Back to Journals » Cancer Management and Research » Volume 12
Clinicopathological Characteristics of Pseudomyxoma Peritonei Originated from Ovaries
Authors Yan F, Shi F , Li X, Yu C, Lin Y, Li Y , Jin M
Received 29 May 2020
Accepted for publication 3 August 2020
Published 21 August 2020 Volume 2020:12 Pages 7569—7578
DOI https://doi.org/10.2147/CMAR.S264474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Fengcai Yan,1,2 Feng Shi,1,2 Xinbao Li,3 Chunkai Yu,1 Yulin Lin,3 Yan Li,1,3 Mulan Jin2
1Department of Pathology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Pathology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Peritoneal Cancer Surgery, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Mulan Jin Email [email protected]
Objective: This study aims to demonstrate clinicopathological characteristics and immunohistopathological phenotypes of pseudomyxoma peritonei (PMP) originated from ovaries.
Methods: The primary origin of PMP was explored by reviewing H&E sections retrospectively and performing a series of immunohistochemical staining on CK7, CK20, CDX2, CEA, Villin, SATB2, CA125, ER, PR, and MUC.
Results: Among 310 PMP patients, a few originated from extra-appendix, whereas eight cases were of ovarian origin (2.6%), including three teratoma-associated ovarian mucinous tumors and five primary ovarian mucinous tumors with spontaneous or iatrogenic rupture, respectively. Most peritoneal metastases were acellular mucin or low-grade mucinous carcinoma peritonei (6/8, 75%), while the rest were high-grade mucinous carcinoma peritonei (2/8, 25%). Tumors were positive for CK20, CDX2, CEA, and Villin. SATB2 was specifically diffuse positive in teratoma-associated ovarian mucinous tumors, and negative in primary ovarian mucinous tumors. Differential expression of MUC was observed in these tumors.
Conclusion: PMP of ovarian origin is extremely rare. The precise diagnosis requires serial sections of the appendix or suspicious tissue to exclude appendiceal mucinous neoplasms, as well as comprehensive analysis of clinical features, surgical findings, histopathological characteristics, and immunohistochemistry on specific biomarkers.
Keywords: pseudomyxoma peritonei, PMP, ovary, mucinous tumor
Introduction
Pseudomyxoma peritonei (PMP) is a unique and extremely rare clinical syndrome with an estimated incidence of 1–2 per million per year. It is characterized by extensive intraperitoneal mucinous effusion resulting from accumulation of gelatinous ascites or disseminated lesions of neoplastic mucinous epithelia. Etiologies of PMP primary tumors are originated most plausibly from mucinous neoplasms of the appendix. Occasionally, PMPs can stem from other abdominal and pelvic organs, in which the ovary is a rare origin.1,2 Most PMPs originated from ovarian mucinous tumors were previously diagnosed clinically according to nonspecific findings from CT and MRI. In fact, these PMPs are metastases from appendiceal mucinous tumors to ovaries.3 However, ovarian mucinous neoplasms are extremely rare sources of PMPs, which should be distinguished from benign causes of mucinous ascites and from appendiceal origin tumors. We performed the present study in an attempt to assess clinicopathological features of PMPs originated from ovaries, for a better understanding and management of these rare diseases.
Materials and Methods
Case Selection
In this retrospective study, 310 PMP cases were included from 2013 to 2020 in Beijing Shijitan Hospital of Capital Medical University. After excluding those without definite primary tumor sources or a lack of preoperative clinicopathological information, only a few such as colorectal, ovary, urachus, and cervix uteri, were identified as primary extra-appendiceal sites. Notably, primary ovarian PMP (eight cases, 2.6%) was the most easily misdiagnosed. Imaging manifestations, clinicopathological findings, immunohistochemical staining, and a long-term follow-up were extracted from an electronic database of PMP patients who underwent cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). The specific scope of CRS involved omentum, peritoneum, ligamentum teres hepatis, appendix, uterus and bilateral appendages, or rectum when appropriate. We had thoroughly inspected specimens and sampled all organs or tissues of each case according to gross sampling protocols. When diagnosed as extra-appendiceal sites, extensive sampling and serial multi-sites sections of the appendix were performed to exclude appendiceal mucinous neoplasms. This study was approved by the institutional review board and ethics committee of Beijing Shijitan Hospital and was conducted in accordance with requirements in the Declaration of Helsinki.
Diagnostic Criteria
The hematoxylin and eosin (H&E) slides of tissue samples from surgical resection were retrieved to confirm histological diagnosis. Diagnosis was classified into four categories according to the 2016 Peritoneal Surface Oncology Group International (PSOGI) criteria:1 acellular mucin (AC), low-grade mucinous carcinoma peritonei (LGMC), high-grade mucinous carcinoma peritonei (HGMC), and high-grade mucinous carcinoma peritonei with signet ring cells (HGMC-S).
Immunohistochemistry on CK7, CK20, CEA, CDX-2, SATB2, CA125, PAX8, WT-1, ER, PR, MUC1, MUC2, MUC5AC, MUC6, and Ki67 (OriGene) was performed on each slide using automated staining equipment (Biocare Medical). Appropriate positive control was established for each antibody according to manufacturer’s instructions, and PBS was used as a blank control. The staining intensity was graded as negative, weak, intermediate, or strong. The staining distribution was classified as minimal, focal, or extensive (<10%, 11–50%, or >50%, respectively, of a tumor section). All the slides were reviewed independently by two surgical pathologists, who were blinded to clinical and follow-up status.
Results
Clinical Features
The clinical manifestations of ovarian origin included abdominal mass, abdominal distention, change in body weight, ascites varying in amount, as well as abdominal or pelvic pain. Surgically, PMP was encountered as grossly visible mucin in the peritoneal cavity.
Most of the tumors exhibited unilateral, cystic solid masses. Patients were followed up for 2–109 months, no recurrence or metastasis was developed in two cases.
Pathological Characteristics
Ovary
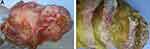
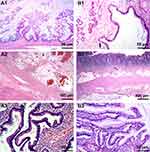
Under macroscopy, ovaries contained multiloculated cystic solid masses of different sizes (4×3×3 cm3–35×28×11 cm3). The cysts had ruptured with jelly-like mucus/ascites overflowing around the abdominal or pelvic cavity. In general, the mucus of ovary origin was much thinner (Figure 1A) than that of appendix origin (Figure 1B). Grossly, none of the cases harbored components of teratoma (eg, skin, grease, or hair). Microscopically, five ovarian mucinous cystadenomas (62.5%) and three mucinous borderline tumors pushed forward or focally infiltrated tumor borders (37.5%, Figure 2A1) in the background, accompanied with partially pseudostratified nuclei at higher magnification. Three cases accompanied with mature ovarian teratomas composed of multiple germ components including skin appendages (Figure 2B1), respiratory epithelium, thyroid gland, and adipose tissue.
Peritoneum and Appendix
When ovarian tumors disseminated through rupture, gelatinous mucus and tumor cells overflowed around the abdominal or pelvic cavity. Jelly-like ascites varying in amount mainly existed in the peritoneum, as well as abdominal and pelvic cavities. The serosal surface of the appendix was normal or slightly rough with complete lumen structure. Microscopically, abundant mucinous pools exhibited in three cases (cases #1, 4, and 8), without any neoplastic epithelium (AC). In cases #3, 5, and 6, tumor components were arranged in a strip or small island shape, and tumor cells were columnar or flat with light heteromorphism (LGMC). In cases #2 and 7, tumor tissue was clustered, adenoid, and papillary, while tumor cells were arranged in multiple layers, with obvious heteromorphism, without necrosis (HGMC). There was no lymph node involvement, angiolymphatic invasion, or perineural invasion.
Four layers of appendiceal structure were identified clearly, and no tumor presented in the mucosa. A small amount of acute/chronic inflammatory cell infiltration presented in the stroma, including a few mucoid (Figure 2A2) or low-grade/high-grade mucinous epithelium tumors (Figure 2B2) in the serosa layer.
Histological characteristics could be divided into two groups, one was teratoma-associated mucinous tumor. Scalloped glands (comprising of irregular and elongated glands dissected into ovarian stroma, which were related to pseudomyxoma ovarii4) were lined by “tall cells” with a low nuclear to cytoplasmic ratio (Figure 2A3). These glands separated the mucinous epithelium from stroma, thus forming a subepithelial cleft. Another one was a purely primary gastrointestinal-type ovarian tumor with conventional mucinous glands containing goblet cells (Figure 2B3), which were surrounded by ovarian-type stroma. Focal papillae and/or subepithelial stromal clefts could be observed occasionally. Scalloped glands or pale histiocyte aggregates (mucin granulomas) were not obvious. In ovarian mucinous borderline tumors, atypical glands displayed complex intraluminal papillary projections; however, their external contours were relatively rounded and circumscribed (Table 1).
 |
Table 1 Main Clinicopathological Features of PMP Originating from Ovaries |
Immunohistochemical Staining Results
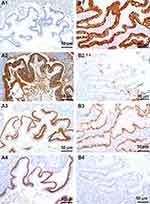
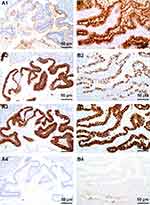
CK7 was negative in three cases, including one case originated from teratoma-associated mucinous tumor (Figure 3A1), whereas it was positive in one case (case #8, Figure 3B1). CK20 was diffuse (Figure 3A2) or partial positive (Figure 3B2) in all cases. CDX2 was positive with different intensity (Figure 3A3 and B3). SATB2 (nuclear matrix binding region binding protein 2) was positive in all three cases originated from teratoma-associated mucinous tumors (Figure 3A4), whereas negative in cases originated from purely ovarian mucinous tumors (Figure 3B4), also focal positive in ovarian mucinous tumors. CEA and Villin were strongly positive in all cases. Pax8, WT-1, ER, and PR were negative. The Ki67 proliferation index was 10–60%. CA125 was focal positive in two cases. Most MUC family members were positive (MUC1: 6/8, MUC2: 7/8; MUC5AC: 8/8; MUC6: focal 2/8, Figure 4A1–B4) (Table 2).
 |
Table 2 Immunohistochemical Markers for Ovarian Teratoma-Associated Mucinous Tumor and Primary Ovarian Mucinous Tumor |
Discussion
PMP is a rare clinical disease, typically characterized by gelatinous myxoperitoneal effusion, progressive abdominal distention, and obstruction. Under macroscopy, a large amount of jelly-like ascites were widely distributed in the abdominal/pelvic cavity. There has been a debate about the origin of the primary tumor. PMPs were considered to arise from many organs, such as the appendix, ovary, fallopian tube, pancreas, urachal tube, colon, and rectum.5–8 Recently, based on clinicopathology, immunohistochemistry, and molecular pathology, researchers have confirmed that the vast majority of PMPs stem from ruptured mucinous tumors of the appendix.9 Usually, low-grade mucinous carcinoma peritonei is related to low-grade appendix mucinous neoplasm (LAMN), whereas high-grade mucinous carcinoma peritonei related to appendiceal mucinous adenocarcinoma. Pathologically, transition from normal epithelium of the appendix to LAMN/HAMN/mucinous adenocarcinoma could provide direct histological evidence for PMP originating from the appendix.10−12
Metastatic ovarian mucinous tumors are complex and vary by exhibiting pathological morphology of multi-directional differentiation, from benign, borderline, to malignant evolution. In female patients with PMP, the appendix and ovary could be involved simultaneously or successively. Because of no exploration or previous operation of the appendix, PMP cases were often misdiagnosed and mistreated as primary ovarian neoplasms. Based on extensive specimen collection of appendices in combination with comprehensive evaluation of medical history, most cases originated from the appendix.13 NCCN has pointed out that primary malignant ovarian mucinous tumors were very rare. It is highly recommended to make comprehensive evaluation of the upper or lower digestive tract to exclude metastasis. Conventional appendectomy is necessary for ovarian mucinous tumors (even if the appendix was grossly normal). However, only a few studies focused on clinicopathological features of PMPs originating from ovaries. Some PMPs originate from teratoma or result from preoperative spontaneous rupture.14–18
Histological morphology,4 such as tall mucin-rich columnar epithelial cells, irregular budding glands, and contracted space around glands, suggests appendiceal origin. In this study, three PMP cases were teratoma-associated ovarian mucinous tumors. These tumors had distinctive histological features involving hypermucinous epithelial cells, scalloped glands, and subepithelial stromal clefts, very similar to classical appendiceal origin. The association of mucinous cystadenomas with teratoma might represent a malignant transformation in the gastrointestinal epithelium of germ-cell origin.
The rest of the PMPs were purely primary ovarian mucinous tumors with preoperative spontaneous rupture or undergoing intraoperative rupture and implantation, which led to mucus dissemination in abdominal and pelvic cavities. Occasionally, rupture of cysts could be obvious under a microscope. Scalloped glands, subepithelial stromal clefts, and histiocyte aggregates were not prominent, however, reactive ovarian-type stroma was more significant, while stromal invasion was absent.
In this study, the majority of PMPs of ovarian origin were acellular mucin or low-grade mucinous carcinoma peritonei (75%). The prognosis of ovarian origin was better than the appendix or colorectum (85.7 vs 72.8 or 34.0 months, P=0.002) during long-term postoperative follow-up according to a previous study in a large patient population of PMPs.19 Surgeons should pay more attention to avoid potential rupture of iatrogenic myxomatous tumor and intraoperative colloidal mucus dissemination in abdominal and pelvic cavities. Rupture of ovarian mucinous tumors can occur without any abdominal symptoms or only with painless abdominal distention.
Immunohistochemical markers CA125, ER, PR, Pax8, and WT1 were expressed to different degrees in primary epithelial ovarian tumors. Most of them failed to distinguish a specific origin of PMP.20 CEA or Villin had low specificity and limited value in identifying original tumor. We used serial makers (CK7, CK20, CDX2, and SATB2) to make a differential diagnosis. SATB2 is a highly specific marker for colorectal and appendiceal tumors. It has a highly specific nuclear expression pattern in malignancies located in the lower gastrointestinal tract.21 SATB2 was negative in primary ovarian mucinous neoplasms (except for teratomas), which could aid in distinguishing metastatic ovarian mucinous neoplasms from primary ovarian mucinous lesions.22–25 In our study, one PMP case originating from purely ovarian mucinous tumor was focal positive for SATB2, probably due to limited material acquisition (insufficient sampling) or teratomatous components.
To sum up, to clarify accurate origin of complicated PMP remains challenging. For PMP patients indicated by imaging, abdominal puncture or laparotomy, appendectomy, and exploration of bilateral ovaries should be performed routinely. Extensive multiple lesions of ovarian tumors should be sectioned and all suspicious appendiceal tissues should be sampled for definite diagnosis. Simultaneously, previous medical history and histopathological sections, intraoperative conditions and histomorphology should be comprehensively analyzed. Scalloped glands, subepithelial clefts, cellular stroma, and histiocyte aggregates in combination with immunohistochemical markers (eg, CK7, CK20, CDX2, and SATB2) may be useful parameters to help distinguish the origin of PMP and to evaluate biological behavior of PMP from different origins (except teratoma-associated tumors).
Declaration of Patient Consent
The authors certify that they have obtained all appropriate patient consent forms. The study protocol was approved by the Medical Ethics Committee of Beijing Shijitan Hospital, Capital Medical University (Beijing, China).
Funding
This project was supported by grants from the Beijing Municipal Natural Science Foundation, China (7172108). We would like to thank all the participants for their contribution to this research project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia the results of the PSOGI. Am J Surg Pathol. 2016;40:14–26. doi:10.1097/PAS.0000000000000535
2. Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. 2017;33:511–519. doi:10.1080/02656736.2017.1310938
3. Ronnett BM, Shmookler BM, Sugarbaker PH, et al. Pseudomyxoma peritonei: new concepts in diagnosis, origin, nomenclature, and relationship to mucinous borderline (low malignant potential) tumors of the ovary. Anat Pathol. 1997;2:197–226.
4. Stewart CJ, Ardakani NM, Doherty DA, et al. An evaluation of the morphologic features of low-grade mucinous neoplasms of the appendix metastatic in the ovary, and comparison with primary ovarian mucinous tumors. Int J Gynecol Pathol. 2014;33:1–10. doi:10.1097/PGP.0b013e318284e070
5. van Ruth S, Acherman YI, van de Vijver MJ, et al. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol. 2003;29:682–688. doi:10.1016/s0748-7983(03)00149-5
6. Pai RK, Beck AH, Norton JA, et al. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol. 2009;33:1425–1439. doi:10.1097/PAS.0b013e3181af6067
7. Agrawal AK, Bobinski P, Grzebieniak Z, et al. Pseudomyxoma peritonei originating from urachus-case report and review of the literature. Curr Oncol. 2014;21:e155–165. doi:10.3747/co.21.1695
8. Hackeng WM, de Guerre L, Kuypers KC, et al. Pseudomyxoma peritonei after a total pancreatectomy for intraductal papillary mucinous neoplasm with colloid carcinoma in lynch syndrome. Pancreas. 2019;48:135–138. doi:10.1097/MPA.0000000000001201
9. Szych C, Staebler A, Connolly DC, et al. Molecular genetic evidence supporting the clonality and appendiceal origin of Pseudomyxoma peritonei in women. Am J Pathol. 1999;154:1849–1855. doi:10.1016/S0002-9440(10)65442-9
10. Dong Y, Li T, Zou W, et al. [Pseudomyxoma peritonei: report of 11 cases with a literature review]. Zhonghua Bing Li Xue Za Zhi. 2002;31:522–525.
11. Guo AT, Song X, Wei LX, et al. Histological origin of pseudomyxoma peritonei in Chinese women: clinicopathology and immunohistochemistry. World J Gastroenterol. 2011;17:3531–3537. doi:10.3748/wjg.v17.i30.3531
12. Kohler F, Rosenfeldt M, Matthes N, et al. Incidental finding of mucinous neoplasia of the appendix: treatment strategies. Chirurg. 2019;90:194–201. doi:10.1007/s00104-018-0768-1
13. Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol. 2000;24:1447–1464. doi:10.1097/00000478-200011000-00001
14. Shen DH, Ng TY, Khoo US, et al. Pseudomyxoma peritonei–a heterogenous disease. Int J Gynaecol Obstet. 1998;62:173–182. doi:10.1016/s0020-7292(98)00095-2
15. Liu L, Sun L, Wang J, et al. Ovarian cystadenocarcinoma and pseudomyxoma peritonei. BMJ Case Rep. 2010;2010. doi:10.1136/bcr.06.2008.0137
16. Mandal S, Kawatra V, Khurana N. Mucinous cystadenocarcinoma arising in mature cystic teratoma ovary and associated pseudomyxoma peritonei: report of a case. Arch Gynecol Obstet. 2008;278:265–267. doi:10.1007/s00404-008-0579-6
17. Stewart CJ, Tsukamoto T, Cooke B, et al. Ovarian mucinous tumour arising in mature cystic teratoma and associated with pseudomyxoma peritonei: report of two cases and comparison with ovarian involvement by low-grade appendiceal mucinous tumour. Pathology. 2006;38:534–538. doi:10.1080/00313020601024078
18. McKenney JK, Soslow RA, Longacre TA. Ovarian mature teratomas with mucinous epithelial neoplasms: morphologic heterogeneity and association with pseudomyxoma peritonei. Am J Surg Pathol. 2008;32:645–655. doi:10.1097/PAS.0b013e31815b486d
19. Yan F, Lin Y, Zhou Q, et al. Pathological prognostic factors of pseudomyxoma peritonei: comprehensive clinicopathological analysis of 155 cases. Hum Pathol. 2020;97:9–18. doi:10.1016/j.humpath.2019.12.008
20. Gleeson EM, Feldman R, Mapow BL, et al. Appendix-derived pseudomyxoma peritonei (PMP): molecular profiling toward treatment of a rare malignancy. Am J Clin Oncol. 2018;41:777–783. doi:10.1097/COC.0000000000000376
21. Magnusson K, de Wit M, Brennan DJ, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35:937–948. doi:10.1097/PAS.0b013e31821c3dae
22. Perez Montiel D, Arispe Angulo K, Cantu-de Leon D, et al. The value of SATB2 in the differential diagnosis of intestinal-type mucinous tumors of the ovary: primary vs metastatic. Ann Diagn Pathol. 2015;19:249–252. doi:10.1016/j.anndiagpath.2015.05.004
23. Moh M, Krings G, Ates D, et al. SATB2 expression distinguishes ovarian metastases of colorectal and appendiceal origin from primary ovarian tumors of mucinous or endometrioid type. Am J Surg Pathol. 2016;40:419–432. doi:10.1097/PAS.0000000000000553
24. Yang C, Sun L, Zhang LX, et al. Diagnostic utility of SATB2 in metastatic krukenberg tumors of the ovary: an immunohistochemical study of 70 cases with comparison to CDX2, CK7, CK20, chromogranin, and synaptophysin. Am J Surg Pathol. 2018;42:160–171. doi:10.1097/PAS.0000000000000951
25. Simons M, Simmer F, Bulten J, et al. Two types of primary mucinous ovarian tumors can be distinguished based on their origin. Mod Pathol. 2020;33:722–733. doi:10.1038/s41379-019-0401-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.