Back to Journals » International Journal of Nanomedicine » Volume 15
Co-Delivery of Paclitaxel and Doxorubicin by pH-Responsive Prodrug Micelles for Cancer Therapy
Authors Jiang Y, Zhou Y, Zhang CY, Fang T
Received 10 February 2020
Accepted for publication 18 April 2020
Published 11 May 2020 Volume 2020:15 Pages 3319—3331
DOI https://doi.org/10.2147/IJN.S249144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Yanhua Jiang,1 Yongjian Zhou,1 Can Yang Zhang,2 Te Fang1
1Department of Anesthesiology, First Affiliated Hospital of China Medical University, Shenyang 110001, People’s Republic of China; 2Singapore-MIT Alliance for Research and Technology, Singapore 138602, Singapore
Correspondence: Te Fang Email [email protected]
Background: It is of great significance to develop intelligent co-delivery systems for cancer chemotherapy with improved therapeutic efficacy and few side-effects.
Materials and Methods: Here, we reported a co-delivery system based on pH-sensitive polyprodrug micelles for simultaneous delivery of doxorubicin (DOX) and paclitaxel (PTX) as a combination chemotherapy with pH-triggered drug release profiles. The physicochemical properties, drug release profiles and mechanism, and cytotoxicity of PTX/DOX-PMs have been thoroughly investigated.
Results and Discussion: The pH-sensitive polyprodrug was used as nanocarrier, and PTX was encapsulated into the micelles with high drug-loading content (25.6%). The critical micelle concentration (CMC) was about 3.16 mg/L, indicating the system could form the micelles at low concentration. The particle size of PTX/DOX-PMs was 110.5 nm, and increased to approximately 140 nm after incubation for 5 days which showed that the PTX/DOX-PMs had high serum stability. With decrease in pH value, the particle size first increased, and thenwas no longer detectable. Similar change trend was observed for CMC values. The zetapotential increased sharply with decrease in pH. These results demonstrated the pHsensitivity of PTX/DOX-PMs. In vitro drug release experiments and study on release mechanism showed that the drug release rate and accumulative release for PTX and DOX were dependent on the pH, showing the pH-triggered drug release profiles. Cytotoxicity assay displayed that the block copolymer showed negligible cytotoxicity, while the PTX/DOX-PMs possessed high cytotoxic effect against several tumor cell lines compared with free drugs and control.
Conclusion: All the results demonstrated that the co-delivery system based on pH-sensitive polyprodrug could be a potent nanomedicine for combination cancer chemotherapy. In addition, construction based on polyprodrug and chemical drug could be a useful method to prepare multifunctional nanomedicine.
Keywords: pH-responsive, polyprodrug micelles, co-delivery, controlled release, cancer therapy
Introduction
Chemotherapy is still currently the most commonly used treatment for various cancers in clinics.1–3 Some efficient chemical anticancer drugs, such as doxorubicin (DOX),4 paclitaxel (PTX),5 and camptothecin (CPT),6 have been developed for cancer chemotherapy. However, a lot of shortcomings have been reported during clinical use.7–10 For example, DOX also induces damage of normal tissues, especially the heart, meanwhile killing the tumor cells.11 Furthermore, small chemical anticancer drug molecules would be cleared quickly by the reticuloendothelial system (RES) and blood/renal during circulation in the body after administration, leading to the quite low concentration at the tumor sites.12–15 When the dose of drug is increased, the adverse side-effects become severe. Therefore, it is of great significance to find a method to solve this dilemma. With the rapid development of bio-nanotechnology recently, a nanoscale stimuli-responsive drug delivery system (DDS) has emerged and attracted more and more attention, and has been deemed to be an effective strategy to increase the drug concentration at the tumor sites and remit the unwanted side-effects of chemical anticancer drugs.16–24 In the past years, various DDSs have been designed and developed for drug delivery, such as polymeric micelles (PMs),25 hybrid nanoparticles (NPs),26 metal-organic framework (MOF),27 liposomes,28 and liquid emulsion.29 Generally, the chemical drugs are physically loaded in the core of the system or chemically linked on the carriers. After administration, the drug-loaded systems would remain stable and compact in the blood circulation in case the cargos are leaked. Once they arrive at the tumor sites by the enhanced permeability and retention (EPR) effect, the drug would be released by responding to the specific tumor microenvironment cues (eg, low pH, high glutathione concentration and special enzyme), followed by inducing the death of tumor cells.30–33 Compared with other nanoscale carriers, PMs which are self-assembled from amphiphilic copolymers have several unique superior advantages.34–36 For instance, it is easy to modify the copolymer to obtain multifunctional carriers. And the PMs possess high drug loading efficacy, high biocompatibility, and low toxicity.37 Compared to the physical drug loading method, the drug molecules are chemically linked on the carriers via sensitive bonds, such as disulfide bonds and hydrazone bonds, resulting in a polyprodrug which could effectively reduce the drug burst release and efficiently protect the drugs during blood circulation.38,39 Combining the advantages of both (PMs and prodrugs), some novel nanomedicines have been reported.40–42 Ma et al prepared a prodrug comprised of mussel-derived biomimetic peptide with fluorescein isothiocyanate for imaging, RGD sequence for targeting, and a pH-sensitive conjugation of antitumor drugs. The prodrug could self-assemble into polyprodrug micelles which were used for active targeted delivery of anticancer drug. The in vivo experimental results showed that this system could effectively deliver drug to tumor cells and suppresstumor growth.43
Recently, several limitations of single drug cancer chemotherapy have been reported such as poor bioavailability, low therapeutic efficacy, and multidrug resistance.44 Therefore, combination chemotherapy of two or more anticancer drugs has been thoroughly investigated and extensively developed in the past few years.45–48 For example, Zhu et al prepared a poly(lactic-co-glycolic acid) nanoparticle (PLGA NPs) for co-delivery of docetaxel (DTX) together with vitamin E TPGS with improved therapeutic efficacy against multidrug resistance.49 Danafar et al reported drug conjugated PEG-PCL NPs for co-delivery of hydrophilic and hydrophobic anticancer drugs. The DOX-conjugated block copolymers were synthesized first, and then curcumin was physically loaded in the polymeric core during the self-assembly in aqueous. The cytotoxicity assay showed that the system had a higher cytotoxic effect against A549 cells compared with free drugs.50 Therefore, co-delivery of multidrugs using intelligent nanoscale carriers could be a potent method to enhance therapeutic efficacy and overcome drug resistance.
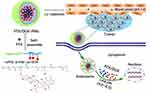
Herein, inspired by the specific tumor microenvironment cues, we designed and prepared a co-delivery system composed of pH-sensitive polyprodrug copolymer and anticancer drug PTX. The DOX-conjugated diblock copolymer was synthesized in our previous work.51 Here, these polyprodrugs were not only used as nanomedicine, but also simultaneously utilized as stimuli-responsive carrier for encapsulation and delivery of PTX with pH-triggered drug release profiles for cancer combination chemotherapy, as shown in Figure 1. The DOX-conjugated copolymer molecules could self-assemble into polymeric micelles together with hydrophobic PTX in aqueous, resulting in PTX-loaded PMs (so-called PTX/DOX-PMs). For PTX/DOX-PMs, the DOX molecules were chemically linked on the pH-sensitive diblock copolymers, and PTX molecules were physically loaded in the micellar core. The PTX/DOX-PMs showed high stability for extended circulation in the body at normal pH (pH 7.4). After deposition at the tumor sites, part of tertiary amine residues in PAE block would be protonated which led to the increase of surface charge of PTX/DOX-PMs, facilitating the cellular uptake by tumor cells. And then, the pH-sensitive bonds between polymer and DOX molecules would be cleaved by responding to the intracellular acidity. All of the tertiary amine residues in PAE block would be protonated, resulting in disassembly of PTX/DOX-PMs to release the encapsulated PTX molecules. Moreover, the drug release mechanisms of chemically loaded DOX and physically loaded PTX were investigated based on a comprehensive semi-empirical equation. The pH-triggered drug release profiles and cytotoxicity of systems against different tumor cell lines were investigated. In addition, some other physicochemical properties of PTX/DOX-PMs, such as particle size, zeta potential and pH -sensitivity, were also studied in this work.
Materials and Methods
Materials
Diblock copolymer poly(ethylene glycol)methyl ether-b-poly(β-amino esters) conjugated with DOX via acid-labile cis-aconityl moiety (mPEG-b-PAE-cis-DOX) was synthesized as reported in our precious work.51 Doxorubicin hydrochloride (DOX-HCl) was purchased from Wuhan Yuan Cheng Gong Chuang Co. Ltd (Wuhan, China). Paclitaxel (PTX) was purchased from Xi’an Helin Pharm Co., Ltd. (Xi’an, China) Methylthiazoltetrazolium (MTT) was purchased from Sigma-Aldrich. Dulbecco’s modified eagle media (DMEM) growth media, fetal bovine serum (FBS), trypsin, penicillin, streptomycin, and other biological agents were all purchased from Invitrogen. A549, MDA-MB-231, A2780 and NCL-H460 cell lines were obtained from the American Type Culture Collection (ATCC). All other chemical and biological reagents were used as received.
Preparation of PTX/DOX-PMs
The PTX/DOX-PMs were prepared using dialysis method. Briefly, polyprodrug mPEG-b-PAE-cis-DOX (20 mg) was dissolved into DMF (20 mL) with stirring for 30 min. Then PTX (5 mg, 10 mg, 20 mg) was added into the solution. The mixed solution was transferred into a pre-swollen cellulose membrane bag (MWCO 3500–4000), followed by placing in a beaker (1 L). The dialysis process was carried out for 48 h against deionized water at room temperature. The deionized water was replaced every 2 h in the first 12 h and then every 6 h. After filtration by 0.45 μm filter and lyophilization, the PTX/DOX-PMs were obtained and stored at −20°C for further studies. DOX-PMs were prepared in a similar way as reported elsewhere.51
Characterization
The particle size of PTX/DOX-PMs and DOX-PMs was measured by dynamic light scattering (DLS, Malvern Zetasizer Nano S, Malvern, UK). As a topical experiment, PTX/DOX-PMs were re-suspended in PBS at different pH values (9.0, 8.0, 7.4, 7.0, 6.8, 6.5, 6.0, 5.5, 5.0, and 4.0) at a concentration of 1.0 mg/mL, and incubated for 2 h. The samples were measured in a 1.0 mL quartz cuvette using a diode laser of 670 nm at room temperature. The zeta potential of samples was measured in a similar method.
To evaluate the serum stability of PTX/DOX-PMs, the samples were prepared as previously mentioned. Then the samples were incubated in PBS with 20% FBS at 37°C for 5 days, the particle size and polydispersity index (PDI) of samples were recorded every day using DLS.
Drug Loading Efficacy
The PTX loading content (LC) and encapsulation efficiency (EE) were analyzed using high- performance liquid chromatography (HPLC, Waters 2690 Separations Module system, Milford, MA) consisting of a P-900 gradient pump system and an Ultra C18 5 μm column (250 × 4.6 mm). Briefly, 1 mg of PTX/DOX-PMs was first dissolved into dichloromethane (DCM) for 30 min. Then, the resultant solution was filtered by 0.45 μm filter. The HPLC analysis was performed using acetonitrile/water (v/v = 4: 1) as the mobile phase at 30°C with a flow rate of 1.0 mL/min and detected at a wavelength of 227 nm (UV detector, Waters). The LC and EE were calculated according to the following equations:

The DOX conjugated efficiency (DCE) has already been confirmed using UV-vis spectrophotometer (UV-2450, Shimadzu, Japan), as reported elsewhere.51
Critical Micelle Concentration (CMC) Measurement
The CMC values of systems were measured by the fluorescence technique using pyrene as a probe dye. In brief, the block copolymer was first dissolved into deionized water at a concentration of 0.1 mg/mL. The systems were mixed with the pre-prepared pyrene solution to prepare a series of mixed solutions with concentrations from 0.0001 to 0.1 mg/mL, and the final concentration of pyrene was 6×10−7 M. Before measurement, the obtained mixture was equilibrated in the dark for one day at 25°C. The excitation spectra of samples were tested with a fluorescence spectrophotometer (F-4500, Hitachi, Japan) at 373 nm with a bandwidth of 0.2 nm.
In vitro Release of PTX and DOX from PMs
To acquire sink conditions, in vitro drug release study was performed at low drug concentration. The release profiles of PTX and DOX from PTX/DOX-PMs were investigated using a dialysis method. Briefly, 2 mg of PTX/DOX-PMs was re-suspended in 2 mL of PBS at pH 7.4, 6.5 or 5.0, and then the solution was transferred into a pre-swollen cellulose membrane bag (MWCO 3500–4000) which was immersed into 38 mL of respective PBS solution in a beaker with stirring of 110 rpm at 37°C. At pre-determined time intervals, 1 mL of sample was drawn from the beaker and 1 mL fresh respective PBS was added. The PTX and DOX concentrations of every sample were analyzed using HPLC and UV-vis spectrophotometer as previously mentioned, respectively. The accumulative release amount of drug (Er) was calculated based on the following equation:
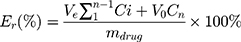
where, mdrug represented the amount of PTX or DOX in the PTX/DOX-PMs, V0 was the whole volume of the release media (V0 = 40 mL), and Ci represented the concentration of PTX or DOX in the ith sample.
Drug Release Mechanism Study
The drug release mechanism from PMs was very complex and not completely understood until now. Peppas et al established a comprehensive semi-empirical equation to roughly analyze the mechanism,52,53 as follows:
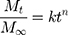
This formula has a transformation, as follows:
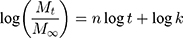
Where, Mt/M∞ was the accumulated amount of drug at time t. k was the constant incorporating structural and geometric characteristics of carrier which indicated the drug release rate, and n was the release exponent indicating the drug release mechanism. The value of n was 0.43 for Fickian diffusion and n < 0.43 corresponded to the combination of diffusion and erosion control, 0.43 < n < 0.85 was for anomalous transport mechanism, and n was 0.85 for swelling-controlled mechanism.
Cell Culture
The cell culture protocols for A549, MDA-MB-231, A2780, NCL-H460 and NIH 3T3 cell lines were similar as reported elsewhere.51,54 The cells were cultured in the medium supplemented with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 37°C in an incubator. After two or three times of passage, the cells were used for future studies.
Cytotoxicity Assay
The cytotoxic effects of diblock copolymer, free DOX, free PTX, mixture of free DOX and PTX, DOX-PMs, PTX/DOX-PMs against these cell lines were evaluated utilizing the standard methylthiazoltetrazolium (MTT) assay. Tumor cells were seeded in a 96-well plate at a density of 1 x 104 cells per well in 200 μL of medium. The 96-well plate was cultured in the incubator for one day. After removing the culture supernatants, the prepared diblock copolymer, free DOX, free PTX, mixed free DOX and PTX, DOX-PMs and PTX/DOX-PMs medium solution with a concentration gradient were added into the wells. Tumor cells with fresh medium were used as blank back-ground. Live tumor cells without any treatment were used as a control with no exogenous cytotoxicity. After incubation for 48 h, 20 μL of MTT solution (5 mg/mL) was added into the wells. The 96-well plate was shaken at 150 rpm for additional 10 min, followed by incubation for 4 h. The supernatants in every well were discarded, and 200 μL of DMSO was added to dissolve the formazan crystals, and the plate was shaken for 15 min. The 96-well plate was measured by a microplate reader (Multiskan Spectrum, Thermo Scientific, Finland) at 490 nm. The relative cell viability (%) was calculated using the following formula:
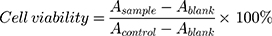
where, A was the absorbance at 490 nm. The cytotoxicity test was performed in replicates of six wells.
Statistical Analysis
The experimental data were presented with average values, expressed as the mean ± standard deviation (S.D.). Statistical analysis was conducted using two-sample Student’s t-test of origin 8.5 or ANOVA analyses, and considered to be significant when p < 0.05.
Results and Discussion
Preparation and Characterization of PTX/DOX-PMs
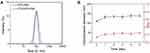
The PTX/DOX-PMs were prepared using dialysis method. The particle sizes of DOX-PMs and PTX/DOX-PMs were recorded by DLS, as shown in Figure 2A. The hydrodynamic diameters of DOX-PMs and PTX/DOX-PMs were 102.3 nm and 110.5 nm, respectively. After PTX loading, the particle size was slightly increased due to the entrapped hydrophobic PTX molecules in the micellar core. As reported, the PMs with size of 50–200 nm were suitable for accumulation at tumor sites through EPR effect in cancer therapy.9 In order to receive high accumulation at the site of tumor, the PTX/DOX-PMs should have high serum stability. Next, the serum stability of PTX/DOX-PMs in PBS containing 20% FBS at pH of 7.4 was evaluated, as shown in Figure 2B. After incubation for 1 day, the particle size was increased from 110.5 nm to approximately 130 nm. Even after incubation for 5 days, the particle size of PTX/DOX-PMs was still less than 150 nm, indicating that the co-delivery system, PTX/DOX-PMs, had high serum stability. In addition, the PDI of PTX/DOX-PMs after incubation for different times was still less than 0.3, suggesting the good uniformity and higher stability. These findings proved that the PTX/DOX-PMs had proper particle size and high serum stability, thereby enhancing the accumulation at tumor sites via EPR effect.
The particle size, PDI, LC and EE of PTX/DOX-PMs at different mass ratios of PTX and polyprodrug copolymer were shown in Table 1. With increase of PTX in feed, the LC of PTX increased, as expected. When the PTX in feed was 5 mg (PTX: carriers= 1: 4, mass ratio), the LC and EE were 16.8 % and 80.3%, respectively. When the mass ratio was 1: 2 (PTX in feed was 10 mg), the LC markedly increased to 25.6%, while the EE was slightly decreased to 75.4%. When the PTX in feed was 20 mg (PTX: carriers= 1: 1, mass ratio), the LC slightly increased to 29.4%, but the EE was sharply decreased to 55.8%. These results showed that the 10 mg of PTX in feed (PTX: carriers= 1: 2, mass ratio) could be the optimal formulation, and these PTX/DOX-PMs were used for the following studies. Additionally, the particle size of PTX/DOX-PMs was slightly increased with increase of PTX in feed, resulting from more PTX molecules in the micellar core thereby enhancing the core of PMs. Moreover, the average number of DOX molecules conjugated on the PAE chain was and 2.5 ± 0.2 per polymer molecule.51
 |
Table 1 Particle Size, Polydispersity Index (PDI), Drug Loading Content (LC) and Encapsulation Efficacy (EE) of PTX/DOX-PMs at Different Mass Ratios of Drug and Carriers |
pH Sensitivity of PTX/DOX-PMs
Next, the pH sensitivity of PTX/DOX-PMs was studied by measurement of particle size, zeta potential and CMC values of systems at different pH conditions. As seen in Figure 3A, when the pH was higher than 7.4, the particle size of PTX/DOX-PMs was quite stable (110 nm-120 nm). When the pH decreased from 7.4 to 6.5, the particle size of PTX/DOX-PMs sharply increased. In particular, the particle size increased to 165.8 nm at pH of 6.5. The reason could be that part of tertiary amine residues in PAE block were protonated at weakly acidic condition which induced the solubility transformation of PAE segment from hydrophobicity to hydrophilicity, resulting in swelling of PTX/DOX-PMs. When the pH decreased sequentially (< 6.5), no particles were detectable, indicating there were no particles. The reason might be that all of the tertiary amine residues in PAE segment were ionized, leading to the hydrophilic PAE segment. Moreover, the pH-sensitive bonds cis-aconityl linker between copolymer and DOX molecules were cleaved, resulting in detachment of the hydrophobic DOX. Therefore, the original amphiphilic block copolymer mPEG-b-PAE-cis-DOX was changed to hydrophilic diblock copolymer mPEG-b-PAE, suggesting the disassembly of PTX/DOX-PMs. Figure 3B shows the zeta potential of PTX/DOX-PMs at different pH values. As expected, the zeta potential rapidly increased (from −7.8 mV to +56.1 mV) with decrease in pH (from 9.0 to 4.0), attributed to the ionization of tertiary amine residues of PAE segment. Figure 3C shows the CMCs of DOX-PMs and PTX/DOX-PMs at different pH conditions. For DOX-PMs, the CMC was increased from 3.6 μg/mL to 45.2 μg/mL when the pH decreased from 7.4 to 6.8. A similar change trend was observed for PTX/DOX-PMs system. The CMCs were 3.16 μg/mL (Figure S1), 18.55 μg/mL and 49.64 μg/mL, respectively, at pH 7.4, 6.8 and 6.5. This was ascribed to part of the tertiary amine residues of PAE segment being protonated which enhanced the molecular polarity, thereby requiring greater driving force for micellization to counteract the greater electrostatic repulsive force. When the pH was lower than 6.5, the CMCs of DOX-PMs and PTX/DOX-PMs were not detectable, resulting from ionized tertiary amine residues in PAE segment and cleavage of pH-sensitive bonds. The micelles were disassembled at this stage. In addition, the CMC of PTX/DOX-PMs was slightly lower than that of DOX-PMs due to the introduction of hydrophobic PTX molecules in the system. In summary, the particle size, zeta potential and CMC of PTX/DOX-PMs were dependent on the pH value. These results proved that the PTX/DOX-PMs possessed pH sensitivity.
pH-Triggered DOX Release in vitro
Since the pH sensitivity of PTX/DOX-PMs has been confirmed, in vitro release profiles of PTX and DOX from PTX/DOX-PMs at different pH values were investigated, as shown in Figure 4. Figure 4A shows the DOX release profiles from PTX/DOX-PMs at different pH conditions (7.4, 6.5 and 5.0). The drug release rate and accumulative release were obviously dependent on the pH values. At pH 7.4, the DOX release rate was quite low, and the accumulative release of DOX was less than 10% (9.8%) for 48 h. Because the DOX molecules were chemically linked on the PAE chains, and the polyprodrug molecules were stable at pH 7.4. When the pH was 6.5, the release rate rapidly accelerated. The accumulative release was about 20% for 2 h and more than 75% at 48 h, respectively, resulting from the cleavage of pH-sensitive bonds between DOX molecules and polymer. When the pH was 5.0, the release rate was much faster in comparison to that at pH 6.5, and the accumulative release was about 30% for 2 h and higher than 95% at 48 h, respectively. The key reason might be that all of the pH-sensitive bonds between DOX and copolymer were broken. Furthermore, the PMs were disassembled, and the free DOX molecules were easily diffused into the solution. Figure 4B shows the PTX release profiles from PTX/DOX-PMs at different pH values (7.4, 6.5 and 5.0). Although the PTX release profiles were dependent on the pH values, the release rate of PTX was faster than that of DOX at pH 7.4. The accumulative release of PTX was more than 10% (12.6%) at 2 h and about 40% (39.8%) at 48 h, respectively. The reason could be that the PTX was physically loaded into the micellar core by hydrophobic interaction during the self-assembly process. Some PTX molecules could be released and diffused into the solution during incubation at pH 7.4 due to the swelling of PMs. When pH was decreased to 6.5 or 5.0, the release rate markedly accelerated compared with that at pH 7.4. The accumulative release of PTX was about 20% and 40% at pH 6.5 and 5.0 (for 2 h), and about 85% and 98% at pH 6.5 and 5.0 (for 48 h), respectively. The reason could be that the tertiary amine residues in PAE segment were gradually protonated and the pH-sensitive bonds were broken, inducing the swelling and disassembly of PTX/DOX-PMs. Collectively, the release rates of DOX and PTX were accelerated by the decrease in pH. Therefore, the drugs’ (DOX and PTX) release profiles from PTX/DOX-PMs were pH-triggered.
Drug Release Mechanism
During preparation of co-delivery system PTX/DOX-PMs, the DOX molecules were chemically linked on the PAE segment, and the PTX molecules were physically loaded in the micellar core. The release profiles were dependent on the pH, but the release mechanism needed to be studied. Here, the drug release mechanism was investigated using a comprehensive semi-empirical model. Based on the experimental data in Figure 4, the drug release process was divided into two stages, one was from 0 h to 12 h, and the other was from 12 h to 48 h. Figure 5 showed the theoretical curves fitted to experimental release data based on the mathematical model. The release exponent (n) and rate constant (k) for two stages of DOX and PTX release profiles were tabulated in Table 2. Good linearity for two stages could be observed in Figure 5. For DOX release mechanism (Figure 5A), the n values for two stages at pH 7.4 were, respectively, 0.279 and 0.362 which were less than 0.43, indicating that the release mechanism corresponded to combination of diffusion and erosion control. Furthermore, the k values for two stages were 0.025 and 0.024, respectively, suggesting the similar drug release rate in two stages over time. At weakly acidic conditions, the n values at first stage were 0.726 and 0.625 at pH 6.5 and 5.0, respectively, which were higher than 0.43 but less than 0.85, indicating the DOX release behavior followed the anomalous transport mechanism in the first 12 h due to the cleavage of pH-sensitive bonds. At the second stage, the n values were 0.067 and 0.073 at pH 6.5 and 5.0, respectively, which were less than 0.43, indicating the DOX release mechanism corresponded with the combination of diffusion and erosion control. The results displayed that the free DOX molecules that detached from PAE chains due to the cleavage of pH-sensitive bonds might diffuse from PTX/DOX-PMs over time, as well as swelling and disassembly of PTX/DOX-PMs. With decrease in pH, the k value increased, which suggested the drug release rate accelerated, showing the DOX release profile was pH-dependent which was consistent with the result of in vitro experiment (Figure 4). Specifically, the k values at pH 5.0 were much higher than those at pH 6.5 and 7.4 at two stages, showing the fastest drug release rate. For PTX release mechanism (Figure 5B), the n value in the first 12 h at pH 7.4 was 0.431 (≈ 0.43) which indicated that the PTX release from PTX/DOX-PMs corresponded to the Fickian diffusion mechanism. At the second stage, the n value was 0.181 (< 0.43), suggesting the PTX release mechanism was changed to the combination of diffusion and erosion control as time went on. At weakly acidic conditions (pH 6.5 and 5.0), the n values at the first stage were, respectively, 0.550 and 0.595 (0.43 < n < 0.85), showing the PTX release mechanism corresponded to anomalous transport mechanism during the process of swelling and disassembly of PTX/DOX-PMs. At the second stage, the n values were 0.155 and 0.030 (< 0.43), respectively, indicating the PTX release behaviors corresponded with the combination of diffusion and erosion control. The change trend of k value was similar to that of DOX release process, demonstrating the PTX release rate accelerated with pH decrease and time increase.
 |
Table 2 Release Exponent (n), Rate Constant (k) for PTX/DOX-PMs in PBS at Different pH Values |
Cytotoxicity Assay
Next, the cytotoxic effects of diblock copolymer, free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs for several cell lines were evaluated using MTT assay, as shown in Figures 6 and 7. As seen in Figure 6, the cytotoxicity of diblock copolymer slightly increased with the increase of copolymer concentration, but the cell viability of A549, MDA-MB-231, A2780, and NCL-H460 cells treated with diblock copolymer was still higher than 90% even at the highest concentration (500 μg/mL). In addition, the cell viability of normal cell line (NIH 3T3, Figure S2) treated with diblock copolymer was also higher than 90% at concentration of 500 μg/mL. These results demonstrated that the diblock copolymer (nanocarrier) showed negligible cytotoxicity. Figure 7 showed the cytotoxicity of free DOX, free PTX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs against four kinds of tumor cells. As for A549 (Figure 7A), the cell viabilities were, respectively, 20.2%, 12.1%, 8.5%, 9.2% and 5.5% for free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs at the highest concentration of drugs (20 μg/mL). The IC50 values were 2.02 µg/mL, 1.21 µg/mL, 0.33 µg/mL, 0.48 µg/mL and 0.25 µg/mL, respectively, for free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs, showing the PTX/DOX-PMs had the highest cytotoxicity in comparison to the other formulations. To further confirm the generality, the cytotoxicities of different formulations for MDA-MB-231 (Figure 7B), A2780 (Figure 7C) and NCL-H460 (Figure 7D) cells were analyzed. For MDA-MB-231, the IC50 values were 3.32 µg/mL, 2.85 µg/mL, 1.41 µg/mL, 1.49 µg/mL and 0.91 µg/mL, respectively, for free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs. For A2780, the IC50 values were 4.02 µg/mL, 3.11 µg/mL, 1.36 µg/mL, 2.01 µg/mL and 0.65 µg/mL, respectively, for free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs. For NCL-H460, the IC50 values were 0.96 µg/mL, 0.75 µg/mL, 0.54 µg/mL, 0.57 µg/mL and 0.38 µg/mL, respectively, for free PTX, free DOX, mixture of free DOX/PTX, DOX-PMs and PTX/DOX-PMs. As a result, the cell viabilities of three kinds of tumor cells were quite low at drug concentration of 20 μg/mL, suggesting the high cytotoxic effect of various drug formulations. Particularly, the cytotoxicity of different drug formulations was in order of free PTX< free DOX< DOX-PMs< free DOX/PTX< PTX/DOX-PMs. Therefore, the findings demonstrated that the PTX/DOX-PMs exhibited the highest cytotoxic effect for tumor cells. These findings showed that the co-delivery system PTX/DOX-PMs could be a promising therapeutic option for cancer treatment with high cytotoxicity.
Conclusion
Combination chemotherapy of multiple anticancer drugs has attracted more and more attention due to the improved therapeutic efficacy. In this work, we designed and prepared a co-delivery anticancer drug system based on pH-sensitive copolymer-based polyprodrug (mPEG-b-PAE-cis-DOX) and hydrophobic chemical drug (PTX) for combination chemotherapy. The co-delivery system was able to self-assemble into polymeric micelles (PTX/DOX-PMs) with suitable particle size (c.a. 110 nm) and low PDI. PTX molecules could be efficiently encapsulated into the core by hydrophobic interaction during the process of self-assembly, resulting in the high drug loading content and encapsulation efficacy. The particle size, zeta potential and CMC of PTX/DOX-PMs were increased with the decrease in pH from normal physiological environment to weakly acidic condition, especially disassembly of the system at pH 5.0, demonstrating the pH sensitivity of PTX/DOX-PMs. The PTX and DOX release profiles in vitro from PTX/DOX-PMs were obviously influenced by change in pH, indicating the pH-triggered drug release property. Furthermore, the drug release mechanism for PTX and DOX was studied based on a comprehensive semi-empirical model, proving the drug release was dependent on pH value. Furthermore, the reasonable drug loading method could be selected for “on-demand” drug delivery. In addition, the cytotoxicity of diblock copolymer, free drugs, single drug delivery system DOX-PMs and co-delivery system PTX/DOX-PMs against different cell lines was extensively studied. The results showed that the PTX/DOX-PMs exhibited the highest cytotoxic effect for tumor cells while the carrier had negligible cytotoxicity. In short, PTX/DOX-PMs could be a promising nanomedicine for combination chemotherapy. By the way, in vivo anticancer efficacies and bio-distributions of PTX/DOX-PMs and other co-delivery systems would be systematically studied in future.
Acknowledgments
This work was financially supported by the Liaoning Provincial Natural science Foundation of China (NO.20180550022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun CY, Shen S, Xu CF, et al. Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. J Am Chem Soc. 2015;137(48):15217–15224. doi:10.1021/jacs.5b09602
2. Pearce TR, Shroff K, Kokkoli E. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv Mater. 2012;24(28):3803–3822. doi:10.1002/adma.201200832
3. Chen W, Hou Y, Tu Z, Gao L, Haag R. pH-degradable PVA-based nanogels via photo-crosslinking of thermo-preinduced nanoaggregates for controlled drug delivery. J Control Release. 2017;259:160–167. doi:10.1016/j.jconrel.2016.10.032
4. Luo Y, Yin X, Yin X, et al. Dual pH/redox-responsive mixed polymeric micelles for anticancer drug delivery and controlled release. Pharmaceutics. 2019;11(4):176. doi:10.3390/pharmaceutics11040176
5. Li Y, Wang H, Wang K, et al. Targeted co-delivery of PTX and TR3 siRNA by PTP peptide modified dendrimer for the treatment of pancreatic cancer. Small. 2017;13(2):1602697. doi:10.1002/smll.201602697
6. Zhang DX, Yoshikawa C, Welch NG, Pasic P, Thissen H, Voelcker NH. Spatially controlled surface modification of porous silicon for sustained drug delivery applications. Sci Rep. 2019;9(1):1367. doi:10.1038/s41598-018-37750-w
7. von Roemeling C, Jiang W, Chan CK, Weissman IL, Kim BYS. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017;35(2):159–171. doi:10.1016/j.tibtech.2016.07.006
8. Elzoghby AO, Hemasa AL, Freag MS. Hybrid protein-inorganic nanoparticles: from tumor-targeted drug delivery to cancer imaging. J Control Release. 2016;243:303–322. doi:10.1016/j.jconrel.2016.10.023
9. Huang X, Liao W, Zhang G, Kang S, Zhang CY. pH-sensitive micelles self-assembled from polymer brush (PAE-g-cholesterol)-b-PEG-b-(PAE-g-cholesterol) for anticancer drug delivery and controlled release. Int J Nanomed. 2017;12:2215–2226. doi:10.2147/IJN.S130037
10. Xu M, Zhang CY, Wu J, et al. PEG-detachable polymeric micelles self-assembled from amphiphilic copolymers for tumor-acidity-triggered drug delivery and controlled release. ACS Appl Mater Interfaces. 2019;11(6):5701–5713. doi:10.1021/acsami.8b13059
11. Alakhov V, Klinski E, Li S, et al. Block copolymer-based formulation of doxorubicin. from cell screen to clinical trials. Colloids Surf B. 1999;16(1–4):113–134. doi:10.1016/S0927-7765(99)00064-8
12. Zhao J, Qin Z, Wu J, Li L, Jin J, Ji J. Zwitterionic stealth peptide-protected gold nanoparticles enable long circulation without the accelerated blood clearance phenomenon. Biomater Sci. 2018;6(1):200–206. doi:10.1039/C7BM00747G
13. Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliver Rev. 1995;17(1):31–48. doi:10.1016/0169-409X(95)00039-A
14. Sun X, Wang G, Zhang H, et al. The blood clearance kinetics and pathway of polymeric micelles in cancer drug delivery. ACS Nano. 2018;12(6):6179–6192. doi:10.1021/acsnano.8b02830
15. Sun X, Yan X, Jacobson O, et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 2017;7(2):319–328. doi:10.7150/thno.18078
16. Feng Q, Zhang Y, Zhang W, et al. Tumor-targeted and multi-stimuli responsive drug delivery system for near-infrared light induced chemo-phototherapy and photoacoustic tomography. Acta Biomater. 2016;38:129–142. doi:10.1016/j.actbio.2016.04.024
17. Yang K, Feng L, Liu Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv Drug Deliver Rev. 2016;105:228–241. doi:10.1016/j.addr.2016.05.015
18. Yin Q, Shen J, Zhang Z, Yu H, Li Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv Drug Deliver Rev. 2013;65(13–14):13–14. doi:10.1016/j.addr.2013.04.011
19. Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi:10.1038/nmat3776
20. Le NTT, Nguyen TNQ, Cao VD, Hoang DT, Ngo VC, Thi TTH. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics. 2019;11(11):591. doi:10.3390/pharmaceutics11110591
21. Alsuraifi A, Curtis A, Lamprou DA, Hoskins C. Stimuli responsive polymeric systems for cancer therapy. Pharmaceutics. 2018;10(3):136. doi:10.3390/pharmaceutics10030136
22. Yang P, Li D, Jin S, et al. Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials. 2014;35(6):2079–2088. doi:10.1016/j.biomaterials.2013.11.057
23. Gulzar A, Gai S, Yang P, Li C, Ansari MB, Lin J. Stimuli responsive drug delivery application of polymer and silica in biomedicine. J Mater Chem B. 2015;3(44):8599–8622. doi:10.1039/C5TB00757G
24. Wu X, Wang Z, Zhu D, et al. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer–lipid bilayer. ACS Appl Mater Interfaces. 2013;5(21):10895–10903. doi:10.1021/am403092m
25. Ahmad Z, Shah A, Siddiq M, Kraatz HB. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014;4(33):17028–17038. doi:10.1039/C3RA47370H
26. Li J, Ma YJ, Wang Y, Chen BZ, Guo XD, Zhang CY. Dual redox/pH-responsive hybrid polymer-lipid composites: synthesis, preparation, characterization and application in drug delivery with enhanced therapeutic efficacy. Chem Eng J. 2018;341:450–461. doi:10.1016/j.cej.2018.02.055
27. Cai W, Wang J, Chu C, Chen W, Wu C, Liu G. Metal-organic framework‐based stimuli‐responsive systems for drug delivery. Adv Sci. 2019;6(1):1801526. doi:10.1002/advs.201801526
28. Han B, Yang Y, Chen J, et al. Preparation, characterization, and pharmacokinetic study of a novel long-acting targeted paclitaxel liposome with antitumor activity. Int J Nanomed. 2020;15:553–571. doi:10.2147/IJN.S228715
29. Sarker DK. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv. 2005;2(4):297–310. doi:10.2174/156720105774370267
30. Park J, Kadasala NR, Abouelmagd SA, et al. Polymer-iron oxide composite nanoparticles for EPR-independent drug delivery. Biomaterials. 2016;101:285–295. doi:10.1016/j.biomaterials.2016.06.007
31. Cao H, Chen C, Xie D, et al. A hyperbranched amphiphilic acetal polymer for pH-sensitive drug delivery. Polym Chem. 2018;9(2):169–177. doi:10.1039/C7PY01739A
32. Zhao J, Yang Y, Han X, et al. Redox-sensitive nanoscale coordination polymers for drug delivery and cancer theranostics. ACS Appl Mater Interfaces. 2017;9(28):23555–23563. doi:10.1021/acsami.7b07535
33. Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):1803618. doi:10.1002/adma.201803618
34. Rafeeq T, Ana MM, Willian PG, Ghaleb AH. Drug delivery systems based on polymeric micelles and ultrasound: a review. Curr Pharm Design. 2016;22(19):2796–2807. doi:10.2174/1381612822666160217125215
35. Muley P, Kumar S, Kourati FE, Kesharwani SS, Tummala H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int J Pharm. 2016;500(1–2):32–41. doi:10.1016/j.ijpharm.2016.01.005
36. Kesharwani SS, Dachineni R, Bhat GJ, Tummala H. Hydrophobically modified inulin-based micelles: transport mechanisms and drug delivery applications for breast cancer. J Drug Deliv Sci Technol. 2019;54:101254. doi:10.1016/j.jddst.2019.101254
37. Zhang CY, Yang YQ, Huang TX, et al. Self-assembled pH-responsive MPEG-b-(PLA-co-PAE) block copolymer micelles for anticancer drug delivery. Biomaterials. 2012;33(26):6273–6283. doi:10.1016/j.biomaterials.2012.05.025
38. Hu X, Liu G, Li Y, Wang X, Liu S. Cell-penetrating hyperbranched polyprodrug amphiphiles for synergistic reductive milieu-triggered drug release and enhanced magnetic resonance signals. J Am Chem Soc. 2015;137(1):362–368. doi:10.1021/ja5105848
39. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaaz7964. doi:10.1126/sciadv.aax7964
40. Li Y, Lu H, Liang S, Xu S. Dual stable nanomedicines prepared by cisplatin-crosslinked camptothecin prodrug micelles for effective drug delivery. ACS Appl Mater Interfaces. 2019;11(23):20649–20659. doi:10.1021/acsami.9b03960
41. Li Z, Wang H, Chen Y, et al. pH-and NIR light-responsive polymeric prodrug micelles for hyperthermia-assisted site-specific chemotherapy to reverse drug resistance in cancer treatment. Small. 2016;12(20):2731–2740. doi:10.1002/smll.201600365
42. Ma Y, Fan X, Li L. pH-sensitive polymeric micelles formed by doxorubicin conjugated prodrugs for co-delivery of doxorubicin and paclitaxel. Carbohyd Polym. 2016;137:19–29. doi:10.1016/j.carbpol.2015.10.050
43. Ma Y, He P, Tian X, Liu G, Zeng X, Pan G. Mussel-derived, cancer-targeting peptide as pH-sensitive prodrug nanocarrier. ACS Appl Mater Interfaces. 2019;11(27):23948–23956. doi:10.1021/acsami.9b09031
44. Zou P, Song J, Jiang B, et al. Epigallocatechin-3-gallate protects against cisplatin nephrotoxicity by inhibiting the apoptosis in mouse. Int J Clin Exp Pathol. 2014;7(8):4607–4616.
45. Duan X, Xiao J, Yin Q, et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7(7):5858–5869. doi:10.1021/nn4010796
46. Zhai S, Hu X, Hu Y, Wu B, Xing D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials. 2017;121:41–54. doi:10.1016/j.biomaterials.2017.01.002
47. Yang J, Zhai S, Qin H, Yan H, Xing D, Hu X. NIR-controlled morphology transformation and pulsatile drug delivery based on multifunctional phototheranostic nanoparticles for photoacoustic imaging-guided photothermal-chemotherapy. Biomaterials. 2018;176:1–12. doi:10.1016/j.biomaterials.2018.05.033
48. Hu X, Zhai S, Liu G, Xing D, Liang H, Liu S. Concurrent drug unplugging and permeabilization of polyprodrug‐gated crosslinked vesicles for cancer combination chemotherapy. Adv Mater. 2018;30(21):1706307. doi:10.1002/adma.201706307
49. Zhu H, Chen H, Zeng X, et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance. Biomaterials. 2014;35(7):2391–2400. doi:10.1016/j.biomaterials.2013.11.086
50. Danafar H, Rostamizadeh K, Davaran S, Hamidi M. Co-delivery of hydrophilic and hydrophobic drugs by micelles: a new approach using drug conjugated PEG–PCL nanoparticles. Drug Dev Ind Pharm. 2017;43(11):1908–1918. doi:10.1080/03639045.2017.1355922
51. Huang X, Liao W, Xie Z, Chen D, Zhang CY. A pH-responsive prodrug delivery system self-assembled from acid-labile doxorubicin-conjugated amphiphilic pH-sensitive block copolymers. Mat Sci Eng C-Mater. 2018;90:27. doi:10.1016/j.msec.2018.04.036
52. He H, Markoutsa E, Zhan Y, Zhang J, Xu P. Mussel-inspired PLGA/polydopamine core-shell nanoparticle for light induced cancer thermochemotherapy. Acta Biomater. 2017;59:181–191. doi:10.1016/j.actbio.2017.07.005
53. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliver Rev. 2004;56(11):1649–1659. doi:10.1016/j.addr.2004.02.014
54. Wang Y, Zhang H, Hao J, Li B, Li M, Wang X. Lung cancer combination therapy: co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2015;23(4):1398–1403. doi:10.3109/10717544.2015.1055619
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.