 6 rats were intranasally inoculated with blank MC-PLGA MPs without HBsAg. Each rat was inoculated three times with formulations in weeks 1, 3 and 6. Blood samples were taken at each sampling time point. Sera were isolated and collected by centrifugation at 5,000 rpm for 10 min, which were stored at −20°C until tested. The nasal and salivary secretions were collected after 12 weeks of primary vaccination. For salivary secretions, saliva was collected using pipette. A nasal wash was collected according to the previously reported method.27 PBS 200 μL (pH 7.4) containing 10 mg/mL bovine serum albumin was used to flush the nasal cavity. The nasal cavity was flushed three times.
ELISA analysis of anti-HBsAg IgG and IgA
IgG, IgG1, IgG2a and IgA antibodies in sera or secretions were serially diluted in phosphate-buffered saline and detected with ELISA test. Serum IgG antibody concentration was measured by a rat HBsAb ELISA kit (Premedical Laboratories Co., Ltd., Beijing, People’s Republic of China). A standard curve was generated using standard solution of various concentrations of rat anti-HBsAg IgG in mIU/mL. IgG2a and IgG1 antibody concentrations were measured with rat IgG2a and IgG1 ELISA kits (Premedical Laboratories Co., Ltd.). Then IgG2a/IgG1 ratio between both IgG subtypes was calculated. IgA antibodies in secretion samples were detected by ELISA method. Briefly, microtiter plates were coated with 100 μL of 2 μg/well HBsAg in carbonate buffer (pH 9.6) and rinsed with PBS (pH 7.4). After blocked with 1% BSA, 100 μL of serially diluted nasal and salivary secretions were added to the wells and incubated for 1 h at 37°C. After washing with PBS, goat anti-rat IgA-HRP (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were 1:2,000 diluted and added to each well. Finally, 3,3′,5,5′-Tetramethylbenzidine was added to each well and absorbance at 450 nm was measured on a microplate reader (Bio-Rad 550). The IgA antibody titer was expressed as the maximum dilution ratio of the sample, which gave an absorbance value at least two times higher than that of the control sample (PBS substituted for secretion).
Determination of cytokine levels by ELISA
Twelve weeks after the final immunization, splenocytes were prepared according to previously published methods.21 Then, 5×105 cells/well spleen cells were cultured in a 24-well plate in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum. To determine overall cytokine production, splenocytes were restimulated with 10 μg/mL HBsAg. Culture supernatants were harvested 5 days later. The levels of IFN-γ and IL-2 in the culture supernatant were determined using ELISA kits.
Statistical analysis
Statistical differences between two experimental groups were determined using one-way analysis of variance (ANOVA) and Student’s t-test. Data were considered to be statistically significant if P<0.05.
Results and discussion
MC-PLGA microparticle preparation and characterization
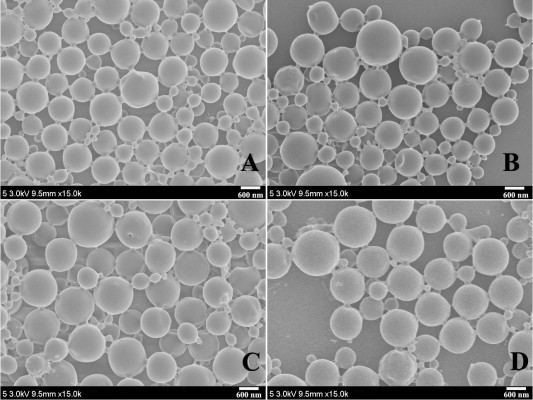
MC-PLGA(HBsAg), MC-PLGA(pI:C), MC-PLGA(PLN) and MC-PLGA(FLN+pI:C) MPs surface modified with COS and mannan were prepared by a modified double emulsion technique. A representative SEM image of MC-PLGA MPs was shown in Figure 1. Four types of MC-PLGA MPs were spherical with smooth surface (Figure 1). As illustrated in Table 1, the average particle sizes of four types of MC-PLGA MPs ranged between 910.8 and 984 nm, in that size, the MPs have been reported to be easily internalized and further facilitated antigen uptake and inducing activation of APCs.28 The zeta potential of MC-PLGA MPs was significantly influenced by environmental pH (Figure S1). The zeta potential of four kinds of MC-PLGA MPs is close to −10 mV at pH 7.4 and increases to almost 0 mV at pH 5 (Figure S1). The change of zeta potential of MC-PLGA MPs with pH may possibly due to surface modification with COS, which possesses the increased positive charge with decrease in solution pH.29
In vitro release of HBsAg, pI:C and FLN from MC-PLGA MPs were studied at pH values 5, 6 and 7.4 to evaluate antigen and TLR ligands release behavior under conditions likely encountered after nasal vaccination and intracellular trafficking following uptake by APCs. As illustrated in Figure 2, cumulative antigenicity of released HBsAg and cumulative amount of TLR ligands released from MC-PLGA MPs by 96 h was low (<20%) under physiological pH value (7.4), mimicking the environment of cytoplasm and body fluids (Figure 2). However, once the MC-PLGA MPs reached the pH milieu of the early endosomes (pH 6) and the late endosomes/lysosomes (pH 5), cumulative antigenicity of released HBsAg and cumulative amount of TLR ligands by 96 h increased to greater than 67% and 82%, respectively (Figure 2). In an acidic milieu, hydrogen ions can permeate into MC-PLGA MPs and reacted with NaHCO3 within MPs. The generated CO2 bubbles would disrupt the MC-PLGA shell wall and rapidly release the encapsulated antigen and TLR ligands.19,20 This pH-sensitive release behavior should particularly be suitable for facilitating the intracellular delivery of antigen and TLR ligands. Ideally, low release rate of antigen and TLR ligands in tissue and body fluids (pH 7.4) may avoid the undesired side effects of antigen and TLR ligands. On the other hand, fast release in acidic endosomes/lysosomes (pH 6–5) should avoid degradation of pI:C by acidic microenvironment within MC-PLGA MPs and facilitate activation of endosomal TLR within APCs.
Cellular uptake of MC-PLGA MPs and intracellular distribution
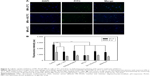
Efficient cellular uptake by APC is a major requirement for induction of adaptive immune responses. In this study, the cellular uptake of MC-PLGA MPs on mouse peritoneal macrophages was estimated. FITC-HSA was used as a fluorescent marker to investigate cellular internalization of MC-PLGA MPs. The uptake of FITC-HSA-loaded MC-PLGA (MC-PLGA(FITC-HSA)) MPs was first evaluated by fluorescent microscopy and the results were illustrated in Figure 3A. After 12 h of incubation, the strong fluorescence signals (green) of MC-PLGA(FITC-HSA) MPs were presented in the macrophages (Figure 3A), suggesting that MC-PLGA MPs can be efficiently internalized into the macrophages. However, low temperature and endocytic inhibitor mannose clearly inhibited cellular uptake of MC-PLGA(FITC-HSA) MPs.
To investigate the uptake mechanism of MC-PLGA MPs, MP uptake by macrophages was further investigated by measuring the fluorescence intensity of Rhodamine B-labeled MC-PLGA MPs within macrophage lysates. Lowering the incubation temperature to 4°C resulted in substantial reduction of MC-PLGA MP uptake by macrophages (P<0.01) (Figure 3B). Likewise, depleting intracellular ATP levels by preincubation of the macrophages with sodium azide and deoxyglucose substantially reduced the cellular internalization of MC-PLGA MPs (P<0.01) (Figure 3B). Moreover, if the cells were preincubated with sodium azide and deoxyglucose at 4°C, the cell uptake of MC-PLGA MPs was further reduced by 49.7% (Figure 3B). The results suggested that the cellular uptake of MC-PLGA MPs by macrophages is possibly through energy-dependent internalization mechanism.23
To further clarify the cellular internalization mechanism, macrophages were pre-treated with some endocytosis inhibitors prior to the addition of MC-PLGA MPs. Pretreatment with a lysosomotropic agent, ammonium chloride, which inhibits endocytosis by increasing the pH of cellular organelles such as late endosomes and lysosomes, substantially decreased the cellular uptake of MC-PLGA MPs (Figure 3B). Hypertonic treatment can block formation of clathrin-coated pit and inhibit endocytosis. When treated with sucrose, cellular uptake of MC-PLGA MPs was significantly reduced (P<0.01) (Figure 3B). Chlorpromazine, which blocks the assembly of clathrin-coated pits on the plasma membrane, also decreased the cellular uptake of MC-PLGA MPs (P<0.01) (Figure 3B). Moreover, if the cells were preincubated with chlorpromazine and sucrose at 4°C, the cell uptake of MC-PLGA MPs was further reduced by 49.4% and 43.5%, respectively (Figure 3B). These results suggested that MC-PLGA MPs are mainly taken up by energy-dependent, clathrin-mediated endocytosis.24,25,30
To investigate the effect of mannose receptor on cellular uptake of MC-PLGA MPs by macrophages, the uptake of MPs were examined in the presence of mannose. As shown in Figure 3B, the cellular uptake of MC-PLGA MPs by macrophages was substantially inhibited by mannose (P<0.01). The results suggested that the uptake of MC-PLGA MPs surface modified by COS and mannan is mediated by mannose receptor-mediated endocytosis. A similar effect of mannose on cellular uptake of mannosylated polymeric NPs has been observed by other researchers.26
To examine the effects of encapsulated cargo on the cellular internalization, uptake efficiencies of MC-PLGA(HBsAg), MC-PLGA(pI:C), MC-PLGA(FLN) and MC-PLGA(FLN+I:C) MPs by macrophages were compared. As shown in Figure S2, uptake efficiencies of MC-PLGA(HBsAg), MC-PLGA(pI:C), MC-PLGA(FLN) and MC-PLGA(FLN+pI:C) MPs by macrophages were similar, which was independent of the encapsulated cargo (P>0.05).
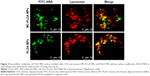
CLSM was used to visualize the intracellular distribution of MC-PLGA MPs within the macrophages. As shown in Figure 4, FITC-HSA-loaded MC-PLGA MPs (green, FITC) existed both in the lysosome (yellow, superposition of green (FITC) and red (Lyso Tracker Red DND-99)) and in the cytoplasm (green, FITC), while PLGA MPs without surface modification mainly existed in the lysosome (yellow). The result was consistent with our previous findings.19
In vitro synergy of FLN and pI:C
To evaluate synergy of TLR3 ligand pI:C and TLR5 ligand FLN, a series of diluted pI:C and FLN solutions were added into cell culture medium and tested for the ability to activate macrophages. As shown in Figure S3A, both FLN and pI:C elicited secretion of IL-6 from rat peritoneal macrophages in a concentration-dependent manner. However, both FLN and pI:C only elicit low concentration of IL12p70 secreted from macrophages (Figure S3B). As FLN engages TLR5 on cell surfaces of APC, FLN in solution should facilitate acting on macrophages. However, ability of FLN to elicit IL12p70 is low, which is consistent with previous study.31 Although TLR3 resides in the membranes of intracellular compartments, in culture medium pI:C still effectively elicits secretion of IL-6 and IL12p70. This should be associated with CD14 mediating pI:C uptake by macrophages and enhancing TLR3 signaling.32 When both FLN and pI:C were added into the culture medium, they synergistically elicited secretion of IL-12p70 and IL-6 from macrophages (Figure S3A and B). When concentration of FLN and pI:C was 0.4 and 2 μg/mL, respectively, the secreted IL-6 elicited by both ligands increased 7.84- and 23.10-fold and the secreted IL-12p70 increased 4.68- and 4.14-fold compared to that elicited by the single ligand FLN and pI:C, respectively. These results suggested that when the same APCs were stimulated by both FLN and pI:C, synergistic signaling by the innate immune system had occurred.
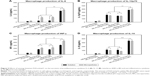
After having optimized context for pI:C and FLN, these were encapsulated into MC-PLGA MPs and the effects of encapsulated TLR ligands on activation of macrophage were investigated. After macrophage incubated with MC-PLGA MPs, the secretion of proinflammatory cytokines IL-6, IL-12, interferon (INF)-γ and anti-inflammatory cytokines IL-10 was analyzed, and the results were illustrated in Figure 5. Compared with TLR ligand in solution in the presence of HBsAg, pI:C and FLN encapsulated into MC-PLGA MPs increased the production of proinflammatory cytokines IL-6 and INF-γ (P<0.05) (Figure 5A and C). However, encapsulation of TLR ligands had no effect on the production of proinflammatory cytokines IL-12p70 and anti-inflammatory cytokines IL-10 compared with TLR ligand in solution in the presence of HBsAg (P>0.05) (Figure 5B and D). Moreover, FLN and pI:C co-encapsulated into MC-PLGA MPs showed significantly synergized activation of macrophages. When incubated with macrophages, MC-PLGA(FLN+pI:C) MPs elicited higher IL-6, IL-12p70, IL-10 and INF-γ than MC-PLGA(FLN) and MC-PLGA(pI:C) MPs (P<0.05) (Figure 5).
After internalization by macrophages, pI:C released from MC-PLGA MPs in the endosome of APCs should facilitate the interaction between pI:C and endosomal TLR3 (Figure 6). On the contrary, FLN released in the cytoplasm of APCs would facilitate passing through the cell membrane and then interact with extracellular TLR 5 (Figure 6). We have observed that pH-sensitive MC-PLGA MPs surface modified with COS and mannan existed both in the lysosome and in the cytoplasm (Figure 4). After uptake by macrophages, TLR ligand-loaded MC-PLGA MPs surface modified with COS and mannan should have two intracellular release patterns: 1) release in late endosomes/lysosomes in favor of interaction between pI:C and endosoma TLR3, 2) release in cytoplasm in favor of interaction between FLN and extracellular TLR5 (Figure 6). Simultaneous stimulation of TLR3 and TLR5 would facilitate synergistic activation of APCs. Synergistic effects between pI:C and FLN might be in favor of reducing the dose of both ligands and then reduce adverse reaction associated with them.15,33
Estimation of IgG and IgA antibody levels
To evaluate the synergistic effects of pI:C and FLN on humoral and mucosal immune responses, rats were immunized with MC-PLGA(HBsAg), MC-PLGA(HBsAg) plus MC-PLGA(pI:C) or MC-PLGA(FLN) or MC-PLGA(FLN+pI:C) MPs (intranasally), and alum-HBsAg vaccine (subcutaneously), and serum IgG levels and IgA levels in secretions were measured with ELISA. As shown in Figure 7A, blank MC-PLGA MPs did not elicit anti-HBsAg IgG antibody production. Intranasal inoculation of MC-PLGA(HBsAg) MP formulation or subcutaneous injections of alum-HBsAg vaccine resulted in a sustained increase of the IgG levels within 10 weeks (Figure 7A). Moreover, MC-PLGA(HBsAg) plus MC-PLGA(FLN+pI:C) MPs induced significantly higher anti-HBsAg IgG levels than MC-PLGA(HBsAg), MC-PLGA(HBsAg) plus MC-PLGA(pI:C) or MC-PLGA(FLN) MPs 4 weeks later (P<0.05) (Figure 7A). At the same time, alum-HBsAg vaccine elicited significantly higher IgG levels than MC-PLGA(HBsAg) plus MC-PLGA(pI:C), MC-PLGA(FLN) or MC-PLGA(FLN+pI:C) MPs (P<0.05) within 11 weeks. At 10 weeks post immunization, IgG levels against HBsAg elicited by MC-PLGA(HBsAg) plus MC-PLGA (FLN+pI:C) MPs was 682.8 mIU/mL, which was slightly lower than the level elicited by the alum-HBsAg vaccine, but no significant difference (P>0.05) (Figure 7A). These results indicated that synergistic effects between FLN and pI:C within MC-PLGA MPs substantially enhanced humoral immune responses.
Mucosal immune responses were investigated by determining IgA levels against HBsAg in nasal and salivary secretions. As shown in Figure S4, HSA-loaded MC-PLGA MPs and alum-HBsAg did not elicit a significant IgA levels against HBsAg. In comparison, IgA levels against HBsAg elicited by MC-PLGA(HBsAg) MPs were significantly higher than that elicited by alum-HBsAg vaccine (P<0.01) (Figure S4). However, MC-PLGA(HBsAg) plus MC-PLGA(pI:C), MC-PLGA(FLN) or MC-PLGA(FLN+pI:C) MPs did not elicit significantly higher levels of anti-HBsAg IgA than MC-PLGA(HBsAg) MPs (P>0.05).
The IgG2a/IgG1 ratio (serum IgG subtypes) provided an insight into the Th1/Th2 polarization of the immune responses activated by MC-PLGA(HBsAg) plus MC-PLGA(FLN+pI:C) MPs. As shown in Figure 7B, the MC-PLGA(pI:C) and MC-PLGA(FLN) MPs displayed significant effect on IgG2a/IgG1 ratio changes. Serum IgG2a/IgG1 ratio induced by alum-HBsAg vaccine and MC-PLGA(HBsAg) MPs was 0.29 and 0.49, respectively, indicating Th2-biased immune response. IgG2a/IgG1 ratio induced by MC-PLGA(HBsAg) plus MC-PLGA(pI:C), MC-PLGA(FLN) and MC-PLGA(FLN+pI:C) MPs was 1.60, 1.47 and 1.63, respectively, suggesting significant Th1-biased immune response.
Estimation of endogenous cytokine levels
To further understand about the effect of TLR ligands on the cell-mediated immune response, in vitro cytokine release was determined by measuring the concentration of Th1 type cytokines IFN-γ and IL-2 in cultured spleen cell supernatants of immunized rats. Without stimulation with HBsAg, splenocytes secreted very low concentrations of IL-2 and IFN-γ (<3.5 pg/mL) (Figure 8A and B). Upon stimulation with HBsAg, the expression of IFN-γ and IL-2 in splenocytes was associated with different types of MP formulations. MC-PLGA(HBsAg) MPs generated higher concentration of IFN-γ and IL-2 compared to alum-HBsAg vaccine group (P<0.05). Splenocytes from rats vaccinated with MC-PLGA(HBsAg) plus MC-PLGA(pI:C), MC-PLGA(FLN) and MC-PLGA(FLN+pI:C) MPs showed a greater ability in secreting IL-2 and IFN-γ cytokines. Approximately 4.3- and 2.36-fold higher IL-2 levels were detected after intranasal vaccination with MC-PLGA(HBsAg) plus MC-PLGA(pI:C) or MC-PLGA(FLN) MPs than MC-PLGA(HBsAg) MPs, respectively. Similarly, approximately 4.91- and 2.63-fold higher IFN-γ levels were detected after intranasal vaccination with MC-PLGA(HBsAg) plus MC-PLGA(pI:C) or MC-PLGA(FLN) MPs than MC-PLGA(HBsAg) MPs, respectively. MC-PLGA(HBsAg) plus MC-PLGA(FLN+pI:C) MPs induced a 1.25- to 2.38-fold increase in secreting IL-2 and IFN-γ cytokines compared to MC-PLGA(HBsAg) plus MC-PLGA(pI:C) or MC-PLGA(FLN) MPs, respectively. The results suggested that both FLN and pI:C facilitated the production of Th1 type cytokines, which is important to facilitate protection against hepatitis B viral infection and elimination of hepatitis B virus.34,35 Moreover, synergistic effects between FLN and pI:C within MC-PLGA MPs substantially enhance the production of Th1 type cytokines IFN-γ and IL-2, consistent with the results from IgG2a/IgG1 ratio.
Conclusion
In this study, synergistic effect between pI:C and FLN within MC-PLGA MPs on activation of macrophages and immune responses induced by nasal delivery of HBsAg was investigated systematically. HBsAg, FLN, pI:C or both ligands were encapsulated within mannan and COS-modified, pH-responsive MC-PLGA-based MPs utilizing a double-emulsion method. Inhibition experiments showed that cellular uptake of MC-PLGA MPs was energy-dependent, mannose receptor-mediated endocytosis. FLN and pI:C in solution and MP formulation could synergize to activate macrophages and induce higher pro- and anti-inflammatory cytokine levels compared to single TLR ligand. In vivo immunogenicity studies indicated that co-delivery of FLN and pI:C within MC-PLGA MPs synergistically induced higher anti-HBsAg IgG levels and Th1 cytokine levels compared with MC-PLGA MPs encapsulated single TLR ligand plus MPs encapsulated HBsAg. These results suggest that synergic TLR3 and TLR5 stimulation might be a promising tool for nasally delivered HBsAg.
Acknowledgment
This work was supported by the Natural Science Foundation of Hebei Province of China (H2014205141).
Disclosure
The authors report no conflicts of interest in this work.
References
Woodrow KA, Bennett KM, Lo DD. Mucosal vaccine design and delivery. Annu Rev Biomed Eng. 2012;14:17–46. | ||
Shakya AK, Chowdhury MY, Tao W, Gill HS. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release. 2016;240:394–413. | ||
Lamichhane A, Azegamia T, Kiyonoa H. The mucosal immune system for vaccine development. Vaccine. 2014;32(49):6711–6723. | ||
Csaba N, Garcia-Fuentes M, Alonso MJ. Nanoparticles for nasal vaccination. Adv Drug Deliv Rev. 2009;61(2):140–157. | ||
Jia Y, Krishnan L, Omri A. Nasal and pulmonary vaccine delivery using particulate carriers. Expert Opin Drug Deliv. 2015;12(6):993–1008. | ||
Riese P, Sakthivel P, Trittel S, Guzmán CA. Intranasal formulations: promising strategy to deliver vaccines. Expert Opin Drug Deliv. 2014;11(10):1619–1634. | ||
Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88(1):8–27. | ||
Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14(9):3754–3779. | ||
Zaman M, Simerska P, Toth I. Synthetic polyacrylate polymers as particulate intranasal vaccine delivery systems for the induction of mucosal immune response. Curr Drug Deliv. 2010;7(2):118–124. | ||
Pavot V, Berthet M, Rességuier J, et al. Poly(lactic acid) and poly(lactic-co-glycolic acid) particles as versatile carrier platforms for vaccine delivery. Nanomedicine (Lond). 2014;9(17):2703–2718. | ||
Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63(10–11):943–955. | ||
Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. | ||
Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–1608. | ||
Kasturi SP, Skountzou I, Albrecht RA, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470(7335):543–547. | ||
Hafner AM, Corthésy B, Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Deliv Rev. 2013;65(10):1386–1399. | ||
Nacer A, Carapau D, Mitchell R, et al. Imaging murine NALT following intranasal immunization with flagellin-modified circumsporozoite protein malaria vaccines. Mucosal Immunol. 2014;7(2):304–314. | ||
Hafner AM, Corthésy B, Textor M, Merkle HP. Surface-assembled poly(I:C) on PEGylated PLGA microspheres as vaccine adjuvant: APC activation and bystander cell stimulation. Int J Pharm. 2016;514(1):176–188. | ||
Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185(10):5677–5682. | ||
Li Z, Xiong F, He J, Dai X, Wang G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: effect of surface modification with chitosan and mannan. Eur J Pharm Biopharm. 2016;109:24–34. | ||
Ke CJ, Su TY, Chen HL, et al. Smart multifunctional hollow microspheres for the quick release of drugs in intracellular lysosomal compartments. Angew Chem Int Ed Engl. 2011;50(35):8086–8089. | ||
Xu W, He J, Wu G, Xiong F, Du H, Wang G. Stabilization and immune response of HBsAg encapsulated within poly(lactic-co-glycolic acid) microspheres using HSA as a stabilizer. Int J Pharm. 2015;496(2):332–341. | ||
Heffernan MJ, Kasturi SP, Yang SC, Pulendran B, Murthy N. The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid). Biomaterials. 2009;30(5):910–918. | ||
He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. | ||
Rezgui R, Blumer K, Yeoh-Tan G, Trexler AJ, Magzoub M. Precise quantification of cellular uptake of cell-penetrating peptides using fluorescence-activated cell sorting and fluorescence correlation spectroscopy. Biochim Biophys Acta. 2016;1858(7 pt A):1499–1506. | ||
Xu ZP, Niebert M, Porazik K, et al. Subcellular compartment targeting of layered double hydroxide nanoparticles. J Control Release. 2008;130(1):86–94. | ||
Chu S, Tang C, Yin C. Effects of mannose density on in vitro and in vivo cellular uptake and RNAi efficiency of polymeric nanoparticles. Biomaterials. 2015;52:229–239. | ||
Pawar D, Mangal S, Goswami R, Jaganathan KS. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur J Pharm Biopharm. 2013;85(3 pt A):550–559. | ||
Jia J, Zhang W, Liu Q, Yang T, Wang L, Ma G. Adjuvanticity regulation by biodegradable polymeric nano/microparticle size. Mol Pharm. 2017;14(1):14–22. | ||
Cabuk M, Yavuz M, Unal HI. Electrokinetic, electrorheological and viscoelastic properties of Polythiophene-graft-Chitosan copolymer particles. Colloids Surf A Physicochem Eng Asp. 2016;510:231–238. | ||
Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485–499. | ||
Didierlaurent A, Ferrero I, Otten LA, et al. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol. 2004;172(11):6922–6930. | ||
Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24(2):153–163. | ||
Van Maele L, Fougeron D, Janot L, et al. Airway structural cells regulate TLR5-mediated mucosal adjuvant activity. Mucosal Immunol. 2014;7(3):489–500. | ||
Jaganathan KS, Vyas SP. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine. 2006;24(19):4201–4211. | ||
Saini V, Jain V, Sudheesh MS, Jaganathan KS, Murthy PK, Kohli DV. Comparison of humoral and cell-mediated immune responses to cationic PLGA microspheres containing recombinant hepatitis B antigen. Int J Pharm. 2011;408(1–2):50–57. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.