Back to Journals » OncoTargets and Therapy » Volume 10
Clinicopathological characteristics and prognostic value of cancer stem cell marker CD133 in breast cancer: a meta-analysis
Authors Li Z, Yin SC, Zhang L, Liu WG, Chen B , Xing H
Received 14 October 2016
Accepted for publication 2 January 2017
Published 14 February 2017 Volume 2017:10 Pages 859—870
DOI https://doi.org/10.2147/OTT.S124733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Faris Farassati
Zhan Li,1,* Songcheng Yin,2,* Lei Zhang,1 Weiguang Liu,1 Bo Chen,1 Hua Xing3
1Department of Breast Surgery, 2Department of Surgical Oncology, The First Hospital of China Medical University, Shenyang, Liaoning, 3Department of Breast Surgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin, People’s Republic of China
*These authors contributed equally to this work
Background: The association of CD133 overexpression with clinicopathological significance and prognosis in patients with breast cancer remains controversial. We thus performed a meta-analysis to evaluate the role of CD133 expression in the development and prognosis of breast cancer.
Methods: The databases PubMed, Embase, and Cochrane Library (updated to August 1, 2016) were searched. Pooled odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (95% CI) were used to evaluate the impact of CD133 expression on clinicopathological features, overall survival, and disease-free survival.
Results: A total of 1,734 patients from 13 studies were subject to final analysis. The results showed a significant association between overexpression of CD133 and estrogen receptor status (OR 0.35, 95% CI 0.18–0.70), progesterone receptor status (OR 0.56, 95% CI 0.43–0.74), human epidermal growth factor-2 status (OR 1.81, 95% CI 1.33–2.45), lymph node metastasis (OR 1.98, 95% CI 1.34–2.92), and tumor histological grade (OR 1.79, 95% CI 1.26–2.54) in breast cancer. However, no significant correlation was found between upregulation of CD133 expression and onset age (OR 1.03, 95% CI 0.70–1.53) or tumor size (OR 1.29, 95% CI 0.80–2.09). Moreover, CD133-positive breast cancer patients had a higher risk of mortality (HR 1.91, 95% CI 1.21–3.03) and disease progression (HR 2.70, 95% CI 1.05–6.95).
Conclusion: This meta-analysis suggested that CD133 might be a predictor of clinical outcomes as well as prognosis and could be a potentially new gene therapy target for breast cancer patients.
Keywords: CD133, CSCs, breast cancer, prognosis, biomarker, meta-analysis
Introduction
Breast cancer is the most commonly occurring malignant tumor in women, with ~1.67 million new cases (25% of all cancers) diagnosed worldwide in 2012. It is the most frequent cause of cancer death (522,000 deaths, 14.7% of total) in females.1 From the time that distinct molecular subtypes were proposed by Perou et al in 2000,2 the combination of traditional pathological morphological classification and molecular subtyping has been applied to determine the optimal therapy for breast cancer patients. However, the prognosis of breast cancer patients remains unsatisfactory. Consequently, it is critical to predict prognosis through novel biomarkers that can serve as potential therapeutic targets in breast cancer patients.
There is a growing realization that a small subpopulation of cells with stem cell-like features resides in the tumor tissue and is known as cancer stem cells (CSCs).3 Their activity is achieved by self-renewal, unlimited proliferation and differentiation potential, and high tumorigenicity.4 Recently, it has been found that CSCs have similar specific cell surface molecular markers to stem cells such as CD44, CD24, ALDH1, and CD133. CD133, which is known as prominin-1, a pentaspan transmembrane cell surface glycoprotein with a molecular weight of 120 kDa, is located in plasma membrane protrusions. It was initially considered to be a marker of hematopoietic stem cells by Yin et al.5 Biological functions of CD133 include tumor initiation, cellular migration, vasculogenic mimicry, and drug resistance.6 Although CD133 has been studied intensely in various types of solid tumors, including lung cancer,7 renal cancer,8 esophageal carcinoma,9 and gastric cancer,10 the role of CD133 in breast cancer has not been verified.
In this meta-analysis, we aimed to evaluate the relationship between CD133 expression in breast cancer and clinicopathological features, including tumor size, lymph node metastasis, histological grade, onset age, receptor status (estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor-2 [HER2]) as well as prognostic significance.
Methods
Literature search
The literature in the following electronic databases – PubMed, Embase, and the Cochrane Library (updated to August 1, 2016) – was systematically searched. We performed our search using the medical subject heading (MeSH) term “CD133” and its synonyms: “fudenine”, “prominin”, “PROML1”, and “AC141 antigen”. These keywords were then combined with “breast”, “mammary”, “cancer”, “neoplasm”, “carcinoma”, “prognosis”, and “survival” using the Boolean “OR” term or the Boolean “AND” term.
Study selection criteria
Study selection inclusion criteria were as follows: 1) patients diagnosed with breast cancer using pathological and histological examinations, 2) full text and published in English, 3) clinicopathological and survival (overall survival [OS] and disease-free survival [DFS]) outcomes were recorded, 4) CD133 expression was detected in primary breast tumors, and 5) outcomes were recorded using odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs).
Exclusion criteria were as follows: 1) meeting abstracts, comments, case reports, reviews, and meta-analyses; 2) experiments on cell lines and animals; 3) metastatic or recurrent cancer; and 4) duplicate studies.
Quality assessment
The selected cohort studies were analyzed from three perspectives, selection, comparability, and outcomes, by two investigators independently, according to the Newcastle–Ottawa scale (NOS). The details of NOS table are shown in Table S1.
Data extraction
The following details were extracted using a predefined form: first author’s name, publication year, country, mean age, tumor stage, total number of included patients, median follow-up time, cutoff value, survival outcome, outcome method, and estimated HR.
Statistical analysis
This meta-analysis was performed using Stata Version 12.0 (Stata Corporation, College Station, TX, USA). For the pooled analysis of clinicopathological features, OR was evaluated. HR was applied as a measure of the prognostic value. Study heterogeneity was evaluated using the chi-square-based Q test and I2 statistic. Studies with an I2>50% or a P<0.05 was considered to have significant heterogeneity, and a random-effects model test was conducted. Otherwise, the fixed-effects model test was selected. Sensitivity analysis was performed to evaluate the stability of the pooled results. Publication bias was assessed using Begg’s funnel plots and Egger’s test. All P-values were two-sided and P<0.05 was considered statistically significant.
Results
Search results
A total of 424 citations were potentially identified for inclusion using the described search strategies. Through reviewing the title and abstracts, 381 papers were excluded. We then systematically read the full text of the remaining 43 articles and filtered out an additional 30 papers. Among the excluded papers, 13 studies were experimental studies, six studies were not correlated with target protein, three studies had overlapped data with other published trials, six studies had no sufficient survival data to analyze, and two studies are reviews. Ultimately, 13 studies11–23 were included (Figure 1).
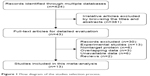  | Figure 1 Flow diagram of the studies selection process. |
Characteristics of included studies
The details of 13 included studies selected from the literature search are summarized in Table 1. In total, 13 eligible articles with 1,734 patients were analyzed for clinicopathological features, and five qualified studies with 879 patients were analyzed for survival outcomes. These cohort studies were conducted in eight regions (Italy, Taiwan, China, New Zealand, Japan, Turkey, Egypt, and Korea) and were published between 2009 and 2016 with a mean patient age ranging from 45.6 years to 61.8 years. Univariate analysis was applied for the survival data.
Meta-analysis of clinicopathological parameters
CD133 expression and ER, PR, and HER2 status
The pooled ORs indicated that overexpression of CD133 was significantly associated with ER status (positive vs negative: OR 0.35, 95% CI 0.18–0.70; Figure 2A), PR status (positive vs negative: OR 0.56, 95% CI 0.43–0.74; Figure 2B), and HER2 status (≥2+ vs 1+, OR 1.81, 95% CI 1.33–2.45; Figure 2C). In the subgroup analysis of ER status, there were no significant heterogeneity in the group of non-Asian (I2=11.1%, P=0.289) and group of mean age was >50 years (I2=0.0%, P=0.689). The details are shown in Table 2.
CD133 expression and age, tumor, node, and grade
Our results showed that there was no significant association between CD133 high expression and onset age (≥50 vs <50 OR 1.03, 95% CI 0.70–1.53; Figure 3A) and tumor size (≥2 cm vs <2 cm, OR 1.29, 95% CI 0.80–2.09; Figure 3B). However, breast cancer with CD133 expression was associated with lymph node metastasis (positive vs negative: OR 1.98, 95% CI 1.34–2.92; Figure 3C) and tumor histological grade (III vs I–II: OR 1.79, 95% CI 1.26–2.54; Figure 3D). We further performed subgroup analysis from three aspects: region, sample size, and mean age. The results showed that in the group of sample size >150, there was a significant correlation between CD133 expression and tumor size (pooled OR 1.70, 95% CI 1.04–2.79). The details of subgroup analysis are shown in Table 2.
Meta-analysis of OS and DFS
The overall analysis of five studies revealed that CD133-positive breast cancer patients had a higher risk of mortality (pooled HR 1.91, 95% CI 1.21–0.03; Figure 4A) with heterogeneity (I2=62.7%, P=0.03). Meanwhile, the pooled results of four studies showed that increased CD133 expression in breast cancer patients had poorer DFS (pooled HR 2.70, 95% CI 1.05–6.95, Figure 4B) with significant heterogeneity (I2=85.3%, P=0.04). We further performed subgroup analysis according to region and sample size. In subgroup analysis of region, we found that there was a different trend between the Asian and non-Asian groups. Patients in the Asian group with tumors that showed high expression of CD133 tended to have a poorer OS (HR 2.22, 95% CI 1.38–3.57; Figure 4A) and DFS (HR 4.01, 95% CI 1.83–8.79; Figure 4B), while there was no significant association between high-level CD133 expression and OS (HR 0.78, 95% CI 0.32–1.93; Figure 4A) or DFS (HR 0.80, 95% CI 0.37–1.73; Figure 4B) in the non-Asian group. The details of subgroup analysis are shown in Table 2.
Sensitivity analysis and publication bias
We further performed sensitivity analysis to gauge the stability of our results with respect to clinicopathological characteristics as well as OS and DFS. The plots illustrated the robustness of our results because excluding any single study did not significantly influence pooled ORs or HRs (Figure 5). Egger’s test and Begg’s funnel plots were used to assess publication bias in this meta-analysis. Both the tests indicated that there was no publication bias for pooled ER (PEgger=0.654), PR (PEgger=0.310), HER2 (PEgger=0.560), age (PEgger=0.784), tumor size (PEgger=0.263), lymph node metastasis, (PEgger=0.523), or histological grade (PEgger=0.166) as well as OS (PEgger=0.806) or DFS (PEgger=0.308).
Discussion
CSCs have been a hot topic of debate in the field of malignant tumor biology since its fundamental theory was put forward. It has been considered that the tumor is composed of tumor cells and CSCs, which are a rare subpopulation of cells in solid tumors with the capability of self-renewal, differentiation potential, and initiating tumors.4,24 CSCs are at the root of tumor formation that can lead to various degrees of differentiation and are the source that enables the tumor to keep growing and spreading.25 This recognition of the importance of CSCs in tumors has not only led to new directions and perspectives that resulted in a reexamination of the causes of tumor initiation, development, and therapeutic resistance but also provided new ideas for early diagnosis and treatment.
CSCs can be distinguished from tumor cells through identification of specific molecular surface markers such as CD24, CD44, ALDH1, and ESA (epithelial specific antigen). Several meta-analysis studies have been performed to evaluate the association between biomarkers of CSCs and prognosis in various malignancies. A meta-analysis performed by Wang et al26 revealed that CD24 overexpression was significantly correlated with shortened OS in breast cancer patients. Wei et al27 conducted a pooled analysis of ALDH1 expression in lung cancer; the results showed that higher ALDH1 levels were associated with decreased DFS and OS.
CD133, a transmembrane cell surface glycoprotein, was initially found in hematopoietic stem cells and is considered to be a specific molecular biomarker of hematopoietic stem cells.28 In recent years, CD133 as a stem cell marker was demonstrated to be expressed in many types of solid tumors, such as liver, colorectal, and ovarian cancers.29–31 However, the prognostic role of CD133 expression in breast cancer is still controversial. Kim et al20 suggested that CD133 high-expression patients had shorter OS and DFS than CD133 low-expression cases. Conversely, Currie et al14 found no significant difference between CD133 high expression and CD133 low expression in breast cancer patients regarding survival time. In view of the inconsistent conclusions on the impact of CD133 expression in breast cancer patients, it was necessary to conduct a meta-analysis to evaluate the prognostic value of CD133 in breast cancer.
Based on our comprehensive analysis of published studies, we found that overexpression of CD133 was significantly associated with ER-positive status, PR-positive status, HER2-positive status, lymph node metastasis, and high histological grade. However, there was no significant association between CD133 high expression and large tumor size or late onset age. Furthermore, the overall analysis of prognosis revealed that CD133-positive breast cancer patients had a higher risk of mortality and a poorer DFS. In subgroup analysis by region, there was a difference between the Asian and non-Asian groups. In the Asian group, there was a significant association between CD133-positive breast cancer and poorer OS and DFS.
It was gradually discovered that several signaling pathways such as Hedgehog, Wnt, Notch, and NF-κB were involved in the CSC development, progression, differentiation, and metastasis.32 Based on the blockade of these signaling pathways, targeted therapy provides a new method to attack surface molecules of CSCs. Before 2010, gemtuzumab/ozogamicin, an antibody–drug conjugate of a recombinant humanized anti-CD33 monoclonal antibody, had been used in targeted therapy for clinical applications in acute myeloid leukemia patients, but has been pulled out of market due to high toxicity.33,34 Moreover, a recently published report in Nature Communications revealed that self-renewal of CD133 cells by IL6/Notch3 signaling regulates therapeutic resistance in metastatic breast cancer.35 Similarly, the results of our meta-analysis demonstrated that patients with high CD133-expressing tumors tended to have poorer survival. Consequently, targeted drugs that act on CD133 have the potential to be applied in the clinic, and breast cancer patients with high CD133 expression levels may benefit from them.
Previously, there was a similar meta-analysis published in 2010, which evaluated the association of CSCs with clinical outcome.36 They presented statistics on two indicators, CD44+/CD24-/low and ALDH1. However, the key point of our study focused on CD133 expression and its association with clinicopathological features and prognosis. Furthermore, statistical analysis of earlier studies on survival data was calculated using risk ratios (RRs). It is believed that the statistics of HRs take into consideration differences in end events, and also take into account the time to reach the end point and censored data. Consequently, the survival data statistic of HRs is calculated in our meta-analysis.
There were limitations in our meta-analysis. First, eligible studies were incorporated with diverse TNM stage and histological grade that may have potentially influenced the results. Second, although we collected all eligible studies for evaluating the association between CD133 expression and survival data, the sample size was not large enough, which in turn weakened the statistical power of the results. Finally, in this present analysis, the influence of bias could not be completely excluded.
Conclusion
The present results provide some evidence on the clinical outcome and prognostic value of CD133 in breast cancer patients. High CD133 expression predicted a worse OS and DFS. CD133 markers may potentially serve as prognostic markers and novel potential therapeutic targets in breast cancer. Large-scale and standard cohort studies are required for further confirmation.
Acknowledgment
This study was supported by the grants from the National Natural Science Foundation of China (no 81372811) and Science and Technology Agency of Liaoning Province (no 2013225049).
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. | ||
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. | ||
Alison MR, Islam S, Wright NA. Stem cells in cancer: instigators and propagators? J Cell Sci. 2010;123(pt 14):2357–2368. | ||
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. | ||
Miraglia S, Godfrey W, Yin AH, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. | ||
Nadal R, Ortega FG, Salido M, et al. CD133 expression in circulating tumor cells from breast cancer patients: potential role in resistance to chemotherapy. Int J Cancer. 2013;133(10):2398–2407. | ||
Wang W, Chen Y, Deng J, et al. The prognostic value of CD133 expression in non-small cell lung cancer: a meta-analysis. Tumour Biol. 2014;35(10):9769–9775. | ||
Cheng B, Yang G, Jiang R, et al. Cancer stem cell markers predict a poor prognosis in renal cell carcinoma: a meta-analysis. Oncotarget. 2016;7(40):65862–65875. | ||
Sui YP, Jian XP, Ma LI, et al. Prognostic value of cancer stem cell marker CD133 expression in esophageal carcinoma: a meta-analysis. Mol Clin Oncol. 2016;4(1):77–82. | ||
Wen L, Chen XZ, Yang K, et al. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One. 2013;8(3):e59154. | ||
Ieni A, Giuffre G, Adamo V, Tuccari G. Prognostic impact of CD133 immunoexpression in node-negative invasive breast carcinomas. Anticancer Res. 2011;31(4):1315–1320. | ||
Lin CH, Liu CH, Wen CH, Ko PL, Chai CY. Differential CD133 expression distinguishes malignant from benign papillary lesions of the breast. Virchows Arch. 2015;466(2):177–184. | ||
Liu Q, Li JG, Zheng XY, Jin F, Dong HT. Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast carcinomas. Chin Med J (Engl). 2009;122(22):2763–2769. | ||
Currie MJ, Beardsley BE, Harris GC, et al. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: relationships with markers of tumor hypoxia and microvascularity. Hum Pathol. 2013;44(3):402–411. | ||
Di Bonito M, Cantile M, Collina F, et al. Overexpression of cell cycle progression inhibitor Geminin is associated with tumor stem-like phenotype of triple-negative breast cancer. J Breast Cancer. 2012;15(2):162–171. | ||
Aomatsu N, Yashiro M, Kashiwagi S, et al. CD133 is a useful surrogate marker for predicting chemosensitivity to neoadjuvant chemotherapy in breast cancer. PLoS One. 2012;7(9):e45865. | ||
Kapucuoglu N, Bozkurt KK, Baspinar S, et al. The clinicopathological and prognostic significance of CD24, CD44, CD133, ALDH1 expressions in invasive ductal carcinoma of the breast: CD44/CD24 expression in breast cancer. Pathol Res Pract. 2015;211(10):740–747. | ||
Zhao P, Lu Y, Jiang X, Li X. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Sci. 2011;102(5):1107–1111. | ||
Mansour SF, Atwa MM. Clinicopathological significance of CD133 and ALDH1 cancer stem cell marker expression in invasive ductal breast carcinoma. Asian Pac J Cancer Prev. 2015;16(17):7491–7496. | ||
Kim SJ, Kim YS, Jang ED, Seo KJ, Kim JS. Prognostic impact and clinicopathological correlation of CD133 and ALDH1 expression in invasive breast cancer. J Breast Cancer. 2015;18(4):347–355. | ||
Liu TJ, Sun BC, Zhao XL, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32(5):544–553. | ||
Lv X, Wang Y, Song Y, Pang X, Li H. Association between ALDH1+/CD133+ stem-like cells and tumor angiogenesis in invasive ductal breast carcinoma. Oncol Lett. 2016;11(3):1750–1756. | ||
Han Z, Chen Z, Zheng R, Cheng Z, Gong X, Wang D. Clinicopathological significance of CD133 and CD44 expression in infiltrating ductal carcinoma and their relationship to angiogenesis. World J Surg Oncol. 2015;13:56. | ||
Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124(10):1929–1939. | ||
Kummermehr JC. Tumour stem cells – the evidence and the ambiguity. Acta Oncol. 2001;40(8):981–988. | ||
Wang Z, Wang Q, Wang Q, Wang Y, Chen J. Prognostic significance of CD24 and CD44 in breast cancer: a meta-analysis. Int J Biol Markers. Epub 2016 Jul 27. | ||
Wei D, Peng JJ, Gao H, Zhang T, Tan Y, Hu YH. ALDH1 expression and the prognosis of lung cancer: a systematic review and meta-analysis. Heart Lung Circ. 2015;24(8):780–788. | ||
Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. | ||
Zhang L, Ge C, Zhao F, et al. NRBP2 overexpression increases the chemosensitivity of hepatocellular carcinoma cells via Akt signaling. Cancer Res. 2016;76(23):7059–7071. | ||
Stanisavljevic L, Myklebust MP, Leh S, Dahl O. LGR5 and CD133 as prognostic and predictive markers for fluoropyrimidine-based adjuvant chemotherapy in colorectal cancer. Acta Oncol. 2016;55(12):1425–1433. | ||
Zhou Q, Chen A, Song H, Tao J, Yang H, Zuo M. Prognostic value of cancer stem cell marker CD133 in ovarian cancer: a meta-analysis. Int J Clin Exp Med. 2015;8(3):3080–3088. | ||
Matsui WH. Cancer stem cell signaling pathways. Medicine (Baltimore). 2016;95(1 suppl 1):S8–S19. | ||
Peterlin P, Guillaume T, Delaunay J, et al. Similarity of fractionated versus single dose(s) of gemtuzumab ozogamicin as part of the MIDAM salvage regimen in relapsed/refractory acute myeloid leukemia patients. Semin Hematol. 2016;53(3):216–217. | ||
Kell J. The addition of gemtuzumab ozogamicin to chemotherapy in adult patients with acute myeloid leukemia. Expert Rev Anticancer Ther. 2016;16(4):377–382. | ||
Sansone P, Ceccarelli C, Berishaj M, et al. Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat Commun. 2016;7:10442. | ||
Zhou L, Jiang Y, Yan T, et al. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122(3):795–801. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







