Back to Journals » Cancer Management and Research » Volume 11
Clinicopathological and prognostic significance of pretreatment thrombocytosis in patients with endometrial cancer: a meta-analysis
Authors Bai YY , Du L, Jing L, Tian T , Liang X, Jiao M, Nan KJ, Guo H, Ruan ZP
Received 5 September 2018
Accepted for publication 8 February 2019
Published 8 May 2019 Volume 2019:11 Pages 4283—4295
DOI https://doi.org/10.2147/CMAR.S186535
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Yi-Yang Bai,1,* Lan Du,2,* Li Jing,1 Tao Tian,1 Xuan Liang,1 Min Jiao,1 Ke-Jun Nan,1 Hui Guo,1 Zhi-Ping Ruan1
1Department of Medical Oncology, The First Affiliated Hospital, College of Medicine of Xi’an Jiaotong University, Xi’an, Shaanxi, People’s Republic of China; 2Department of Obstetrics and Gynecology, Xi’an Angel Women’s and Children’s Hospital, Xian, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Background: The prognostic and clinicopathological role of pretreatment thrombocytosis in cancer has been widely studied, but conclusions in endometrial cancer (EnCa) remain controversial. Therefore, we conducted a meta-analysis to assess the pathologic and prognostic impacts of pretreatment thrombocytosis in patients with EnCa.
Methods: We searched PubMed, Embase, SpringerLink, ScienceDirect and China National Knowledge Infrastructure databases. Pooled HR or OR with their 95% CIs were applied to assess the association of pretreatment thrombocytosis with survival outcomes and clinical parameters of EnCa patients.
Results: In total, 10 studies containing 2,995 cases of EnCa met the criteria. The results suggested that pretreatment thrombocytosis was significantly associated with high International Federation of Gynecology and Obstetrics (FIGO) stage (pooled OR 3.45, 95% CI 1.68–7.08, P=0.001), poor tumor differentiation (pooled OR 2.00, 95% CI 1.22–3.29, P=0.006), lymph-vascular space invasion (pooled OR 2.04, 95% CI 1.35–3.07, P=0.001); myometrial invasion (pooled OR 2.14, 95% CI 1.39–3.32, P=0.001); cervical involvement (pooled OR 2.54, 95% CI 1.56–4.15, P=0.000) and lymph node metastasis (OR 3.15, 95% CI 1.71–5.80, P=0.001). No significant difference existed between pretreatment thrombocytosis and overall survival (P=0.012), cancer/disease-specific survival (P=0.07) or disease-free survival (P=0.25).
Conclusion: pretreatment thrombocytosis was associated with advanced clinicopathological features in patients with EnCa, which may serve as a potential therapeutic target for EnCa.
Keywords: thrombocytosis, endometrial cancer, prognosis, meta-analysis
Introduction
Endometrial cancer (EnCa) is the most common gynecological malignancy with a rising incidence in developed countries.1 Most patients (80%) are commonly diagnosed at the early stage and can be surgically cured. However, patients with metastatic or recurrent disease portend a poor prognosis, with a 5-year survival rate of 5.3–20.1%, as there are limited treatment options.2,3 Prognostic assessment is essential for treatment decision-making. Clinically, the prognosis of EnCa is heterogeneous due to variations in tumor biology.4 Some patients with the same stage or pathologic prognostic factors have various clinical courses and survival outcomes.5 Therefore, additional prognostic markers are needed to guide therapeutic options and surveillance strategies.
Recently, studies have shown that tumor–platelet interactions is associated with tumorigenesis. Specifically, elevated platelet count or thrombocytosis has been identified as a marker of cancer prognosis and may reflect tumor burden. Todenhöfer T et al reported thrombocytosis could be used as a prognostic parameter and constructed a more accurate prognostic model according to pretreatment platelet count and established pathological factors.6 Several studies have suggested that preoperative thrombocytosis associated with poor survival in gynecological malignancies, such as ovarian and cervicalcancer.7,8 So the relationship between thrombocytosis and prognosis of EnCa is worth further study Pretreatment thrombocytosis has been reported by most researches to be correlated with poor prognosis of EnCa. However, conflicting results exist and a consensus cannot be achieved. Takahashi et al9 suggested that pretreatment thrombocytosis significantly predicted unfavorable survival. Heng and Benjapibal10 reported that thrombocytosis was not a prognostic factor of EnCa in the multivariate analysis. Against this background, we performed a comprehensive and quantitative evaluation of the literature about the relationships between pretreatment thrombocytosis and survival and clinicopathological features in EnCa.
Methods
The study was performed according to the PRISMA statement.11
Search strategy
Our search was restricted to the English and Chinese using databases from PubMed, Embase, SpringerLink, ScienceDirect and China National Knowledge Infrastructure up to July 15, 2018. Both medical subject heading (Mesh) terms and free-text terms included EnCa, thrombocytosis and prognosis. The full search strategy is available in the Supplementary materials. The bibliographies of the retrieved articles were also manually scrutinized for potential related articles.
Selection criteria
The criteria for inclusion were as follows: 1) prospective or retrospective studies analyzed the relationship between thrombocytosis and clinicopathological factors or prognosis of EnCa; 2) the cutoff values of thrombocytosis were reported; and 3) the most complete study was included if multiple studies described the same cohorts studies were excluded based on the following criteria: (1) studies for the lack of information for further analysis; (2) laboratory articles; and (3) non-research articles (abstracts, letters, comments or reviews).
Definitions and data extraction
Overall survival (OS) was defined as the interval between the initial surgical procedure and the death or the last follow-up. Cancer/disease-specific survival (DSS) was defined as the time from initial diagnosis to date of death attributed to EnCa. Disease-free survival (DFS) was measured from the day of surgery to the time of local/distant disease progression or the date of last follow-up. The following data were collected: (1) publication details: first author’s surname, publication year, country of study, age and sample size; (2) study design: study type (prospective/retrospective study), cutoff points; (3) patients characteristics (patients number and age); and (4) follow-up data (median/mean follow-up duration, survival analysis).
Quality assessment
The Newcastle-Ottawa Scale (NOS) criterion12 was used to evaluate the quality of the included studies (Table S1). The scores were judged based on the three aspects of NOS, namely, selection, comparability and outcomes. Studies achieving scores ≥6 were defined as high quality (Table S2).
Statistical analyses
For the quantitative aggregation of results, pooled OR with 95% CI were used to evaluate the association of thrombocytosis with clinicopathological features of patients. The HR with 95% CI were used to analyze postoperative OS, cancer/DSS, or DFS, which was directly retrieved from each of included article. Pooled OR and HR were calculated using random-effect model. Heterogeneity was performed by using chi-square-based Q-test. The I2 value indicated the degree of heterogeneity. A P-value <0.10 or I2>50% indicated significant heterogeneity. All statistical analyses were carried out by Review Manager 5.3 (Cochrane Collaboration, London, UK).
Results
Characteristics of included studies
The detail search process is shown in Figure 1. The initial search retrieved a total of 339 published studies. Out of which, 146 studies were excluded due to duplicate records. After screening titles and abstracts, we removed 162 publications including 148 irrelevant studies and 14 reports without clinical specimens. Further evaluating, finally, we included 10 studies in the final meta-analysis.9,10,13–20 Sample sizes ranged from 68 to 1166 patients. All included studies including 2995 patients were counted for the incidence of thrombocytosis ranged from 2.3% to 18.2%. The study by Njølstad et al15 was a prospective study. The remaining studies were retrospective. All the included studies did not report the methods to measure pretreatment thrombocytosis in detail. Three studies10,16,19 stated that platelet count in each case was obtained within 14 days before surgery. The detailed characteristics of eligible studies are described in Table 1.
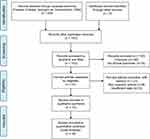 | Figure 1 Flow diagram of the search strategy. |
 | Table 1 Baseline characteristics of studies included in the meta-analysis |
Correlation of preoperative thrombocytosis and clinicopathological feature
Four studies9,10,13,17 involving 1133 patients reported the association of FIGO stage with preoperative thrombocytosis. The pooled OR revealed that patients with preoperative thrombocytosis were more likely to have high FIGO stage categories (OR 3.45, 95% CI 1.68–7.08, P=0.001; Figure 2A). Five studies9,10,13,15,17 including 1261 patients provided information regarding histological grade. The pooled analysis showed that thrombocytosis was linked to high histological grading (pooled OR 2.00, 95% CI 1.22–3.29, P=0.006; Figure 2B). Four studies9,10,15,17 described preoperative thrombocytosis according to histologic subtype and lymph-vascular space invasion (LVSI). The pooled data showed thrombocytosis correlated with LVSI (pooled OR 2.04, 95% CI 1.35–3.07, P=0.001; Figure 2C), but there were no significant associations of histologic subtype (pooled OR 0.79, 95% CI 0.39–1.60, P=0.52; Figure 3A). The combined results showed that thrombocytosis was significantly associated with cervical involvement (pooled OR 2.54, 95% CI 1.56–4.15, P=0.000; Figure 3B) and myometrial invasion (pooled OR 2.14, 95% CI 1.39–3.32, P=0.001; Figure 3C) and in three studies.9,10,15 The pooled OR revealed that preoperative thrombocytosis was associated with lymph node metastasis (OR 3.15, 95% CI 1.71–5.80, P=0.001 Figure 3D) by analyzing two studies.9,10
 | Figure 2 Association of pretreatment thrombocytosis with clinicopathological parameters. (A) FIGO stage; (B) tumor differentiation; and (C) lymph-vascular space invasion. |
 | Figure 3 Association of pretreatment thrombocytosis with clinicopathological factors. (A) histologic subtype; (B) cervical involvement; (C) myometrial invasion; and (D)lymph node metastasis. |
Impact of preoperative thrombocytosis on survival
As seen in Figure 4, six9,10,13,16,19,20 of the included studies showed was preoperative thrombocytosis was not associated with OS in EnCa patients (pooled HR =1.65, 95% CI: 0.88–3.11, P=0.012, I2=82%, Figure 4A). The synthesized data from four studies10,13,17,20 suggested that thrombocytosis did not correlate with poor DFS (pooled HR =1.66, 95% CI: 0.96–2.89, P=0.07, I2=59%, Figure 4B). There was no association between preoperative thrombocytosis and DSS (pooled HR =1.37, 95% CI: 0.80–2.36, P=0.25, I2=40%, Figure 4C). Particularly, Andersen et al14 divided the preoperative platelet count into two categories of thrombocytosis (mild, platelet count=400–550×109/L; severe, platelet count >550×109/L). The study reported that mild and severe preoperative thrombocytosis was all not associated with cancer-specific mortality.
 | Figure 4 The association between thrombocytosis and survival outcomes (all multivariate analysis). (A) overall survival; (B) disease-free survival; and (C) cancer/disease-specific survival. |
Subgroup and sensitivity analysis
Stratified analysis was conducted to assess the prognostic value of thrombocytosis on OS and DFS according to geographic region, sample size, and NOS score. As shown in Table 2, stratified analysis did not alter the prognostic role of preoperative thrombocytosis on OS, except for the subgroup small sample size (pooled HR =1.88, 95% CI: 1.26–2.80, P=0.002, I2=0%). However, the EnCa patients with preoperative thrombocytosis showed a significant worse DFS in subgroups of Asian patients (pooled HR =2.21, 95% CI: 1.17–4.21, P=0.02, I2=0%), NOS scores >7 (pooled HR=2.16, 95% CI: 1.34–3.56, P=0.002, I2=0%). We further performed a sensitivity analysis to gauge the stability of the results. The pooled effects of OS was significantly altered when the study by Heng and Benjapibal10 was omitted (pooled HR=2.04, 95% CI=1.11–3.73, P=0.02, Table 3). When the study by Moeini et al20 was removed, the pooled results for DFS were significantly altered (pooled HR=2.23, 95% CI=1.45–3.42, P=0.000, Table 3). We did not evaluate publication bias since the number of included studies was limited.
 | Table 2 Subgroup analyses for OS and DFS |
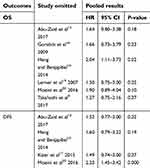 | Table 3 Sensitivity analysis for OS and DFS |
Discussion
Thrombocytosis in cancer patients is a common finding and preoperative thrombocytosis has a strong connection to cancer outcomes.21,22 However, conflicting studies exist regarding the prognostic effects of thrombocytosis on EnCa patients.9,10 So we performed the meta-analysis to reassess the association of preoperative thrombocytosis with clinicopathological factors and prognosis of EnCa. In the current study, the pooled effects indicated that preoperative thrombocytosis was positively correlated with high FIGO stage, high histological grading, LVSI, myometrial invasion, cervical involvement and lymph node metastasis in EnCa patients. Nonetheless, preoperative thrombocytosis was not associated with poor OS, DFS, and DSS (all included studies were used multivariate analysis). According to NOS quality assessment, all included studies were high quality and the scores of ranged from 6 to 9 (median 7). So we defined the cutoff value of NOS quality as 7 when performing stratification analysis. EnCa patients with preoperative thrombocytosis showed a reduced DFS in subgroups of Asian patients and studies with NOS scores larger than 7.
The prognosis remains dismal for patients with recurrent or metastatic EnCa. In order to improve survival, it is imperative that we identify the tumors with aggressive behaviors and treat them appropriately. Recently, studies have been reported that circulating biomarkers can predict the course of EnCa. Indices of systemic inflammation such as elevated platelet/lymphocyte ratio, platelet count and platelet volume have shown potential for prognostic surveillance.23,24 In addition, such circulating markers are readily monitored by relatively noninvasive means. Therefore, it is of great clinical significance to identify new circulating markers, combining with the established clinicopathologic prognostic factors, to improve the outcomes of patients with EnCa.
The mechanisms by which preoperative thrombocytosis correlates clinicopathological features of EnCa patients remains to be not fully elucidated. Several studies which put forward to plausible hypotheses may explain the correlations. Platelets can infiltrate into tumor tissue and contribute to tumor growth by secreting pro-angiogenic and pro-tumorigenic factors including vascular endothelial growth factor, insulin-like growth factor 1 and 2 .25 Platelet-tumor cell adhesion established a pro-metastatic microenvironment that protects cancer cells from immune surveillance.26 Orellana et al27 found that platelets acted as chemo-attractants to facilitate cancer cells migration through co-cultivating cancer cells with human platelets. The authors concluded that platelet–cancer interactions contributed to the cancer metastasis. On the one hand, a variety of tumor-related cytokines stimulates thrombopoiesis in cancer. Among them are IL-1, IL-6, and thrombopoietin (TPO).28 Stone et al29 established mouse models of ovarian cancer and demonstrated that tumor-derived IL-6 stimulates hepatic production of TPO, which stimulates megakaryocyte growth and thrombopoiesis. In addition, Inhibition of IL-6 by neutralizing antibody reduced tumor growth and enhanced the therapeutic efficacy of paclitaxel. Recently, Guillem-Llobat et al30 reported that low-dose aspirin which inhibits platelet activation prevented colorectal cancer metastasis in mice. Other antiplatelet agents such as heparinoids may be beneficial for cancer patients.
Limitations
Certain limitations exist in the current study. First, the cutoff point of preoperative thrombocytosis is still not established. Most of the included studies set cutoff point as platelet count >400×109/L, but Njølstad et al18 report thrombocytosis as platelet count >390×109 platelets/L, which may lead to inter-study heterogeneity. Besides, the majority of included studies were retrospective, so the possibility of selection bias cannot be ruled out. Second, several disease conditions such as inflammatory hematological diseases may affect platelet count, but some included studies did not control these confounding factors. Third, the sample size of the included studies ranged from 68 to 714, which may result in between-study heterogeneity. The limited number of included studies might impact the validity of our analysis, so further studies are warranted. These limitations may also contribute to the conflicting results of the prognostic significance of thrombocytosis in EnCa. Besides, a study by Oge T et al24 reported that platelet volume can be used as a parameter for platelet activation and prediction of advanced-stage EnCa. All included studies highlighted on platelet count, rather than function. Thus, the association of platelet volume with the survival of EnCa deserves further investigation.
Conclusion
In summary, the current meta-analysis shows that preoperative thrombocytosis is correlated with high FIGO stage, poor tumor differentiation, LVSI, myometrial invasion, cervical involvement, and lymph node metastasis. No significance was found between thrombocytosis and OS, DFS, and DSS. However, further studies are needed to update our results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi:10.1016/S0140-6736(15)00130-0
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
3. Pectasides D, Pectasides E, Economopoulos T. Systemic therapy in metastatic or recurrent endometrial cancer. Cancer Treat Rev. 2007;33(2):177–190. doi:10.1016/j.ctrv.2006.10.007
4. Gupta D. Clinical behavior and treatment of endometrial cancer. Adv Exp Med Biol. 2017;943:47–74. doi:10.1007/978-3-319-43139-0_2
5. Murali R, Soslow RA, Weigelt B, et al. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–78. doi:10.1016/S1470-2045(13)70591-6
6. Todenhöfer T, Renninger M, Schwentner C, Stenzl A, Gakis G. A new prognostic model for cancer-specific survival after radical cystectomy including pretreatment thrombocytosis and standard pathological risk factors. BJU Int. 2012;110(11Pt B):E533–40. doi:10.1111/bju.2012.110.issue-11b
7. Cohen JG, Tran AQ, Rimel BJ, et al. Thrombocytosis at secondary cytoreduction for recurrent ovarian cancer predicts suboptimal resection and poor survival. Gynecol Oncol. 2014;132(3):556–559. doi:10.1016/j.ygyno.2014.01.003
8. Koulis TA, Kornaga EN, Banerjee R, et al. Anemia, leukocytosis and thrombocytosis as prognostic factors in patients with cervical cancer treated with radical chemoradiotherapy: A retrospective cohort study. Clin Transl Radiat Oncol. 2017;4:51–56. doi:10.1016/j.ctro.2017.05.001
9. Takahashi R, Mabuchi S, Kuroda H, et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial cancer. Int J Gynecol Cancer. 2017;27(7):1399–1407. doi:10.1097/IGC.0000000000001019
10. Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac J Cancer Prev. 2014;15(23):10231–10236.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z
13. Abu-Zaid A, Alsabban M, Abuzaid M, AlOmar O, Salem H, Al-Badawi IA. Preoperative thrombocytosis as a prognostic factor in endometrioid-type endometrial carcinoma. Ann Saudi Med. 2017;37(5):393–400. doi:10.5144/0256-4947.2017.393
14. Andersen CL, Eskelund CW, Siersma VD, et al. Is thrombocytosis a valid indicator of advanced stage and high mortality of gynecological cancer? Gynecol Oncol. 2015;139(2):312–318. doi:10.1016/j.ygyno.2015.09.017
15. Gücer F, Moser F, Tamussino K, et al. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol Oncol. 1998;70(2):210–214. doi:10.1006/gyno.1998.5078
16. Gorelick C, Andikyan V, Mack M, Lee YC, Abulafia O. Prognostic significance of preoperative thrombocytosis in patients with endometrial carcinoma in an inner-city population. Int J Gynecol Cancer. 2009;19(8):1384–1389. doi:10.1111/IGC.0b013e3181a47d47
17. Kizer NT, Hatem H, Nugent EK, et al. Chemotherapy response rates among patients with endometrial cancer who have elevated serum platelets. Int J Gynecol Cancer. 2015;25(6):1015–1022. doi:10.1097/IGC.0000000000000453
18. Njølstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013;131(2):410–415. doi:10.1016/j.ygyno.2013.08.032
19. Lerner DL, Walsh CS, Cass I, Karlan BY, Li AJ. The prognostic significance of thrombocytosis in uterine papillary serous carcinomas. Gynecol Oncol. 2007;104(1):91–94. doi:10.1016/j.ygyno.2006.07.020
20. Moeini A, Machida H, Takiuchi T, et al. Association of nonalcoholic fatty liver disease and venous thromboembolism in women with endometrial cancer. Clin Appl Thromb Hemost. 2016;23(8):1018–1027. doi:10.1177/1076029616665925
21. Wang YH, Deng SJ, Yang YD, et al. The pretreatment thrombocytosis may predict prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Biomark Med. 2017;11(2):195–210. doi:10.2217/bmm-2016-0214
22. Wang YH, Kang JK, Zhi YF, et al. The pretreatment thrombocytosis as one of prognostic factors for gastric cancer: A systematic review and meta-analysis. Int J Surg. 2018;53:304–311. doi:10.1016/j.ijsu.2018.03.084
23. Cummings M, Merone L, Keeble C, et al. Preoperative neutrophil: lymphocyteand platelet: lymphocyteratios predict endometrial cancer survival. Br J Cancer. 2015;113(2):311–320. doi:10.1038/bjc.2015.200
24. Oge T, Yalcin OT, Ozalp SS, Isikci T. Platelet volume as a parameter for platelet activation in patients with endometrial cancer. J Obstetrics Gynaecol. 2013;33(3):301–304. doi:10.3109/01443615.2012.758089
25. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. doi:10.1007/s10555-017-9673-1
26. Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer. 2016;138(9):2078–2087. doi:10.1002/ijc.29847
27. Orellana R, Kato S, Erices R, et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer. 2015;15:290. doi:10.1186/s12885-015-1584-3
28. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–269. doi:10.1007/s10555-014-9498-0
29. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi:10.1056/NEJMoa1110352
30. Guillem-Llobat P, Dovizio M, Bruno A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7(22):32462–32477. doi:10.18632/oncotarget.8655
31. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute. Available from:
Supplementary materials
 | Table S1 Newcastle-Ottawa quality assessment scale |
 | Table S2 Assessment of Newcastle-Ottawa Scale methodological quality of cohort studies |
Search strategy
PubMed:
#1: Search (((((((((((((((((((Endometrial Neoplasm[Title/Abstract]) OR Neoplasm, Endometrial[Title/Abstract]) OR Neoplasms, Endometrial[Title/Abstract]) OR Endometrial Carcinoma[Title/Abstract]) OR Carcinoma, Endometrial[Title/Abstract]) OR Carcinomas, Endometrial[Title/Abstract]) OR Endometrial Carcinomas[Title/Abstract]) OR Endometrial cancer[Title/Abstract]) OR Cancer, Endometrial[Title/Abstract]) OR Cancers, Endometrial[Title/Abstract]) OR Endometrial Cancers[Title/Abstract]) OR Endometrium Cancer[Title/Abstract]) OR Cancer, Endometrium[Title/Abstract]) OR Cancers, Endometrium[Title/Abstract]) OR Cancer of the Endometrium[Title/Abstract]) OR Carcinoma of Endometrium[Title/Abstract]) OR Endometrium Carcinoma[Title/Abstract]) OR Endometrium Carcinomas[Title/Abstract]) OR Cancer of Endometrium[Title/Abstract]) 38,079
#2: Search (((((((Thrombocytosis[Title/Abstract]) OR Thrombocytoses[Title/Abstract]) OR Thrombocythemia[Title/Abstract]) OR Thrombocythemias[Title/Abstract]) OR Increased platelets[Title/Abstract]) OR Increased platelet[Title/Abstract]) OR platelets count[Title/Abstract]) OR platelet count[Title/Abstract] 29,942
#3: Search (((((((((((((((“Prognosis”[Mesh]) OR Prognosis[Title/Abstract]) OR Prognoses[Title/Abstract]) OR Prognostic[Title/Abstract]) OR Outcome[Title/Abstract]) OR Survival[Title/Abstract]) OR Overall survival[Title/Abstract]) OR OS[Title/Abstract]) OR Cancer-specific survival[Title/Abstract]) OR CSS[Title/Abstract]) OR Progression-free survival[Title/Abstract]) OR PFS[Title/Abstract]) OR Disease-free survival[Title/Abstract]) OR DFS[Title/Abstract]) OR Mortality[Title/Abstract]) OR Recurrence[Title/Abstract] 3,215,415
#4: #1 and #2 and #3 38
Embase:
(“thrombocytosis”:ab,ti OR “thrombocytoses”:ab,ti OR “thrombocythemia”:ab,ti OR “thrombocythemias”:ab,ti OR “increased platelets”:ab,ti OR “increased platelet”:ab,ti OR “platelets count”:ab,ti OR “platelet count”:ab,ti) AND [1966-2018]/py 49,707
“prognosis”/exp/mj OR prognosis:ab,ti OR prognoses:ab,ti OR prognostic:ab,ti OR outcome:ab,ti OR survival:ab,ti OR “overall survival”:ab,ti OR os OR “cancer-specific survival”:ab,ti OR css:ab,ti OR “progression-free survival”:ab,ti OR pfs:ab,ti OR “disease-free survival”:ab,ti OR dfs:ab,ti OR mortality:ab,ti OR recurrence:ab,ti 3,462,505
(“endometrial cancer”:ab,ti OR “endometrial neoplasm”:ab,ti OR “neoplasm, endometrial”:ab,ti OR “neoplasms, endometrial”:ab,ti OR “endometrial carcinoma”:ab,ti OR “carcinoma, endometrial”:ab,ti OR “carcinomas, endometrial”:ab,ti OR “endometrial carcinomas”:ab,ti OR “cancer, endometrial”:ab,ti OR “cancers, endometrial”:ab,ti OR “endometrial cancers”:ab,ti OR “cancer, endometrium”:ab,ti OR “cancers, endometrium”:ab,ti OR “cancer of the endometrium”:ab,ti OR “carcinoma of endometrium”:ab,ti OR “endometrium carcinoma”:ab,ti OR “endometrium carcinomas”:ab,ti OR “cancer of endometrium”:ab,ti) AND [1966-2018]/py 31,021
#4: #1 and #2 and #3 35
Springerlink 178
ScienceDirect 88
References
1. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in metaanalyses. Ottawa Hospital Research Institute. Available from:
2. Abu-Zaid A, Alsabban M, Abuzaid M, AlOmar O, Salem H, Al-Badawi IA. Preoperative thrombocytosis as a prognostic factor in endometrioid-type endometrial carcinoma. Ann Saudi Med. 2017;37 (5):393–400. doi:10.5144/0256-4947.2017.393
3. Andersen CL, Eskelund CW, Siersma VD, et al. Is thrombocytosis a valid indicator of advanced stage and high mortality of gynecological cancer? Gynecol Oncol. 2015;139(2):312–318. doi:10.1016/j.ygyno.2015.09.017.
4. Gücer F, Moser F, Tamussino K, et al. Thrombocytosis as a prognostic factor in endometrial carcinoma. Gynecol Oncol. 345 1998;70(2):210–214. doi:10.1006/gyno.1998.5078
5. Gorelick C, Andikyan V, Mack M, Lee YC, Abulafia O. Prognostic significance of preoperative thrombocytosis in patients with endometrial carcinoma in an inner-city population. Int J Gynecol Cancer. 2009;19(8):1384–1389. doi:10.1111/IGC.0b013e3181a47d47
6. Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac J Cancer Prev. 2014;15(23):10231–10236
7. Kizer NT, Hatem H, Nugent EK, et al. Chemotherapy response rates among patients with endometrial cancer who have elevated serum platelets. Int J Gynecol Cancer. 2015;25(6):1015–1022. doi:10.1097/IGC.0000000000000453
8. Lerner DL, Walsh CS, Cass I, Karlan BY, Li AJ. The prognostic significance of thrombocytosis in uterine papillary serous carcinomas. Gynecol Oncol. 2007;104(1):91–94. doi:10.1016/j.ygyno.2006.07.020
9. Moeini A, Machida H, Takiuchi T, et al. Association of nonalcoholic fatty liver disease and venous thromboembolism in women with endometrial cancer. Clin Appl Thromb Hemost. 2016;23(8):1018–1027. doi:10.1177/107602961666592510.
10. Njølstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. 355 Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecol Oncol. 2013;131 (2):410–415. doi:10.1016/j.ygyno.2013.08.032
11. Takahashi R, Mabuchi S, Kuroda H, et al. The significance of pretreatment thrombocytosis and its association with neutrophilia in patients with surgically treated endometrial cancer. Int J Gynecol Cancer. 2017;27(7):1399–1407. doi:10.1097/IGC.0000000000001019
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
