Back to Journals » Cancer Management and Research » Volume 12
Clinicopathological Characteristics and Treatment Outcomes of Pregnancy Complicated by Malignant Ovarian Germ Cell Tumors
Authors Zong X, Yang JX , Zhang Y, Cao DY, Shen K
Received 2 December 2019
Accepted for publication 13 February 2020
Published 24 February 2020 Volume 2020:12 Pages 1347—1354
DOI https://doi.org/10.2147/CMAR.S240793
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Xuan Zong, Jia-Xin Yang, Ying Zhang, Dong-Yan Cao, Keng Shen
Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Jia-Xin Yang
Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuaifuyuan, Dongcheng District, Beijing, People’s Republic of China
Tel +86 10 6915 4022
Fax +86 10 6915 5635
Email [email protected]
Purpose: This study aimed to analyze the clinicopathological features, treatment, and feto-maternal outcomes of pregnancy complicated by malignant ovarian germ cell tumors (MOGCTs), to increase the awareness on this condition.
Patients and Methods: We retrospectively reviewed the medical records of patients diagnosed with MOGCTs during pregnancy, who were treated and followed-up at Peking Union Medical College Hospital from January 2000 to December 2017. The demographic characteristics, pathological features, treatment and prognosis were analyzed.
Results: The histological subtypes varied in 14 patients (dysgerminoma, n=1; immature teratoma, n=4; yolk sac tumor, n=6; and mixed germ cell tumors, n=3). Ten (71.4%) patients, including three who opted for conservative therapy until childbirth, one who only received salvage chemotherapy during pregnancy, and six who underwent cystectomy or unilateral salpingo-oophorectomy during pregnancy, desired fetal preservation. After undergoing surgery, four patients chose surveillance instead of timely adjuvant chemotherapy. Eight patients delivered their babies, and the preterm delivery rate was 50.0%. One newborn died of premature birth. The median follow-up period was 44 (range: 13 to 86) months. During the current study period, 12 patients had survived and did not report any diseases. However, two died due to disease progression.
Conclusion: Pregnant women with MOGCTs had favorable outcomes. However, when a malignant tumor is suspected, surgery cannot be avoided. Thus, instead of timely postoperative adjuvant chemotherapy, close surveillance may be an acceptable alternative for pregnant women with low-risk MOGCTs.
Keywords: pregnancy, germ cell tumor, expectant management, retrospective studies
Introduction
The occurrence of ovarian cancer in pregnant women is extremely rare, with a rate of approximately 0.2–3.8 per 100,000 pregnancies, and the incidence may increase with the rising trend of childbirth at a late maternal age.1–3 Generally, malignant ovarian germ cell tumors (MOGCTs) only account for 3% of all ovarian tumors. However, the proportion of women whose pregnancy is complicated by MOGCTs is increasing, due to the early age of onset. MOGCTs is characterized by heterogeneous histological subtypes and chemotherapy sensitivity.4 Almost all types of MOGCTs, except stage Ⅰdysgerminoma and stage ⅠgradeⅠA immature teratoma, are managed with timely postoperative chemotherapy.5 With chemotherapy treatment, the rate of survival in cases of MOGCTs has significantly improved. However, the side effects of certain chemotherapy drugs for conditions, such as pulmonary fibrosis and ovarian failure, can cause permanent injury. Although in-utero chemotherapy exposure is not associated with a higher rate of neonatal mortality, it might increase the risk of other complications, such as preterm delivery and small for gestational age.6–8 Thus, chemotherapy during pregnancy should be managed cautiously when considering potential harm to the fetus. Most previous studies are case reports, and a standardized guideline has not been established due to the lack of knowledge on this rare condition. In this study, we reviewed the clinical courses of 14 patients, with a focus on the treatment options for MOGCTs in pregnant women to minimize harm to the fetus without compromising the prognosis of the patient. In addition, we also reviewed relevant studies to increase our knowledge on the management of this rare condition.
Materials and Methods
We retrospectively reviewed the medical records of 447 patients diagnosed with MOGCTs from January 1, 2000 and December, 31, 2017 at Peking Union Medical College Hospital (PUMCH). Thereafter, 14 cases of MOGCTs were confirmed via pathological examination and detected during pregnancy or delivery in our hospital. The exclusion criteria included normal, non-pregnant women and/or those with primary tumors with other non-germ cell tumor histological components. This study was approved by the Ethics Committee of PUMCH and was conducted in accordance with the Helsinki Declaration. A written informed consent was obtained from all patients.
The clinicopathologic characteristics of the participants were age at diagnosis, gravidity, parity, gestational age, levels of serum tumor markers (alpha-fetoprotein [AFP] and cancer antigen 125 [CA125]) at diagnosis, histological subtype, tumor size, and International Federation of Gynecology and Obstetrics (FIGO) stage. The obstetric outcomes were gestational age at delivery, route of delivery, and neonatal complications. Meanwhile, the oncological management and maternal outcomes included timing and type of surgery for the primary tumor, administration of chemotherapy, recurrence of disease, follow-up period, and survival. All patients underwent fertility-sparing surgery with or without fetal conservation.
All the patients were followed-up either through the Outpatient Surveillance System of PUMCH or via telephone interviews until June 2019. Overall survival (OS) was defined as the interval between the date of diagnosis and date of death or send date of the study. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 23.0 statistical (IBM Corporation, Armonk, NY, the USA).
Results
Clinicopathological Characteristics of the Patients
Tables 1 and 2 depict the clinicopathological characteristics of the patients. The mean age was 27.5 (range: 18–34) years, and there were 12 (85.7%) nulliparous patients. Ultrasonic examination of all patients was conducted to obtain the diagnosis, and the tumor was diagnosed in the first, second, and third trimester in 10, 3, and 1 patient, respectively. The histological subtypes varied in 14 patients (dysgerminoma, n=1; immature teratoma, n=4; yolk sac tumor, n=6; and mixed germ cell tumors, n=3). Six patients with pure yolk sac tumor had high serum AFP levels (> 1000 ng/mL). Meanwhile, the other patients had normal or slightly elevated serum AFP levels (about 100 ng/mL). In 10 patients, data on CA125 values (range: 8.8–702 U/mL) were available, and only one patient had a normal CA125 level. One patient presented with disease recurrence; and in the remaining 13 patients who were first diagnosed during pregnancy, all tumors were unilateral, and the tumor in 11 of 13 (84.6%) patients was confined to the ovary. Eight (61.5%) tumors ruptured; of these, six ruptured spontaneously, and two during surgery. Four patients underwent systematic lymph node dissection. However, only one presented with a histological metastasis of para-aortic lymph node.
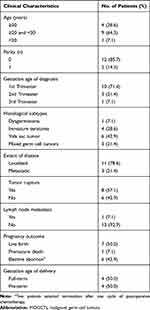 |
Table 1 Patient Clinicopathological Characteristics and Obstetric Outcomes |
 |
Table 2 Clinical Details and Treatments of Cases in Present Study |
Tumor Treatment and Patient Outcomes
Treatments during pregnancy and patient outcomes are summarized in Table 2. Six patients underwent surgery with fetal conservation for the primary tumor in the second trimester of pregnancy, and half of the patients had laparoscopic surgery. Moreover, they underwent unilateral salpingo-oophorectomy or cystectomy instead of comprehensive staging, and the occurrence of metastasis or residual diseases was not observed. Only two of six patients received adjuvant chemotherapy. Four patients refused further treatment, and the surveillance time during pregnancy ranged from 11 to 25 weeks. Two patients had full-term delivery with no additional postpartum tumor treatments and recurrence. However, the other two patients presented with disease recurrence at 24 and 35 weeks of gestation. Case 7 received three cycles of cisplatin, etoposide, and bleomycin (PEB) chemotherapy and underwent elective cesarean and secondary cytoreduction procedure at 35 weeks of gestation. Case 14 insisted on close surveillance until full term. Both patients (cases 7 and 14) received salvage therapy with secondary cytoreductive surgery and chemotherapy, and the overall survival times were 80 and 64 months.
Three patients underwent primary oncological surgery at the time of cesarean delivery in the third trimester. Two patients (cases 1 and 2) were diagnosed with ovarian tumor earlier. However, both chose surveillance until full term or nearly full term. The duration of expectant management was 21 and 15 weeks, and the size of the tumor increased from 7 to 16 and 9 to 20 cm. In case 12, a pelvic mass was detected during the 36th week of pregnancy, and she underwent emergency cesarean section and concurrent tumor debulking surgery. The FIGO stage of the tumor was ⅢC, and the effect of primary cytoreduction surgery was optimal. The patients received timely adjuvant chemotherapy. However, new scattered metastases were detected at 3 months after completion of the initial treatment, and the patient eventually died.
Case 11 completed primary tumor treatment at local hospital. The patient’s AFP level was normal before pregnancy, and tumor recurrence was detected in the second trimester. She was referred to our hospital and received one cycle of PEB chemotherapy because the hepatic metastatic tumor was large. Thereafter, the patient’s amniotic fluid was decreasing rapidly. Thus, emergency cesarean section was conducted at 35 weeks of gestation. The patients underwent salvage surgery and received chemotherapy after delivery. However, the serum AFP level increased again 3 months after the completion of treatment. She refused further treatment and died after 3 months.
As for primary tumor treatment, four (28.6%) patients underwent primary comprehensive surgical staging, and 10 (71.4%) received timely postoperative chemotherapy. Four patients received antenatal chemotherapy. The median follow-up period was 44 (range: 13–86) months. At the time of review, 12 (85.7%) patients survived and did not present with any disease, and four (28.6%) patients experienced recurrence. Of these patients, two patients died due to disease progression.
Obstetric and Neonatal Outcomes
Six (42.9%) patients, including two who finally decided to terminate the pregnancy after the primary surgery and after one cycle of PEB chemotherapy, chose elective termination. The remaining four patients chose elective abortion in the first trimester.
Eight (57.1%) patients delivered live newborns. Among them, two gave birth via vaginal delivery. The preterm birth rate was 50%. Neonatal malformations or complications were not observed in these singleton livebirths. However, three babies who were preterm were admitted to the neonatal intensive care unit (NICU). The other premature baby who was exposed to in-utero chemotherapy died within the first week of life (Table 2).
Discussion
Adnexal masses are often detected incidentally during pregnancy, and they are usually benign or functional. Malignant tumors only account for about 1–6% of all cases.9 Tumor marker levels and imaging findings (pelvic ultrasonography and magnetic resonance imaging) can be used in obtaining a differential diagnosis. Of note, the use of AFP and β-HCG as tumor markers is not recommended, as they are usually elevated during pregnancy. Both CA125 and human epididymis protein 4 (HE4) levels are elevated in the first trimester. However, the serum HE4 level is relatively normal during the entire pregnancy.10
The treatment options are based on the type of adnexal tumors. Surveillance is acceptable for benign tumors, and active surger is recommended if a malignant tumor is suspected. A histopathological diagnosis is obtained to guide the next step of treatment and to prevent tumor torsion or rupture.11,12
Surgery is indispensable in the management of MOGCTs. Operation during pregnancy is safe and is usually performed in the second trimester, when the risk of miscarriage is low and there is still space to operative.13 Previous studies have shown that the incidence of adverse fetal events (miscarriage, stillbirth, and preterm delivery) is significantly lower in laparoscopic surgery than in open surgery, and the duration of surgery and hospitalization is significantly shorter.14 However, laparoscopic surgery may lead to hypercapnia, uterine perforation, and decreased blood flow.15 Therefore, the operative time should be limited within 90–120 mins, and the abdominal pressure should be maintained around 10–13 mmHg. As for surgical methods, unilateral salpingo-oophorectomy is recommended for adnexal masses to prevent rupture during cystectomy.16
Chemotherapy is administered at 14 weeks of gestation. Previous studies have shown that the incidence rate of neonatal malformation in in-utero chemotherapy exposure group was similar to that in the general population. However, chemotherapy during pregnancy is not completely safe. Hann et al have conducted an analysis of 1170 pregnant patients with cancer, and results showed that chemotherapy during pregnancy might increase the risk of certain neonatal complications, such as small for gestational age and NICU admission.8 Moreover, the administration of chemotherapy increases maternal stress.17 In our series, four patients received chemotherapy during pregnancy. However, only one livebirth was recorded. Although the sample size was small, we cannot disregard the side effects of antenatal chemotherapy on both the fetus and mother.
There is a debate as to whether patients with early-stage MOGCTs should receive timely adjuvant chemotherapy. In the past few years, due to the long-term side effects of chemotherapy regimens, oncologists, particularly pediatric oncologists, have attempted to reduce the use of toxic treatments. Thus, the surgery-only approach is used for all stage I MOGCTs. The research results of this close surveillance conducted in different European medical centers have been successively reported. The recurrence rate is about 20–50%, and most patients with disease relapse can be salvaged. Moreover, complete remission is achieved.18–21 Gobel et al have analyzed and shown that recurrent MOGCTs usually have a yolk sac tumor component.20 Newton et al have first presented immature teratoma in adult patients with minimal response to chemotherapy. However, the condition could be well controlled with surgery alone. In addition, 25 chemo-naïve patients with FIGO stage I of other histological subtypes had long survival. However, 10 (40%) patients presented wit recurrence.22 Although this non-chemotherapy policy for early- stage MOGCTs is not universally accepted, it can provide new insights in managing pregnant women diagnosed with MOGCTs. Chemotherapy can be postponed in cases of pregnancy complicated by MOGCTs. Based on our literature review, seven patients did not want to receive chemotherapy after the initial tumor surgery (Table 3).23–29 All patients had livebirths, and three were full-term infants. Moreover, no neonatal complication was observed. Five patients finished adjuvant treatments after delivery. However, the other two were under close surveillance. Two patients experienced relapse during pregnancy. However, they were all salvaged by further treatments. Our retrospective study has indicated that expectant management can have favorable maternal and fetal outcomes. Four patients opted for surveillance after resection of the tumor, and three of these patients had livebirths.
 |
Table 3 Literature Review of Seven Cases of MOGCTs Postponing Chemotherapy During Pregnancy |
For patients with MOGCTs who are at high risk for recurrence, chemotherapy after delivery is required.5 Considering that chemotherapy may damage the ovaries, patients can undergo some fertility preservation procedures before chemotherapy, including cryopreservation of oocytes, embryos, or ovarian tissue.30 Oocyte harvested via in oocyte or embryo cryopreservation requires an ovarian stimulation, which may delay anticancer treatment. Recently, Ben-Haroush et al have reported about procedures, such as the direct small follicles aspiration during cesarean section and in vitro maturation (IVM) to mature oocyte.31 Meanwhile, other researchers have reported about live births after IVM and normal fertilization.32–34 This result indicates that pregnant women with MOGCTs have options for the preservation for their fertility.
One of the most common complications of cancer in pregnant women is preterm delivery, and the rate is up to 50%. In our study, the incidence rate of premature birth was 50%, and this result is consistent with that of previous reports. Thus, special neonatal follow-up management is required for preterm infants.35 The administration of cisplatin during pregnancy may cause irreversible damage to fetal hearing.36 However, long-term follow-up data about pediatric outcomes after in-utero chemotherapy exposure are not available.
Conclusion
The management of pregnancy complicated by MOGCTs is challenging, as it requires the cooperation of a multidisciplinary team composed of obstetricians, gynecologists, and neonatologists. To date, no standardized guidance has been established. To confirm the diagnosis of a suspected malignant tumor, surgery cannot be avoided. However, instead of timely postoperative adjuvant chemotherapy, close surveillance may be an acceptable option for patients with MOGCTs during pregnancy.
Consent
The project and consent process were approved by the Ethics Committee of Peking Union Medical College Hospital, Beijing. The written informed consent was obtained from participants.
Acknowledgment
This study was supported by the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-12M).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006;101(2):315–321. doi:10.1016/j.ygyno.2005.10.022
2. Machado F, Vegas C, Leon J, et al. Ovarian cancer during pregnancy: analysis of 15 cases. Gynecol Oncol. 2007;105(2):446–450. doi:10.1016/j.ygyno.2007.01.002
3. Aggarwal P, Kehoe S. Ovarian tumours in pregnancy: a literature review. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):119–124. doi:10.1016/j.ejogrb.2010.11.023
4. Brown J, Friedlander M, Backes FJ, et al. Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S48–S54. doi:10.1097/IGC.0000000000000223
5. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17(8):896–909. doi:10.6004/jnccn.2019.0039
6. Van Calsteren K, Heyns L, De Smet F, et al. Cancer during pregnancy: an analysis of 215 patients emphasizing the obstetrical and the neonatal outcomes. J Clin Oncol. 2010;28(4):683–689. doi:10.1200/JCO.2009.23.2801
7. Amant F, Vandenbroucke T, Verheecke M, et al. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373(19):1824–1834. doi:10.1056/NEJMoa1508913
8. de Haan J, Verheecke M, Van Calsteren K, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19(3):337–346. doi:10.1016/S1470-2045(18)30059-7
9. Hoover K, Jenkins TR. Evaluation and management of adnexal mass in pregnancy. Am J Obstet Gynecol. 2011;205(2):97–102. doi:10.1016/j.ajog.2011.01.050
10. Han SN, Lotgerink A, Gziri MM, Van Calsteren K, Hanssens M, Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: a systematic review. BMC Med. 2012;10:86. doi:10.1186/1741-7015-10-86
11. Froyman W, Landolfo C, De Cock B, et al. Risk of complications in patients with conservatively managed ovarian tumours (IOTA5): a 2-year interim analysis of a multicentre, prospective, cohort study. Lancet Oncol. 2019;20(3):448–458. doi:10.1016/S1470-2045(18)30837-4
12. Resapu P, Rao Gundabattula S, Bharathi Bayyarapu V, Pochiraju M, Surampudi K, Dasari S. Adnexal torsion in symptomatic women: a single-centre retrospective study of diagnosis and management. J Obstet Gynaecol. 2019;39(3):349–354. doi:10.1080/01443615.2018.1494702
13. Mazze RI, Kallen B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178–1185. doi:10.1016/0002-9378(89)90659-5
14. Shigemi D, Aso S, Matsui H, Fushimi K, Yasunaga H. Safety of laparoscopic surgery for benign diseases during pregnancy: a nationwide retrospective cohort study. J Minim Invasive Gynecol. 2019;26(3):501–506. doi:10.1016/j.jmig.2018.06.008
15. Ye P, Zhao N, Shu J, et al. Laparoscopy versus open surgery for adnexal masses in pregnancy: a meta-analytic review. Arch Gynecol Obstet. 2019;299(3):625–634. doi:10.1007/s00404-018-05039-y
16. Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol. 2019;30(10):1601–1612. doi:10.1093/annonc/mdz228
17. Mielcarek P, Nowicka-Sauer K, Kozaka J. Anxiety and depression in patients with advanced ovarian cancer: a prospective study. J Psychosom Obstet Gynaecol. 2016;37(2):57–67. doi:10.3109/0167482X.2016.1141891
18. Baranzelli MC, Bouffet E, Quintana E, Portas M, Thyss A, Patte C. Non-seminomatous ovarian germ cell tumours in children. Eur J Cancer. 2000;36(3):376–383. doi:10.1016/S0959-8049(99)00317-2
19. Mann JR, Raafat F, Robinson K, et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol. 2000;18(22):3809–3818. doi:10.1200/JCO.2000.18.22.3809
20. Gobel U, Calaminus G, Schneider DT, Koch S, Teske C, Harms D. The malignant potential of teratomas in infancy and childhood: the MAKEI experiences in non-testicular teratoma and implications for a new protocol. Klin Padiatr. 2006;218(6):309–314. doi:10.1055/s-2006-942275
21. Billmire DF, Cullen JW, Rescorla FJ, et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children’s Oncology Group. J Clin Oncol. 2014;32(5):465–470. doi:10.1200/JCO.2013.51.1006
22. Newton C, Murali K, Ahmad A, et al. A multicentre retrospective cohort study of ovarian germ cell tumours: evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur J Cancer. 2019;113:19–27. doi:10.1016/j.ejca.2019.03.001
23. Sayedur Rahman M, Al-Sibai MH, Rahman J, et al. Ovarian carcinoma associated with pregnancy. A review of 9 cases. Acta Obstet Gynecol Scand. 2002;81(3):260–264. doi:10.1034/j.1600-0412.2002.810313.x
24. Shimizu Y, Komiyama S, Kobayashi T, Nakata K, Iida T. Successful management of endodermal sinus tumor of the ovary associated with pregnancy. Gynecol Oncol. 2003;88(3):447–450. doi:10.1016/S0090-8258(02)00075-6
25. Aoki Y, Higashino M, Ishii S, Tanaka K. Yolk sac tumor of the ovary during pregnancy: a case report. Gynecol Oncol. 2005;99(2):497–499. doi:10.1016/j.ygyno.2005.06.022
26. Mekaru K, Kamiyama S, Masamoto H, et al. Squamous cell carcinoma arising in an ovarian mature cystic teratoma complicating pregnancy: a case report. Arch Gynecol Obstet. 2008;278(3):287–290. doi:10.1007/s00404-008-0573-z
27. Pafilis I, Haidopoulos D, Rodolakis A, et al. Management of a pregnancy complicated by yolk sac tumor. Arch Gynecol Obstet. 2009;280(5):803–806. doi:10.1007/s00404-009-0977-4
28. Budiman HD, Burges A, Ruhl IM, Friese K, Hasbargen U. Squamous cell carcinoma arising in a dermoid cyst of the ovary in pregnancy. Arch Gynecol Obstet. 2010;281(3):535–537. doi:10.1007/s00404-009-1193-y
29. Mendivil AA, Brown JV
30. Fisch B, Abir R. Female fertility preservation: past, present and future. Reproduction. 2018;156(1):F11–F27. doi:10.1530/REP-17-0483
31. Ben-Haroush A, Abir R, Sapir O, Garor R, Fisch B. Aspiration of immature oocytes during cesarean section for fertility preservation. J Matern Fetal Neonatal Med. 2017;30(17):2112–2114. doi:10.1080/14767058.2016.1238895
32. Prasath EB, Chan ML, Wong WH, et al. First pregnancy and live birth resulting from cryopreserved embryos obtained from in vitro matured oocytes after oophorectomy in an ovarian cancer patient. Hum Reprod. 2014;29(2):276–278. doi:10.1093/humrep/det420
33. Segers I, Mateizel I, Van Moer E, et al. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–1231. doi:10.1007/s10815-015-0528-9
34. Uzelac PS, Delaney AA, Christensen GL, Bohler HC, Nakajima ST. Live birth following in vitro maturation of oocytes retrieved from extracorporeal ovarian tissue aspiration and embryo cryopreservation for 5 years. Fertil Steril. 2015;104(5):1258–1260. doi:10.1016/j.fertnstert.2015.07.1148
35. Kodama M, Grubbs BH, Blake EA, et al. Feto-maternal outcomes of pregnancy complicated by ovarian malignant germ cell tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;181:145–156. doi:10.1016/j.ejogrb.2014.07.047
36. Geijteman EC, Wensveen CW, Duvekot JJ, van Zuylen L. A child with severe hearing loss associated with maternal cisplatin treatment during pregnancy. Obstet Gynecol. 2014;124(2Pt 2 Suppl 1):454–456. doi:10.1097/AOG.0000000000000389
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
