Back to Journals » OncoTargets and Therapy » Volume 10
Clinicopathological significance of ZEB-1 and E-cadherin proteins in patients with oral cavity squamous cell carcinoma
Authors Yao X, Sun S, Zhou X, Zhang Q, Guo W, Zhang L
Received 3 May 2016
Accepted for publication 31 October 2016
Published 13 February 2017 Volume 2017:10 Pages 781—790
DOI https://doi.org/10.2147/OTT.S111920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Xiaofeng Yao,1,2 Shanshan Sun,1,2 Xuan Zhou,1,2 Qiang Zhang,1,2 Wenyu Guo,1,2 Lun Zhang1,2
1Department of Maxillofacial and Otorhinolaryngology Head and Neck Surgery, Tianjin Medical University Cancer Institute and Hospital, 2Key Laboratory of Cancer Prevention and Therapy, National Clinical Research Center of Cancer, Tianjin, People’s Republic of China
Background: Zinc-finger E-box binding homeobox 1 (ZEB-1), a member of the ZFH family, plays a key role in epithelial–mesenchymal transition during tumor progression in various cancers. However, little information is available on ZEB-1 expression in oral cavity squamous cell carcinoma (OSCC).
Methods: The expression levels of ZEB-1 and E-cadherin were assessed by immunohistochemistry in a cohort of 120 patients with OSCC treated by curative operation, and then the correlations between ZEB-1 and E-cadherin expression and clinical factors were evaluated, including patient prognosis. Quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed to assess mRNA levels of ZEB-1 and E-cadherin in 20 matched OSCC specimens.
Results: Patients were followed up for a median period of 66 months (range 8-116 months), and 5-year overall survival was 68.3%. Positive ZEB-1 and E-cadherin immunostaining reactivity was detected in 64 (53.3%) and 53 (44.2%) patients, respectively. There was a negative correlation between ZEB-1 expression and E-cadherin expression. In addition, overexpression of ZEB-1 was significantly associated with recurrence, lymph node metastasis, and pathologic grading of patients, loss of E-cadherin was significantly associated with lymph node metastasis and pathologic grading of patients. Univariate analysis showed that increased ZEB-1 expression, loss of E-cadherin expression, lymph node metastasis, recurrence, and pathology grade were prognostic factors. In multivariate analysis, increased ZEB-1 expression and recurrence remained independent prognostic factors. In particular, patients with both ZEB-1 positivity and loss of E-cadherin expression had a poorer prognosis. qRT-PCR showed that ZEB-1 mRNA expression was higher in OSCC compared to the adjacent nontumorous tissues, while E-cadherin mRNA expression was lower in tumor tissues.
Conclusion: This study shows that overexpression of ZEB-1 and loss of E-cadherin expression are significantly correlated with poor survival in OSCC patients, and ZEB-1 expression might serve as an independent prognostic biomarker of OSCC.
Keywords: ZEB-1, E-cadherin, EMT, oral squamous cell carcinoma, prognosis
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a frequently occurring carcinoma with high mortality rates, and approximately 30% arise in the oral cavity, which is characterized by a high degree of local invasiveness and lymph node metastasis. There are approximately 260,000 new cases of oral cavity squamous cell carcinoma (OSCC) and 124,000 deaths worldwide annually.1 Despite advances in treatments, including adjuvant chemotherapy, radiotherapy, and targeted therapy, the overall 5-year survival rate has not improved significantly during the last two decades.2 Cervical lymph node metastasis is a major determinant of prognosis of OSCC. Approximately 50% of patients were detected with metastasis to regional lymph nodes at the time of diagnosis.3 Therefore, it is urgent to study the underlying mechanisms of metastasis and evaluate the factors involved in poor prognosis of OSCC.
It is generally believed that epithelial–mesenchymal transition (EMT) plays a key role in tumor progression and metastasis.4,5 EMT is a complex process of epithelial cells changing into mesenchymal cells, including modification in cellular construction, morphology, and adhesion.6 A key event of EMT is loss of E-cadherin expression, which is one of the important adhesion molecules and plays a crucial role in the maintenance of epithelial integrity7 and overexpression of mesenchymal molecules such as N-cadherin and vimentin.8 It has been reported that repression of E-cadherin is associated with increased tumor migration and invasion both in vitro and in vivo.9–11 The expression of E-cadherin is regulated by a variety of transcription factors, including zinc-finger E-box binding homeobox 1 (ZEB-1), ZEB-2, Snail, SIP-1, and Twist,12,13 which can downregulate the expression of E-cadherin by specifically binding to the E-boxes domain and then inducing EMT in several cancers.14,15
ZEB-1 (also called Tcf8, dEF-1, Nil-2-a, Bzp, Areb6, Meb-1, Zfhep) is a member of the ZFH family. ZEB-1 encodes a transcription factor, which plays a critical role in invasion and metastasis of several types of human cancer by inducing EMT.16 It has been shown that ZEB-1 promotes EMT in several ways by regulating target genes through its protein binding domains, including the Smad interaction domain, CtBP interaction domain, and p300-P/CAF binding domain.17,18 In addition, ZEB-1 contains two zinc-finger domains at the N- and C-termini, by which ZEB-1 binds E-box-like sequences within the E-cadherin promoter region with high specificity and then regulates the expression of E-cadherin.19 Downregulation of E-cadherin by the aberrant expression of ZEB-1 has been revealed in several human cancers, including lung cancer, colorectal cancer, breast cancer, prostate cancer, and pancreatic cancer.20–24 In OSCC, it is well documented that reduced E-cadherin expression will lead to the loss of epithelioid cell morphology and significantly relates with nodal metastasis, advanced stages, and poor prognosis.25 Till date, limited opinions about the clinical significance of ZEB-1 expression and its relationship with E-cadherin have been reported in OSCC.
The aim of this study was to investigate the expression of ZEB-1 and E-cadherin in OSCC by immunohistochemistry (IHC) and analyze their relationship with the clinicopathological parameters. Moreover, we present evidence showing that overexpression of ZEB-1 and loss of E-cadherin expression are significantly correlated with poor survival in OSCC patients and that ZEB-1 expression might serve as an independent prognostic biomarker of OSCC.
Materials and methods
Patients and tumor samples
The Human Ethics Committee of Affiliated Cancer Hospital of Tianjin Medical University approved the study. Written consent for using the samples for research purpose was obtained from all patients prior to surgery.
A total of 120 cases of primary OSCC were diagnosed over a period of 5 years (2005–2010) at Tianjin Medical University Cancer Institute and Hospital. Primary tumors were confirmed by both preoperative and postoperative pathologic examinations, and formalin-fixed paraffin-embedded blocks of those tumors were used in this study. For each patient, data regarding the following clinicopathological factors were collected: gender, age, smoker, alcohol, tumor site, clinical T stage, pathologic differentiation, lymph node metastasis, and recurrence. The stage of disease was determined after the surgical resection of the tumor according to the tumor–node–metastasis classification criteria for malignant tumors (International Union Against Cancer).26
Treatments
All patients had received radical resection of tumor and neck lymph node dissection. Patients without clinical nodal metastasis underwent selective neck dissection, involving levels I-III. Patients with clinical nodal metastasis underwent radical neck dissection, involving levels I-V, and contralateral neck dissection was done if primary tumor involved the median line or if contralateral neck lymph node metastasis was suspected. Patients with poor prognostic factors were treated with postoperative radiotherapy or chemoradiotherapy.
Immunohistochemistry
The streptavidin–biotin–peroxidase immunohistochemical staining method was used in this study. Four-micrometer sections of formalin-fixed paraffin-embedded tissue were mounted on poly-L-lysine-coated slides. The slides were deparaffinized, and then endogenous peroxidase activity was blocked with 3% hydrogen peroxide in 50% methanol for 10 min at room temperature. The slides were rehydrated and washed with phosphate-buffered saline (PBS) and then pretreated with citrate buffer (0.01 M citric acid, pH 6.0) for 20 min at 100°C in a microwave oven. After nonspecific binding sites were blocked by incubating in 2% normal goat serum in PBS for 15 min at 37°C, the slides were incubated overnight at 4°C with the primary rabbit ZEB-1 antibody (dilution 1:100; Abcam) and rabbit E-cadherin antibody (dilution 1:200; Abcam). The slides were washed with PBS and incubated with biotin-labeled secondary antibody (dilution 1:100; Zhongshan Biotechnology) for 1 hour at 37°C and then incubated with avidin–biotin-conjugated peroxidase and diaminobenzidine (Zhongshan Biotechnology), and counterstained with hematoxylin. Slides were dehydrated with different concentrations of alcohol and soaked in xylene, and then mounted with neutral balsam and visualized using light microscope.
Evaluation of immunohistochemistry
The results were evaluated by measuring both the staining intensity and the number of positive cells. The intensity of the positive reaction was scored as negative (0), weak (1), moderate (2), and intense (3), and reactivity was assessed by the percentage of positively stained cells as 0%–5% positive cells (0), 6%–25% positive cells (1), 26%–50% positive cells (2), 51%–75% positive cells (3), and 75%–100% positive cells (4). The scores for the intensity and the percentage of positive cells were multiplied to work out at a weighted score for each case, giving a minimum-to-maximal score of 0–12. The expressions of ZEB-1 and E-cadherin were dichotomized to low (0–6) and high (7–12) values for outcome analyses.
Quantitative real-time polymerase chain reaction
Twenty pairs of OSCC specimens and corresponding adjacent nontumorous tissues were collected and stored in liquid nitrogen until RNA extraction. Total RNA was extracted by using Trizol (Invitrogen) according to the manufacturer’s protocol. cDNA was synthesized from 1 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The mRNA expression of ZEB-1 and E-cadherin was determined using SYBR green-based real-time polymerase chain reaction (RT-PCR) on a PikoReal RT-PCR System (Thermo Fisher Scientific). The primer sequences for ZEB-1 and E-cadherin were designed as follows: ZEB-1 sense primer 5′-AGACATGTGACGCAGTCTG-3′, antisense primer 5′-ATGTGTGAGCTATAGGAGC-3′; E-cadherin sense primer 5′-AGGGGTTAAGCACAACAGCA-3′, antisense primer 5′-GGTATTGGGGGCATCAGCAT-3′. GAPDH gene was used as an internal control. The primers were 5′-ATGTTCGTCATGGGTGTGAA-3′ and 5′-GGTGCTAAGCAGTTGGTGGT-3′. A melting curve analysis was performed to monitor PCR product purity and the 2−ΔΔCt method was used to quantify the expression of the ZEB-1 and E-cadherin genes.
Statistical analysis
Data were analyzed using IBM SPSS software version 21.0. The interrelationship of ZEB-1 and E-cadherin expression with clinicopathological parameters was analyzed by the χ2 test or Fisher’s exact test. Spearman’s correlation test was used to analyze the correlation between ZEB-1 and E-cadherin expression. Kaplan–Meier and log-rank tests were used for univariate survival analysis. Cox proportional hazards model was used for multivariate analysis. For all tests, differences were considered as significant at the value of P<0.05.
Results
Characterization of OSCC patients
As shown in Table 1, among the 120 OSCC patients, 75 were males (62.5%) and 45 were females (37.5%). The age ranged from 26 to 82 years, with a median of 57.6 years. The oral tongue was the most common primary tumor site (43.3%), followed by oral floor (21.7%). Seventy-three (60.8%) patients had early T-stage (stage 1/2) disease, and 58 (48.3%) patients had pathological cervical lymph node metastasis. Thirty-nine (32.5%) tumors showed well differentiation and 81 (67.5%) showed moderate/poor differentiation. Fifty-five (45.8%) patients experienced local or distant recurrence during the follow-up period; of the 63 (52.5%) dead patients, 49 (77.7%) died of tumor recurrence and 14 (22.3%) died of other causes.
  | Table 1 Clinicopathological characteristics of 120 OSCC patients |
Expression of ZEB-1 and E-cadherin in OSCC
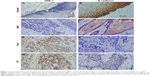
Using immunohistochemical staining, we evaluated the biological significance of ZEB-1 and E-cadherin in OSCC tissues. ZEB-1 staining was mainly located in the cellular nuclei of tumor cells, and E-cadherin was observed predominantly in the cellular membranes. The typical immunohistochemical expression patterns of ZEB-1 and E-cadherin are shown in Figures 1 and S1. Of 120 patients, 64 (53.3%) had a high IHC reactivity score for ZEB-1, and 53 (44.2%) had a high IHC reactivity score for E-cadherin.
All noncancerous oral squamous epithelial cells were ZEB-1 negative, and out of the positive samples, 26 (40.6%) cases were detected in the tumor infiltrative margin. E-cadherin was detected in the tumor cells and the normal squamous epithelial cells, and tumor cells with a staining intensity equal to or greater than that of normal oral squamous epithelial cells were considered to show positive expression, and those with a weaker staining intensity than normal oral squamous epithelial cells or with no expression at all were considered to show reduced expression. Twenty (37.7%) cases with the loss of E-cadherin expression were detected in the tumor infiltrative margin.
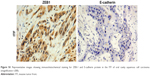
The results of quantitative RT-PCR also confirmed that the relative expression level of ZEB-1 mRNA in OSCC was significantly higher than that in adjacent noncancerous tissues, and E-cadherin mRNA expression was lower in OSCC than in adjacent noncancerous tissues. The relative expression levels of ZEB-1 and E-cadherin mRNA are shown in Figure 2.
Relationship between ZEB-1 and E-cadherin expression and clinicopathologic parameters
As shown in Table 2, when we compared ZEB-1 and E-cadherin expression by clinicopathological factors, we found that positive expression of ZEB-1 was significantly associated with recurrence (P=0.008), lymph node metastasis (P=0.000), and pathologic grading (P=0.000), and loss of E-cadherin was significantly associated with lymph node metastasis (P=0.003) and pathologic grading (P=0.004). Further analysis revealed that reduced E-cadherin expression was detected in 48 of 64 cases with positive ZEB-1 expression. There was a negative correlation between positive ZEB-1 expression and reduced E-cadherin expression (P=0.000).
  | Table 2 Clinicopathologic correlation of ZEB-1 and E-cadherin expression in OSCC |
Univariate and multivariate analyses for recurrence and survival
The results of univariate and multivariate analyses of factors affecting patient outcomes are shown in Table 3. Poorer differentiation, lymph node metastasis, positive ZEB-1 expression, recurrence, and reduced E-cadherin expression were significantly associated with poor overall survival (OS). In multivariate analysis, increased ZEB-1 expression and recurrence remained independent prognostic factors. In particular, patients with both ZEB-1 positivity and loss of E-cadherin expression had a poorer prognosis.
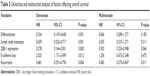  | Table 3 Univariate and multivariate analysis of factors affecting overall survival |
Prognostic impact of ZEB-1 and E-cadherin expression
At the conclusion of the study, 57 patients (47.5%) were still alive, while 63 patients (52.5%) died. The 5-year OS of patients was 68.3%. Figure 3A–C shows the OS curves after surgery according to ZEB-1 and E-cadherin expression. Specifically, Figure 3C displays that the 5-year OS rates were significantly poorer in the ZEB-1 positive and loss of E-cadherin expression groups than in other expression groups (P=0.000). Figure 3D–F shows that patients with moderate/poor differentiation, lymph node metastasis, and recurrence have worse prognosis.
Discussion
Metastasis is the primary cause of death in most cancer patients. This process involves modification in cellular construction and morphology, which promotes invasion and metastasis, and is regulated by a mechanism known as EMT. EMT is a complex process involving many pathways, and decreased expression of E-cadherin is a key event which can induce the loss of adhesion among epithelial cells.7 Loss of E-cadherin in HNSCC has been shown to correlate with EMT and with poor patient prognosis.10 In the present study, we found that the E-cadherin protein was significantly decreased in 44.2% of OSCC specimens, and loss of E-cadherin was significantly associated with lymph node metastasis and pathologic grading, which is consistent with those reported by a previous study.25
ZEB-1 is one of the transcription factors that can downregulate E-cadherin expression. Previous studies showed that ZEB-1 overexpression was significantly correlated with aggressiveness and poor clinical prognosis, as indicated by increased metastasis and posttreatment recurrence in other human malignancies.27,28 Consistent with this finding, our study showed that ZEB-1 was overexpressed in 53.3% of OSCC specimens, and the expression was significantly associated with recurrence, lymph node metastasis, and pathologic grading. Ho et al29 reported that depletion of ZEB-1 by lentiviral-mediated knockdown reversed the arecoline-stimulated oncogenicity including migration, invasion, and clonogenicity of OSCC cells both in vitro and in vivo, and the level of ZEB-1 expression was higher in recurrent OSCC tumor samples but lower in primary lesions. We also showed that the positive rate was higher in recurrent patients. Therefore, ZEB-1 may serve as a relapse marker in OSCC.
Lose of E-cadherin expression has been continually reported in OSCC, especially about its role in tumor invasion and migration. However, the expression of ZEB-1 in OSCC was not widely investigated in these reported studies. Haddad et al30 reported that ZEB-1 manipulated the mesenchymal phenotype in HNSCC. However, the underlying mechanism is unknown. Our study thoroughly examined the expression of ZEB-1 and E-cadherin in OSCC, and we analyzed the relationship with clinical factors, including patient prognosis. The result showed that increased ZEB-1 expression was associated with reduced expression of E-cadherin, which was consistent with other studies. Those findings demonstrated that overexpression of ZEB-1 increased migration and invasiveness of OSCC through suppressing E-cadherin expression. In this study, we also found that overexpression of ZEB-1 and reduced expression of E-cadherin were significantly associated with poor prognosis. Particularly, when compared with other groups of patients, the subgroup of patients with upregulated ZEB-1 and downregulated E-cadherin was more prone to metastasis and showed the worst prognosis than other groups.
Local invasion and metastasis are important prognostic factors in patients with OSCC. It has been reported that different parts of the tumor tissue often exhibit great heterogeneity, including aberrant expression of some cell molecules.31 Bryne et al32 first proposed the invasive tumor front (ITF) concept, which was defined as the most progressed 3–6 tumor cell layers or detached tumor cell groups at the advancing edge of the tumor. Piffko et al33 had observed that tumor cells at the most invasive parts of OSCC differed substantially from center and superficial ones. It is believed that more information can be obtained from ITF where the most aggressive tumor cells are present and where crucial molecular interactions take place.32 EMT has also been previously reported to occur at the ITF, and principal characters can be indicated by the expression of specific proteins, including the loss of E-cadherin expression.34 Wang et al35 found that reduced expression of E-cadherin at the ITF influenced disease-free survival, and a statistically significant correlation was observed between E-cadherin loss at the ITF and poor survival of OSCC patients. To our knowledge, no studies have yet been performed to analyze the expression of ZEB-1 at the ITF of OSCC. In human prostate cancer, Sethi et al36 reported that ZEB-1 showed higher expression at the ITF than at the center of the tumor. In the present study, we found that the altered expression of ZEB-1 and E-cadherin was prominent at the ITF than at the center of the OSCC. Indeed, we observed a trend where overexpression of ZEB-1 (40.6%) and loss of expression of E-cadherin (37.7%) at the ITF were associated with poor prognosis, although this did not reach statistical significance. These data suggest that overexpression of ZEB-1 and loss of E-cadherin expression at the ITF are important events in the progression of OSCC, so further studies are necessary to examine the role of ZEB-1 and E-cadherin at ITF as useful markers of OSCC in larger patient samples.
Conclusion
In summary, positive expression of ZEB-1 and decreased expression of E-cadherin are frequently detected in OSCC by IHC, and the abnormal expression is associated with lymph node metastasis and pathologic grading. Moreover, our study found that patients who have higher ZEB-1 and lower E-cadherin expression would have worst prognosis. Thus, further studies are necessary to confirm that these two are promising markers for prognostic prediction in OSCC.
Acknowledgment
This work was supported by a grant from the National Natural Science Foundation of China (Grant No 81572492).
Disclosure
The authors report no conflicts of interest in this work.
References
Almeida MR, Pérez-Sayáns M, Suárez-Peñaranda JM, et al. p27Kip1 expression as a prognostic marker for squamous cell carcinoma of the head and neck. Oncol Lett. 2015;10(5):2675–2682. | ||
Allen CT, Clavijo PE, Van Waes C, Chen Z. Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel). 2015;7(4):2397–2414. | ||
Kim JW, Roh JL, Park Y, et al. Cytoplasmic iASPP expression as a novel prognostic indicator in oral cavity squamous cell carcinoma. Ann Surg Oncol. 2015;22(2):662–669. | ||
Savagner P. Epithelial–mesenchymal transitions: from cell plasticity to concept elasticity. Curr Top Dev Biol. 2015;112:273–300. | ||
Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49(4):287–292. | ||
Zhang W, Shi X, Peng Y, et al. HIF-1alpha promotes epithelial–mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS One. 2015;10(6):e129603. | ||
Sakamoto K, Imanishi Y, Tomita T, et al. Overexpression of SIP1 and downregulation of E-cadherin predict delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma after partial glossectomy. Ann Surg Oncol. 2012;19(2):612-619. | ||
Nijkamp MM, Span PN, Hoogsteen IJ, van der Kogel AJ, Kaanders JH, Bussink J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother Oncol. 2011;99(3):344–348. | ||
Yoshida R, Morita M, Shoji F, et al. Clinical significance of SIP1 and E-cadherin in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2015;22(8):2608–2614. | ||
Xie CG, Wei SM, Chen JM, et al. Down-regulation of GEP100 causes increase in E-cadherin levels and inhibits pancreatic cancer cell invasion. PLoS One. 2012;7(5):e37854. | ||
Wen CL, Chen KY, Chen CT, Chuang JG, Yang PC, Chow LP. Development of an AlphaLISA assay to quantify serum core-fucosylated E-cadherin as a metastatic lung adenocarcinoma biomarker. J Proteomics. 2012;75(13):3963–3976. | ||
Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. | ||
Fan CC, Wang TY, Cheng YA, et al. Expression of E-cadherin, Twist, and P53 and their prognostic value in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139(10):1735–1744. | ||
Balasundaram P, Singh MK, Dinda AK, Thakar A, Yadav R. Study of beta-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn Pathol. 2014;9:145. | ||
Chow V, Yuen AP, Lam KY, et al. A comparative study of the clinicopathological significance of E-cadherin and catenins (α, β, γ) expression in the surgical management of oral tongue carcinoma. J Cancer Res Clin Oncol. 2001;127(1):59–63. | ||
Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial–mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14(4):481–487. | ||
Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28(1-2):151–166. | ||
Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773–787. | ||
Romero-Pérez L, López-García MÁ, Díaz-Martín J, et al. ZEB1 overexpression associated with E-cadherin and microRNA-200 downregulation is characteristic of undifferentiated endometrial carcinoma. Mod Pathol. 2013;26(11):1514–1524. | ||
Miyahara S, Hamasaki M, Hamatake D, et al. Clinicopathological analysis of pleomorphic carcinoma of the lung: diffuse ZEB1 expression predicts poor survival. Lung Cancer. 2015;87(1):39–44. | ||
Paek AR, Lee CH, You HJ. A role of zinc-finger protein 143 for cancer cell migration and invasion through ZEB1 and E-cadherin in colon cancer cells. Mol Carcinog. 2014;53:E161–E168. | ||
Chaffer CL, Marjanovic ND, Lee T, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154(1):61–74. | ||
Drake JM, Strohbehn G, Bair TB, et al. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell. 2009;20(8):2207–2217. | ||
Wellner U, Brabletz T, Keck T. ZEB1 in pancreatic cancer. Cancers (Basel). 2010;2(3):1617–1628. | ||
Zhou J, Tao D, Xu Q, et al. Expression of E-cadherin and vimentin in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;18(3):3150–3154. | ||
Sobin LH, Gospodarowicz MK, Witterkind C, editors. TNM classification of malignant tumors. 7th ed. New York: John Wiley & Sons; 2009. | ||
Ran J, Lin DL, Wu RF, et al. ZEB1 promotes epithelial–mesenchymal transition in cervical cancer metastasis. Fertil Steril. 2015;103(6):1606–1614. | ||
Mock K, Preca BT, Brummer T, et al. The EMT-activator ZEB1 induces bone metastasis associated genes including BMP-inhibitors. Oncotarget. 2015;6(16):14399–14412. | ||
Ho CM, Hu FW, Lee SS, et al. ZEB1 as an indicator of tumor recurrence for Areca Quid chewing-associated oral squamous cell carcinomas. J Oral Pathol Med. 2015;44(9):693–698. | ||
Haddad Y, Choi W, McConkey DJ. Delta-crystallin enhancer binding factor 1 controls the epithelial to mesenchymal transition phenotype and resistance to the epidermal growth factor receptor inhibitor erlotinib in human head and neck squamous cell carcinoma lines. Clin Cancer Res. 2009;15(2):532–542. | ||
Mehendiratta M, Solomon MC, Boaz K, Guddattu V, Mohindra A. Clinico-pathological correlation of E-cadherin expression at the invasive tumor front of Indian oral squamous cell carcinomas: an immunohistochemical study. J Oral Maxillofac Pathol. 2014;15(2):217–222. | ||
Bryne M, Boysen M, Alfsen CG, et al. The invasive front of carcinomas. The most important area for tumour prognosis? Anticancer Res. 1998;18(6B):4757–4764. | ||
Piffko J, Bankfalvi A, Ofner D, et al. Prognostic value of histobiological factors (malignancy grading and AgNOR content) assessed at the invasive tumour front of oral squamous cell carcinomas. Br J Cancer. 1997;75(10):1543–1546. | ||
Masuda R, Kijima H, Imamura N, et al. Laminin-5γ2 chain expression is associated with tumor cell invasiveness and prognosis of lung squamous cell carcinoma. Biomed Res. 2012;33(5):309-317. | ||
Wang C, Huang H, Huang Z, et al. Tumor budding correlates with poor prognosis and epithelial–mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40(7):545–551. | ||
Sethi S, Macoska J, Chen W, et al. Molecular signature of epithelial–mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3(1):90–99. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.