Back to Journals » Cancer Management and Research » Volume 10
Clinicopathologic characteristics and clinical outcomes of pure type and mixed type of tubular carcinoma of the breast: a single-institution cohort study
Authors Zhang WW , Wu SG, Ling YH, Sun JY, Long ZQ , Hua X , Dong Y, Li FY, He ZY , Lin HX
Received 13 June 2018
Accepted for publication 4 September 2018
Published 11 October 2018 Volume 2018:10 Pages 4509—4515
DOI https://doi.org/10.2147/CMAR.S177046
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Wen-Wen Zhang,1,* San-Gang Wu,2,* Yi-Hong Ling,3 Jia-Yuan Sun,1 Zhi-Qing Long,1 Xin Hua,1 Yong Dong,4 Feng-Yan Li,1 Zhen-Yu He,1 Huan-Xin Lin1
1Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 2Department of Radiation Oncology, Xiamen Cancer Hospital, The First Affiliated Hospital of Xiamen University, Xiamen, People’s Republic of China; 3Department of Pathology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, People’s Republic of China; 4Department of Radiation Oncology, the Third People’s Hospital of Dongguan, Dongguan, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Introduction: We aimed to evaluate the clinicopathologic characteristics and clinical outcomes of the mixed type versus the pure type of tubular carcinoma (TC) of the breast in a retrospective cohort study.
Materials and methods: Patients were categorized into the following three groups: patients with pure TC of the breast (the PTC group), patients with TC and carcinoma in situ of the breast (the TC-CIS group), and patients with TC and other invasive carcinomas of the breast (the TC-IC group). We compared the clinicopathologic characteristics and treatment outcomes of the three groups. The primary end point of this study was breast cancer-specific survival (BCSS). Secondary end points included distant metastasis-free survival (DMFS) and locoregional recurrence (LRR).
Results: A total of 68 patients were included in this study, including 31 patients in the PTC group, 12 in the TC-CIS group, and 25 in the TC-IC group. Our data showed that PTC and TC-CIS were more likely to be smaller in size (P=0.014) and had substantially less nodal involvement (P=0.019), compared with TC-IC. The median follow-up time was 64.3 months (range, 3.78–223.2 months) for all patients. No locoregional relapse was observed in any group during the follow-up period. The 10-year BCSS of the PTC, TC-CIS, and TC-IC groups was 100%, 100%, and 95.2%, respectively, and the 10-year DMFS was 92.3%, 100%, and 96.0%, respectively. There was no significant difference in terms of BCSS (P=0.53) or DMFS (P=0.84) between the three groups.
Conclusion: This study indicates that both the pure type and mixed type of TC of the breast show very low LRR and distant metastasis rate and have excellent survival. The TC-IC group is likely to show good prognosis similar to the PTC group. Further clinical trials with larger sample sizes as well as molecular and genetic studies are warranted.
Keywords: pure type of tubular carcinoma, mixed type of tubular carcinoma, clinicopathologic characteristics, clinical outcomes
Introduction
Breast cancer is the most frequently diagnosed cancer among females,1–4 and globally, it is also the leading cause of cancer death in female.4 Breast cancer is a heterogeneous disease with different histological subtypes, which may represent quite different prognosis. Tubular carcinoma (TC) of the breast is a rare but distinct type of breast cancer with a particularly favorable prognosis, which accounts for 1%–4% of invasive breast cancer, depending on series.5–8 It is characterized by well-differentiated tubular structures with open lumina lined by a single layer of cells.9 However, TC commonly occurs in association with ductal carcinoma in situ (DCIS), invasive ductal carcinoma, and less commonly, lobular neoplasia.10–12 There is no consensus concerning the proportion of tubular structures necessary for diagnoses of TC yet. However, it is recommended that tumors with at least 90% of the tubule formation present should be regarded as the pure type, while tumors exhibiting between 50% and 90% tubules admixed with another morphology should be regarded as mixed type.9 Pure TC (PTC) is generally considered as having a better prognosis compared with invasive ductal carcinoma or lobular carcinoma, which has been well described in previous studies.12–16 Several studies showed that TC had superior survival even compared with grade I ductal carcinoma of the breast.12,14–16 TC also exhibits favorable clinicopathologic characteristics even compared with DCIS.17 However, it is still vague whether the mixed type of TC possesses different biology and natural history. Due to the low incidence and excellent prognosis of TC, it is quite difficult to carry out prospective studies. Extensive analysis of pure and mixed type of TC in large databases, such as the Surveillance, Epidemiology, and End Results database, might bring uncertainties as the pathologic diagnoses are done by numerous pathologists from so many hospitals. To further our knowledge of the clinicopathologic characteristics and clinical outcomes of the pure type and mixed type of TC of the breast, we conducted a retrospective cohort study in Sun Yat-sen University Cancer Center (SYSUCC).
Materials and methods
Study population
We reviewed the documents of patients treated in SYSUCC between January 1998 and December 2017 and searched for patients diagnosed with a TC component. After Ethical Review Board of SYSUCC approval, data of those patients from the pathology databases and medical records were reviewed thoroughly. Written informed consent was obtained from every patient for the treatment and publication of this report before initial treatment. After treatment, patients were followed-up every 3–6 months up to 5 years and then annually or when clinically indicated. Medical history and physical examination were performed at each follow-up visit. Ultrasound, mammography, laboratory tests, and other imaging studies depended on the discretion of the treating physicians. Clinical information was obtained from the database, including patients’ characteristics (eg, gender, age at diagnosis, clinical history, past medical history), tumor characteristics (eg, primary tumor size, the TNM stage, histological tumor type, hormone receptor status, human epidermal growth factor receptor 2 [HER2] status), treatments (type of surgery, use of chemotherapy, use of radiotherapy, use of endocrine therapy), vital status, and survival. TNM stage of all patients was reassessed according to the 7th edition of the American Joint Commission on Cancer staging system.
Pathology of all patients was re-evaluated by one experienced pathologist under a same outlined procedure defined below. We defined PTC as tumors with at least 90% of the tubule formation present and mixed type of TC as tumors with <90% of tubule formation.18 When the tumor was composed of TC and ductal/lobular carcinoma in situ, without any other invasive carcinoma component, we called it TC and carcinoma in situ (TC-CIS). On the other hand, when the tumor was composed of TC and other invasive histological types, with or without carcinoma in situ, we call it TC and invasive carcinoma (TC-IC).
Survival analysis
Breast cancer-specific survival (BCSS) was calculated from the date of operation, with death from breast cancer or death with active breast cancer being scored as an event; patients who died from any other causes or were still alive were censored at the time of last follow-up. Distant metastasis-free survival (DMFS) was defined as the interval between the operation and distant metastasis, with distant metastasis scored as an event; patients who died from any causes or were still alive were censored at the time of last follow-up. Locoregional recurrence (LRR) was defined as tumor arising in the treated breast or chest wall, the ipsilateral axillary lymph nodes, the ipsilateral internal mammary lymph nodes, or the ipsilateral supraclavicular or subclavian area.
Statistical methods
We utilized the SPSS statistical software for Windows (version 23.0; IBM Corporation, Armonk, NY, USA) to perform statistical analysis. Baseline clinical characteristics were compared using the independent-samples t-test or Mann–Whitney U-test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. Survival analysis was performed using the Kaplan–Meier method and log-rank test. A two-sided P-value of <0.05 was considered significant. Estimates were reported with the 95% CI where appropriate. The date of treatment initiation was used as the starting point for all time to event variables. The primary end point of this study was BCSS. Secondary end points included DMFS and LRR.
Results
A total of 68 patients met the criteria and were included in this study. After reviewed by the pathologist, the patients were categorized as the following three groups: the PTC group, included 31 patients; the TC-CIS group, included 12 patients; and the TC-IC group, included 24 patients. Among the 68 patients in this study, 1 patient was diagnosed with TC and mucinous carcinoma of the breast, and she was included in the TC-IC group.
Clinicopathologic characteristics
Baseline clinicopathologic characteristics of the 68 patients are listed in Table 1. All patients in this cohort were female (100%). The median ages of patients with PTC, TC-CIS, and TC-IC were 46 (range, 28–67), 44.5 (range, 37–60), and 47 (range, 27–73), respectively (P=0.658). In all three groups, tumors tended to arose more frequently in the upper outer quadrant of the breast (P=0.780). Only one (3.2%) patient in the PTC group and two (8.0%) patients in the TC-IC group showed elevated carcinoembryonic antigen in the serum when diagnosed. PTC and TC-CIS groups were more likely to be smaller in size (P=0.014) and had substantially less nodal involvement (P=0.019) compared with TC-IC group. Most patients in all three groups were estrogen receptor (ER) positive, progesterone receptor (PR) positive, and HER2 negative.
Treatments
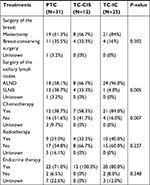
Surgical procedures of the breast were characterized as either breast-conserving surgery (BCS) or mastectomy, while surgical procedures of the axilla were either sentinel lymph node biopsy or axillary lymph node dissection (ALND). All patients who received radiotherapy were treated with external beam radiation using high-energy photons generated from a linear accelerator.
Treatment information, including surgery of the breast and axillary lymph nodes, chemotherapy, radiotherapy, and endocrine therapy, of the three groups is listed in Table 2. A similar proportion, approximately one-third, of patients with PTC and TC-CIS received BCS, which seems to be higher than that of the TC-IC group; however, there was no significant difference. Twenty-four out of 25 patients (96.0%) in the TC-IC group received ALND, while about one-third of patients in the other two groups omitted ALND (P=0.005). Patients in the TC-IC group were more likely to receive chemotherapy (P=0.007). All patients who received chemotherapy were given adjuvant chemotherapy, except one patient in the TC-IC group, who received neoadjuvant chemotherapy. On the other hand, 29.0%, 33.3%, and 40.0% of the patients received radiotherapy after surgery in the PTC group, TC-CIS group, and TC-IC group, respectively (P=0.227). Most patients in all three groups received endocrine therapy (P=0.348).
Clinical outcomes
The median follow-up time was 64.3 months (range, 3.78–223.2 months) for all patients. The median survival was not reached in any group. No treatment-related death was observed. Only one patient died of breast cancer in the TC-IC group, who had bone metastasis when diagnosed. All patients in the PTC group and TC-CIS group were alive until the day of last follow-up. There was no significant difference between the three groups in terms of BCSS (P=0.53) (Figure 1A). The 10-year BCSS of the PTC, TC-CIS, and TC-IC group was 100%, 100%, and 95.2%, respectively (Figure 1A).
No locoregional relapse was observed in any group during the follow-up period. One patient in the TC-IC group had bone metastasis when diagnosed, while one patient in the PTC group developed bone metastasis about 6 years after treatment completion. No patient in the TC-CIS group experienced distant metastasis. DMFS was also comparable between the three groups (P=0.84) (Figure 1B). The 10-year DMFS was 92.3%, 100%, and 96.0% in PTC, TC-CIS, and TC-IC groups, respectively (Figure 1B).
Discussion
PTC of the breast is considered to have an excellent prognosis. However, TC is not always pure; it sometimes appears together with other malignant components, such as carcinomas in situ or other invasive carcinomas. Because of its low incidence and heterogeneity, there is a paucity of studies characterizing the clinical and biological features and the natural history of mixed type of TC. To our knowledge, this is the first report evaluating the clinical characteristics and treatment outcomes of TC subgrouped as PTC, TC-CIS, and TC-IC in a single-institution cohort. Data from our cancer center illustrated that PTC and TC-CIS were more likely to be smaller in size and had substantially less nodal involvement, compared with TC-IC. Other characteristics such as age, ER status, PR status, and HER2 status were all comparable. Prognosis of patients in the three groups was generally excellent, with only one patient died of breast cancer during the follow-up period. There was no significant difference in terms of BCSS (P=0.53) or DMFS (P=0.84) between the three groups.
Tumor size
Previous studies demonstrated that TCs of the breast were usually small, with about 88%–100% of tumors smaller than 2 cm.12,14,15,19–21 An interesting finding of this study is that tumor size of TC-IC is not as small as that of PTC or TC-CIS (P=0.014). Twenty-two (71.0%) patients in the PTC group and 11 (91.7%) in the TC-CIS group had T1 disease (tumor ≤2 cm and without skin involvement), whereas in the TC-IC group, as high as 40% of patients had tumors larger than 2 cm. Thus, for TC larger than 2 cm, we should be cautious whether it is mixed with another type of invasive carcinoma. A similar proportion of patients in PTC (9.7%) and TC-IC (8.0%) had skin involvement.
Nodal involvement
As previous studies reported, PTC has a low incidence of lymph node metastasis, ranging from 2% to 12%.12,18,20,22–25 In our study, two (6.4%) patients in the PTC group had axillary lymph node metastasis, which is in consistency with previous studies. All 12 patients in the TC-CIS group were node negative. One study reported that axillary lymph node metastasis developed in only 6% of patients in the PTC, while occurred in 29% in the mixed type.18 In the present study, the TC-IC group also showed a higher rate of axillary lymph node metastasis than the PTC group and the TC-CIS group (P=0.019). In the TC-IC group, 10 (40%) patients had axillary lymph node involvement, including 7 (28%) with N1 disease, 2 (8%) with N2 disease, and 1 (4%) with N3 disease. This is an important finding, which suggests ALND should be considered in patients with TC-IC.
Molecular subtype
Previously published series have demonstrated that PTC is commonly ER positive (>85%)12,15,17,26,27 and without HER2 overexpression. As reported, HER2 overexpression rate ranged from 0% to 13% in TC.12,15,23 In the present study, 27 (87.1%) patients in the PTC group were ER positive. An important finding was that ER-positive rate was also quite high in the mixed type, which was 100% in the TC-CIS group and 96% in the TC-IC group, respectively. On the other hand, all three groups had very low HER2 overexpression rate, with only two (8%) patients in the TC-IC group and none in the other two groups. Therefore, we assume that breast cancers with a TC component are usually ER positive and HER2 negative and probably respond to endocrine therapy but not to trastuzumab.
Treatment outcomes
Treatment outcomes of all three groups were excellent. After surgery with or without adjuvant radiotherapy, no patients developed locoregional relapse. As previous studies reported, for PTC, the locoregional relapse rate ranged from 0% to 13%.15,23,24,26,28–30 The results of our study further indicate that even the mixed type of TC rarely encounters locoregional relapse. Additionally, it is reported that the 10-year BCSS was 97.2%–98% and the 10-year DMFS was 99% for PTC.15,24 Likewise, the 10-year BCSS and 10-year DMFS in our study were very high for all three groups. For the TC-IC group, the 10-year BCSS and 10-year DMFS were 95.2% and 96.0%, respectively.
An interesting finding of this study is that, even mixed with invasive ductal carcinoma or invasive lobular carcinoma, the prognosis of TC is generally quite good, despite the moderate axillary lymph node metastasis rate. It is unexpected because we thought the prognosis of mixed tumors would depend on the more aggressive part, invasive ductal carcinoma, or invasive lobular carcinoma in this case. However, TC-IC in the present study showed prognosis as good as PTC. A possible explanation is that TC-IC usually exhibits favorable clinical characteristics, such as small tumor size, high hormone receptor expression rate, and low HER2 overexpression rate. However, is there any molecular mechanism by which TC-IC has a propensity to show prognosis close to PTC but not the more aggressive component? We believe that molecular and genetic studies are warranted to uncover the underlying mechanisms.
Several limitations existed in this study, including the relatively small sample size, the retrospective nature, and the inadequate follow-up period. In addition, because of the long recruitment time, treatment strategies were sort of heterogenous.
Conclusion
The results of the present retrospective study indicate that both the pure type and mixed type of TC show very low LRR and distant metastasis rate and have excellent survival. However, as this is a small sample-sized study, further trials with larger sample sizes are warranted. Interestingly, we find TC-IC are likely to show good prognosis similar to PTC. To discover the underlying mechanisms, molecular and genetic studies might be necessary.
Acknowledgments
This work was supported by grants from the Science and Technology project of Guangdong Province, People’s Republic of China (No. 2017A030310422). The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2018000738.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1128–1135. | ||
Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17(5):1442–1448. | ||
Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology. 1992;20(6):479–489. | ||
Mcboyle MF, Razek HA, Carter JL, Helmer SD. Tubular carcinoma of the breast: an institutional review. Am Surg. 1997;63(7):639–644; discussion 644–645. | ||
Lakhani SEI, Schnitt S. WHO Classification of Tumours of the Breast, 4th edition. Lyon: IARC Press; 2012. | ||
Fernández-Aguilar S, Simon P, Buxant F, Simonart T, Noël JC. Tubular carcinoma of the breast and associated intra-epithelial lesions: a comparative study with invasive low-grade ductal carcinomas. Virchows Arch. 2005;447(4):683–687. | ||
Goldstein NS, O’Malley BA. Cancerization of small ectatic ducts of the breast by ductal carcinoma in situ cells with apocrine snouts: a lesion associated with tubular carcinoma. Am J Clin Pathol. 1997;107(5):561–566. | ||
Rakha EA, Lee AH, Evans AJ, et al. Tubular carcinoma of the breast: further evidence to support its excellent prognosis. J Clin Oncol. 2010;28(1):99–104. | ||
Li CI, Moe RE, Daling JR. Risk of mortality by histologic type of breast cancer among women aged 50 to 79 years. Arch Intern Med. 2003;163(18):2149–2153. | ||
Romano AM, Wages NA, Smolkin M, Fortune KL, Atkins K, Dillon PM. Tubular carcinoma of the breast: institutional and SEER database analysis supporting a unique classification. Breast Dis. 2015;35(2):103–111. | ||
Liu GF, Yang Q, Haffty BG, Moran MS. Clinical-pathologic features and long-term outcomes of tubular carcinoma of the breast compared with invasive ductal carcinoma treated with breast conservation therapy. Int J Radiat Oncol Biol Phys. 2009;75(5):1304–1308. | ||
Kader HA, Jackson J, Mates D, Andersen S, Hayes M, Olivotto IA. Tubular carcinoma of the breast: a population-based study of nodal metastases at presentation and of patterns of relapse. Breast J. 2001;7(1):8–13. | ||
Min Y, Bae SY, Lee HC, et al. Tubular carcinoma of the breast: clinicopathologic features and survival outcome compared with ductal carcinoma in situ. J Breast Cancer. 2013;16(4):404–409. | ||
Deos PH, Norris HJ. Well-differentiated (tubular) carcinoma of the breast. A clinicopathologic study of 145 pure and mixed cases. Am J Clin Pathol. 1982;78(1):1–7. | ||
Poirier É, Desbiens C, Poirier B, et al. Characteristics and long-term survival of patients diagnosed with pure tubular carcinoma of the breast. J Surg Oncol. 2018;117(6):1137–1143. | ||
Lea V, Gluch L, Kennedy CW, Carmalt H, Gillett D. Tubular carcinoma of the breast: axillary involvement and prognostic factors. ANZ J Surg. 2015;85(6):448–451. | ||
Li B, Chen M, Nori D, Chao KS, Chen AM, Chen SL. Adjuvant radiation therapy and survival for pure tubular breast carcinoma – experience from the SEER database. Int J Radiat Oncol Biol Phys. 2012;84(1):23–29. | ||
Fedko MG, Scow JS, Shah SS, et al. Pure tubular carcinoma and axillary nodal metastases. Ann Surg Oncol. 2010;17(Suppl 3):338–342. | ||
Sullivan T, Raad RA, Goldberg S, et al. Tubular carcinoma of the breast: a retrospective analysis and review of the literature. Breast Cancer Res Treat. 2005;93(3):199–205. | ||
Livi L, Paiar F, Meldolesi E, et al. Tubular carcinoma of the breast: outcome and loco-regional recurrence in 307 patients. Eur J Surg Oncol. 2005;31(1):9–12. | ||
Green I, Mccormick B, Cranor M, Rosen PP. A comparative study of pure tubular and tubulolobular carcinoma of the breast. Am J Surg Pathol. 1997;21(6):653–657. | ||
Vo T, Xing Y, Meric-Bernstam F, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194(4):527–531. | ||
Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046–1052. | ||
Thurman SA, Schnitt SJ, Connolly JL, et al. Outcome after breast-conserving therapy for patients with stage I or II mucinous, medullary, or tubular breast carcinoma. Int J Radiat Oncol Biol Phys. 2004;59(1):152–159. | ||
Cabral AH, Recine M, Paramo JC, McPhee MM, Poppiti R, Mesko TW. Tubular carcinoma of the breast: an institutional experience and review of the literature. Breast J. 2003;9(4):298–301. | ||
Winchester DJ, Sahin AA, Tucker SL, Singletary SE. Tubular carcinoma of the breast. Predicting axillary nodal metastases and recurrence. Ann Surg. 1996;223(3):342–347. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.