Back to Journals » OncoTargets and Therapy » Volume 13
Clinical Value of Tumor Marker Index Based on Preoperative CYFRA 21-1 and SCC-Ag in the Evaluation of Prognosis and Treatment Effectiveness in Patients with Esophageal Squamous Cell Carcinoma
Received 19 December 2019
Accepted for publication 9 April 2020
Published 13 May 2020 Volume 2020:13 Pages 4135—4143
DOI https://doi.org/10.2147/OTT.S243038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Nanchang Yin,1 Wei Liu2
1Department of Thoracic Surgery, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, People’s Republic of China; 2Department of Medical Oncology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, People’s Republic of China
Correspondence: Wei Liu
Department of Medical Oncology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, People’s Republic of China
Tel +86 416-4673536
Fax +86 416-4580464
Email [email protected]
Background: This study aimed to investigate the prognostic value of tumor marker index (TMI) based on preoperative cytokeratin 19 fragment (CYFRA 21– 1) and squamous cell carcinoma antigen (SCC-Ag) and the relationship between preoperative TMI and treatment effectiveness of postoperative adjuvant chemotherapy for patients with esophageal squamous cell carcinoma (ESCC).
Patients and Methods: Between January 2009 and December 2014, a total of 267 patients with ESCC who underwent radical resection were retrospectively enrolled. The TMI was defined as the geometric mean of normalized CYFRA 21– 1 and SCC-Ag levels. The clinical and prognostic values of TMI were determined using univariate and multivariate survival analyses.
Results: Preoperative TMI level was associated with age, tumor size, pT stage, pN stage, and CYFRA 21– 1, SCC-Ag, neutrophil-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR) levels. The 5-year overall survival rate of patients with high TMI was significantly lower than that of patients with low TMI (P < 0.001). Univariate and multivariate analyses revealed that TMI (P = 0.031) was an independent prognostic factor. Patients with ESCC with high TMI level who underwent surgery combined with postoperative chemotherapy had a significantly better prognosis than those who underwent surgery alone (P = 0.015). However, no significant difference was observed in patients with low TMI level (P = 0.682).
Conclusion: TMI as a prognostic indicator of ESCC is superior to CYFRA 21– 1 and SCC-Ag. The TMI might be useful in predicting the therapeutic effectiveness of postoperative chemotherapy and selecting patients who may benefit from postoperative chemotherapy.
Keywords: esophageal squamous cell carcinoma, CYFRA 21-1, SCC-Ag, tumor marker index, adjuvant chemotherapy
Introduction
Esophageal cancer is the eighth most commonly diagnosed cancer worldwide.1 In China, esophageal squamous cell carcinoma (ESCC) is the predominant histopathological type. Despite the recent improvements in surgical techniques and adjuvant therapies, esophageal cancer ranked fifth and fourth among all cancers for incidence and mortality, respectively.2 Curative surgical resection is the best approach in the treatment of patients with localized carcinoma. The National Comprehensive Cancer Network (NCCN) guidelines for the diagnosis and treatment of esophageal cancer recommend that, regardless of pT or pN stage, no additional treatment is needed for patients who have undergone R0 resection.3 Neither prospective clinical trials nor retrospective studies regarding the influence of postoperative chemotherapy on the survival of patients with ESCC have obtained a conclusion.4,5 Therefore, it is of great importance to identify potential predictive biomarkers for the benefit of postoperative adjuvant therapy so that some patients could avoid the burden of toxic chemotherapy treatment and improve their outcomes.
Tumor-related proteins that are generated by cancer cells and secreted into the peripheral circulation could be detected.6 In the clinical, peripheral proteins are commonly used as noninvasive tools to detect cancer, identify tumor progression, and predict prognosis.7 Presently, cytokeratin 19 fragment (CYFRA 21–1), squamous cell carcinoma antigen (SCC-Ag), carbohydrate antigen 72–4 are useful biomarkers for diagnosis, progression, and prognosis evaluation of ESCC.8–11 Besides, several studies have reported the association between tumor markers (CYFRA 21–1 and SCC-Ag) and postoperative adjuvant chemotherapy in ESCC.11,12 Therefore, these biomarkers may guide the selection of patients with ESCC who may benefit from postoperative chemotherapy. However, the sensitivities and specificities of these tumor markers are unacceptably low. Additionally, the best biomarker for the selection patients for adjuvant chemotherapy remains unknown. Therefore, if these two biomarkers are used in combination, it may result in a more accurate and useful biomarker for guiding adjuvant therapy of ESCC. Recently, tumor marker index (TMI) based on the CYFRA 21–1 and CEA levels was introduced in non-small cell lung cancer (NSCLC) by Muley et al13 and showed more strong prognostic value in resected NSCLC.13,14 Tomita et al15 found that TMI based on preoperative serum CEA and KL-6 levels might be useful in the prediction of the prognosis of patients with NSCLC. TMI, which is based on CYFRA 21–1 and SCC-Ag, was reported to be an independent prognostic factor for patients with ESCC.16 From this point of view, the TMI, which was established by combining CYFRA 21–1 and SCC-Ag, may be a more useful predictive biomarker for the selection of patients with ESCC who may benefit from postoperative adjuvant chemotherapy.
Therefore, in the present study, we examined the clinical and prognostic significance of TMI based on preoperative CYFRA 21–1 and SCC-Ag in patients with ESCC. More importantly, we explored if the TMI could be a predictor for selecting patients who may benefit from postoperative adjuvant chemotherapy.
Patients and Methods
Patients
We retrospectively reviewed patients with ESCC who underwent radical esophagectomy with lymph node dissection at the First Affiliated Hospital of Jinzhou Medical University between January 2009 and December 2014. Finally, a total of 267 patients with ESCC were enrolled in this study according to the following eligibility criteria: 1) histologically proven ESCC; 2) absence of distant metastasis and other malignant tumors; 3) absence of neoadjuvant therapy, including chemotherapy, radiotherapy, or chemoradiotherapy; 4) radical esophagectomy; and 5) complete clinical pathology and follow-up data.
The depth of tumor invasion, lymph node metastasis, and tumor-node-metastasis (TNM) staging were classified according to the 7th edition of the UICC/AJCC TNM classification.17 Histological grade was classified according to the World Health Organization classification of esophageal tumors.18 Clinical data, including demographic and clinicopathological features, surgery, and postoperative therapy, were collected from our medical records. This study was approved by the ethics committee of the First Affiliated Hospital of Jinzhou Medical University. All patients provided written informed consent.
Postoperative Adjuvant Chemotherapy Regimens
Based on the NCCN, Japanese Esophageal Society, and Chinese Anti-Cancer Association guidelines,3,19,20 we did not have a standardized protocol for postoperative adjuvant therapy during the study period. In the present study, a total of 95 (35.6%) patients with ESCC underwent curative surgery alone, while 172 (64.4%) underwent surgery combined with postoperative adjuvant chemotherapy. Among the 172 patients who received adjuvant chemotherapy, 108 (62.8%) received 5-fluorouracil infusion plus cisplatin chemotherapy, 48 (27.9%) received paclitaxel plus platinum chemotherapy, and 16 (9.3%) received an irregular regimen chemotherapy.
Measurement of Tumor Markers and TMI
Blood samples were obtained from all included patients within 1 week preoperatively. CYFRA 21–1 and SCC-Ag as the routine preoperative examination items were detected in the clinical laboratory in our hospital by commercially available enzyme immunoassays using a Roche E170 modular immunoassay analyzer (USA). The normal upper limits were 3.3 μg/L for CYFRA 21–1 and 1.5 μg/L for SCC-Ag. According to the normal upper limits of tumor markers, patients were divided into low and high groups.
The TMI was defined by determining the geometric mean of the normalized values of the serum CYFRA 21–1 and SCC-Ag levels.16 Normalization was performed by dividing individual marker values by corresponding diagnostic cut-off points, which were 3.3 μg/L for CYFRA 21–1 and 1.5 μg/L for SCC-Ag. The TMI was calculated as previously described.16
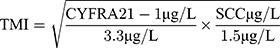
Follow-Up
After surgery, all patients were followed up in the outpatient department every 3 months for the first 2 years and then annually until death or the last follow-up. The follow-up included recording the medical history, physical examinations, chest computed tomography, and endoscopy (if necessary). The last follow-up date was December 2018, and the follow-up rate was 91.8%. The cases lost to follow-up were treated as censored data for the analysis of survival. The median follow-up duration of the 267 patients with ESCC was 36.0 months (range, 3–120 months). Overall survival (OS) was defined as the time from the date of surgery to the date of death or final clinical follow-up.
Statistical Analysis
The receiver operating characteristics (ROC) curve was used to determine the optimal cutoff value of TMI used for prognostic prediction. The correlations between preoperative TMI level and clinicopathological characteristics were assessed using the χ2 test. Survival curves were obtained according to the Kaplan-Meier method, and comparison of survival curves was conducted using the Log rank test. Furthermore, factors deemed as potentially significant by univariate analysis were subjected to a multivariate analysis with Cox proportional hazards model to identify the independent prognostic factors. A P-value < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 17.0 software (SPSS, Inc., Chicago, IL).
Results
Clinicopathological Characteristics of Patients
The clinicopathological characteristics of patients are summarized in Table 1. There were 219 men and 48 women, with a median age of 60 years (range, 44–79 years). A total of 185 (69.3%) patients were former or current smokers. Moreover, 22 patients had tumors in the upper third of the esophagus, 150 in the middle third, and 95 in the lower third. Tumors of 143 (53.6%) patients were ≥ 4.0 cm in size, pT3 (94, 35.2%) and pT4a (97, 36.3%) diseases were observed in 71.5% of the patients, and positive lymph nodes were observed in 122 (45.7%) patients. There were 119 (44.6%) patients with TNM stage I–II and 148 (55.4%) patients with TNM stage III.
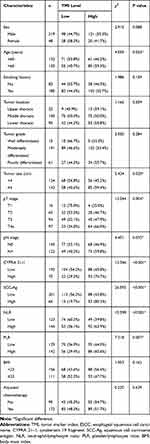 |
Table 1 Correlations Between the TMI Level and Clinicopathological Features of Patients with ESCC (n=267) |
Correlations Between Preoperative TMI and Clinicopathological Characteristics
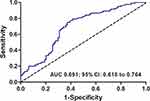
The best cutoff value for TMI was 0.599 (sensitivity, 75.0%; specificity, 62.8%; and area under the ROC curve, 0.691; Figure 1). Using this cutoff value, the patients were subdivided into two subgroups: 141 (52.8%) patients with TMI > 0.599 (high TMI group) and 126 patients (47.2%) with TMI ≤ 0.599 (low TMI group). The correlations between the preoperative TMI level and clinicopathological characteristics are shown in Table 1. An increased preoperative TMI level correlated closely with age (P = 0.033), tumor size (P = 0.020), pT stage (P = 0.004), pN stage (P = 0.035), CYFRA 21–1 level (P < 0.001), SCC-Ag level (P < 0.001), NLR level (P < 0.001), and PLR level (P = 0.007). However, no statistically significant association was observed between preoperative TMI level and sex, smoking history, tumor location, tumor grade, body mass index (BMI), and postoperative adjuvant chemotherapy (P > 0.05).
Prognostic Value of Preoperative TMI
The 1-, 3-, and 5-year OS rates in all patients were 84.6%, 51.7%, and 37.3%, respectively. Kaplan–Meier analysis showed that the low-CYFRA 21–1 group had a markedly higher 5-year OS rate than the high-CYFRA 21–1 group (40.5% vs. 28.7%; P = 0.012; Figure 2A) and the low-SCC-Ag group had a markedly higher 5-year OS rate than the high-SCC-Ag group (39.2% vs. 31.2%; P = 0.025; Figure 2B). Furthermore, the 5-year OS rate in patients with ESCC with a high TMI level was 25.7%, which was significantly lower than that in patients with a low TMI level (50.2%, P < 0.001; Figure 2C).
The results of the univariate analysis revealed that age; smoking history; tumor grade; tumor size; pT stage; pN stage; CYFRA 21–1, SCC-Ag, TMI, NLR, and PLR levels; and postoperative chemotherapy were significantly related to patients’ prognosis (P < 0.05). Further multivariate analysis revealed that tumor grade (HR, 1.495; 95% CI, 1.089–2.053; P = 0.013), pT stage (HR, 1.247; 95% CI, 1.027–1.515; P = 0.026), pN stage (HR, 1.699; 95% CI, 1.235–2.336; P = 0.001), TMI levels (HR, 1.453; 95% CI, 1.035–2.040; P = 0.031), and postoperative chemotherapy (HR, 0.686; 95% CI, 0.490–0.961; P = 0.028) were independent prognostic factors in patients with ESCC (Table 2). However, preoperative serum CYFRA 21–1 (HR, 1.263; 95% CI, 0.896–1.779; P = 0.182) and SCC-Ag (HR, 1.049; 95% CI, 0.655–1.386; P = 0.801) levels were not independent prognostic factors. These results showed that, compared with preoperative CYFRA 21–1 and SCC-Ag, the preoperative TMI was a better indicator significantly associated with OS of patients with ESCC.
 |
Table 2 Univariate and Multivariate Analyses for Overall Survival in 267 Patients with ESCC |
Association of Preoperative TMI with Postoperative Adjuvant Chemotherapy
In the present study, postoperative adjuvant chemotherapy was performed in 172 (64.4%) patients. To further evaluate the prognostic value of TMI in different subgroups of patients with ESCC, the patients were further classified according to the postoperative adjuvant chemotherapy status. In patients with ESCC who underwent surgery alone, the 5-year OS rate in patients with a high TMI level was 16.9%, which was significantly lower than that in patients with a low TMI level (50.7%, P = 0.001; Figure 3A). In patients with ESCC who underwent surgery and postoperative adjuvant chemotherapy, the 5-year OS rate in patients with a high TMI level was significantly lower than that in patients with a low TMI level (31.0% vs. 49.8%, P = 0.006; Figure 3B).
The 5-year OS rate in 172 patients who received adjuvant chemotherapy was 40.2%, which was significantly higher than that in patients who did not received adjuvant chemotherapy (31.9%, P = 0.038, Figure 4A). Furthermore, we explored whether the TMI is correlated with the therapeutic effect of postoperative chemotherapy in patients with ESCC. Our results revealed that, in patients with ESCC with high TMI level, those who underwent surgery combined with postoperative adjuvant chemotherapy had a significantly better prognosis than those who underwent surgery alone (χ2= 5.931, P = 0.015, Figure 4B). However, this difference of OS between the two groups cannot be observed in patients with low TMI level (χ2= 0.168, P = 0.682, Figure 4C).
Discussion
Currently, the efficacy of postoperative treatment, especially postoperative chemotherapy for patients with ESCC remains controversial, with no consensus being reached.21,22 Several studies have indicated that postoperative adjuvant chemotherapy compared with surgery alone could improve the outcome of patients with lymph nodes metastasis.22,23 However, there are substantial differences in survival for patients with similar TNM stage with the same treatment regimens.24 With regard to the toxicity and different survival benefit of postoperative adjuvant chemotherapy, potential predictive biomarkers for patients with ESCC are needed to identify those who could benefit from adjuvant chemotherapy. In this study, we investigated the associations between TMI based on preoperative CYFRA 21–1 and SCC-Ag levels and clinicopathological characteristics and survival in patients with ESCC. We initially reported that preoperative TMI may be helpful in predicting the efficacy of postoperative adjuvant chemotherapy in patients with ESCC.
Blood tumor biomarkers have advantages of being inexpensive easily accessible and allowing measurements in the clinic. Besides, tumor biomarkers have potential applications in cancer diagnosis, relapse detection, and prognosis prediction.25 It is well known that CYFRA 21–1 and SCC-Ag are important tumor markers for ESCC and correlated with clinicopathological parameters and prognosis.26,27 In this study, we analyzed the associations between CYFRA 21–1 and SCC-Ag and clinicopathological variables and prognosis in patients with ESCC. Our results showed that increased preoperative CYFRA 21–1 and SCC-Ag levels were related to depth of tumor invasion, lymph node metastasis, and TNM stage in patients with ESCC. Our results were consistent with those of the studies by Mao et al28 and Cao et al.24 Although the univariate analysis showed that increased CYFRA 21–1 and SCC-Ag levels were related to poor prognosis in patients with ESCC, we failed to show the independent prognostic value in the multivariate analysis. The results of the survival analyses were consistent with those of the study by Qiao et al.16 This result may be explained by relatively low sensitivity of CYFRA 21–1 and SCC-Ag in ESCC.
To improve the accuracy of tumor markers in evaluating patients’ prognosis, Muley et al13 initially introduced an algorithm based on CYFRA 21–1 and CEA, which was known as the TMI. By analogy, Qiao et al16 also established a new TMI based on CYFRA 21–1 and SCC-Ag and revealed that TMI was an independent prognostic factor in ESCC. In the present study, the multivariate survival analysis also demonstrated the powerful prognostic value of TMI based on CYFRA 21–1 and SCC-Ag in patients with ESCC.
The JCOG 9204 clinical trial and several studies have demonstrated that there is a significant disease-free survival benefit from surgery combined with adjuvant chemotherapy compared with surgery alone in patients with advanced stage ESCC.22,23,29 However, adjuvant chemotherapy is also associated with considerable side effects and morbidity, and some patients will not benefit from adjuvant chemotherapy. Therefore, there is an urgent need to seek biomarkers for identifying what therapy is optimum for a given patient. Some studies reported that CYFRA 21–1 is valuable in predicting the efficacy of chemotherapy, radiotherapy, or chemoradiotherapy in patients with ESCC.12,30 Yang et al11 reported that patients with ESCC with low CA19–9, CEA, SCC-Ag levels may be more likely to benefit from the postoperative chemotherapy. Besides, some molecular biomarkers in ESCC have also been reported to be able to predict efficacy of therapy.31,32 However, to date, the optimum biomarker for predicting efficacy of chemotherapy is unknown. Therefore, our aim was to identify predictive biomarkers to individualize adjuvant chemotherapy in patients with ESCC.
The measurement of serum CYFRA 21–1 and SCC-Ag levels is inexpensive and routinely available in the clinical. Our results demonstrated that the TMI, by combining CYFRA 21–1 and SCC-Ag, could increase the prognostic evaluation. Therefore, it seems that the TMI may also increase the predictive value and serve as a new variable for predicting efficacy of adjuvant chemotherapy in patients with ESCC. Our results showed that postoperative adjuvant chemotherapy in patients with ESCC with high TMI level prolonged survival compared with surgery alone. However, in patients with low TMI level, adjuvant chemotherapy could not prolong the outcomes. Our results indicated that patients with ESCC with high TMI level may benefit from postoperative adjuvant chemotherapy. Thus, from these results, it can be hypothesized that the TMI based on CYFRA 21–1 and SCC-Ag may be as a valuable marker for identifying patients with ESCC who could benefit from postoperative chemotherapy. However, further large-scale sample and prospective studies in this area are warranted to validate these results.
To the best of our knowledge, this is the first study to demonstrate that the TMI based on CYFRA 21–1 and SCC-Ag has predictive implications for patients with ESCC with respect to postoperative adjuvant chemotherapy. However, our study has some limitations. First, this was a retrospective study, and all data were collected from one single institution. Thus, selection bias might be underestimated. Second, patients with T4a disease in the study did not receive neoadjuvant therapy, which may influence the treatment effect. Moreover, we acknowledge that tumor biology is complex and the TMI alone will not determine the treatment plan. In the clinical setting, more clinicopathological variables are needed to be taken into consideration.
Conclusion
The TMI, which is based on preoperative CYFRA 21–1 and SCC-Ag, appears to be an independent prognostic factor for patients with ESCC. Furthermore, a close correlation was observed between TMI level and the efficacy of postoperative adjuvant chemotherapy. The TMI may help in the selection of patients with ESCC who may benefit from adjuvant chemotherapy.
Acknowledgments
This study was supported by Basic Research Projects in Colleges and Universities of Liaoning Province (JYTQN201702, JYTQN201705), and the Science and Technology Research Project of Liaoning Province (21080550406).
Disclosure
All authors state that they have no conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–883. doi:10.6004/jnccn.2019.0033
4. Zhang SS, Yang H, Xie X, et al. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus. 2014;27(6):574–584. doi:10.1111/dote.12073
5. Zhao P, Yan W, Fu H, Lin Y, Chen KN. Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: a meta-analysis. Thorac Cancer. 2018;9(8):1048–1055. doi:10.1111/1759-7714.12787
6. Baek AR, Seo HJ, Lee JH, et al. Prognostic value of baseline carcinoembryonic antigen and cytokeratin 19 fragment levels in advanced non-small cell lung cancer. Cancer Biomark. 2018;22(1):55–62. doi:10.3233/CBM-170885
7. Zhong H, Qian Y, Fang S, Wang Y, Tang Y, Gu W. Prognostic value of plasma fibrinogen in lung cancer patients: a meta-analysis. J Cancer. 2018;9(21):3904–3911. doi:10.7150/jca.26360
8. Kawaguchi H, Ohno S, Miyazaki M, et al. CYFRA 21-1 determination in patients with esophageal squamous cell carcinoma: clinical utility for detection of recurrences. Cancer Am Cancer Soc. 2000;89(7):1413–1417.
9. Zhang HQ, Wang RB, Yan HJ, et al. Prognostic significance of CYFRA21-1, CEA and hemoglobin in patients with esophageal squamous cancer undergoing concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2012;13(1):199–203. doi:10.7314/apjcp.2012.13.1.199
10. Ma Z, Wu X, Xu B, et al. Development of a novel biomarker model for predicting preoperative lymph node metastatic extent in esophageal squamous cell carcinoma. Oncotarget. 2017;8(62):105790–105799. doi:10.18632/oncotarget.22399
11. Yang Y, Huang X, Zhou L, et al. Clinical use of tumor biomarkers in prediction for prognosis and chemotherapeutic effect in esophageal squamous cell carcinoma. BMC Cancer. 2019;19(1):526. doi:10.1186/s12885-019-5755-5
12. Nakamura T, Ide H, Eguchi R, Hayashi K, Takasaki K, Watanabe S. CYFRA 21-1 as a tumor marker for squamous cell carcinoma of the esophagus. Dis Esophagus. 2017;11(1):35–39. doi:10.1093/dote/11.1.35
13. Muley T, Fetz TH, Dienemann H, et al. Tumor volume and tumor marker index based on CYFRA 21-1 and CEA are strong prognostic factors in operated early stage NSCLC. Lung Cancer. 2008;60(3):408–415. doi:10.1016/j.lungcan.2007.10.026
14. Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Prognostic significance of tumour marker index based on preoperative CEA and CYFRA 21-1 in non-small cell lung cancer. Anticancer Res. 2010;30(7):3099–3102.
15. Tomita M, Ayabe T, Chosa E, Nose N, Nakamura K. Prognostic significance of a tumor marker index based on preoperative serum carcinoembryonic antigen and krebs von den lungen-6 levels in non-small cell lung cancer. Asian Pac J Cancer Prev. 2017;18(1):287–291. doi:10.22034/APJCP.2017.18.1.287
16. Qiao Y, Chen C, Yue J, Yu Z. Tumor marker index based on preoperative SCC and CYFRA 21-1 is a significant prognostic factor for patients with resectable esophageal squamous cell carcinoma. Cancer Biomark. 2019;25(3):243–250. doi:10.3233/CBM-190058
17. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
18. Flejou JF. WHO classification of digestive tumors: the fourth edition. Ann Pathol. 2011;31(5 Suppl):S27–S31. doi:10.1016/j.annpat.2011.08.001
19. Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus-Tokyo. 2015;12:1–30. doi:10.1007/s10388-014-0465-1
20. Chinese Society of Esophageal Cancer, Chinese Anti-Cancer Association. Clinical Practice Guidelines for the Diagnosis and Treatment of Esophageal Cancer. Beijing: Peking Union Medical College Press; 2011. doi:10.1111/j.1572-0241.1999.00767.x
21. Saeed NA, Mellon EA, Meredith KL, et al. Adjuvant chemotherapy and outcomes in esophageal carcinoma. J Gastrointest Oncol. 2017;8(5):816–824. doi:10.21037/jgo.2017.07.10
22. Qin RQ, Wen YS, Wang WP, Xi KX, Yu XY, Zhang LJ. The role of postoperative adjuvant chemotherapy for lymph node-positive esophageal squamous cell carcinoma: a propensity score matching analysis. Med Oncol. 2016;33(4):31. doi:10.1007/s12032-016-0746-8
23. Pasquer A, Gronnier C, Renaud F, et al. Impact of adjuvant chemotherapy on patients with lymph node-positive esophageal cancer who are primarily treated with surgery. Ann Surg Oncol. 2015;22(Suppl 3):S1340–S1349. doi:10.1245/s10434-015-4658-1
24. Cao X, Zhang L, Feng GR, et al. Preoperative Cyfra21-1 and SCC-Ag serum titers predict survival in patients with stage II esophageal squamous cell carcinoma. J Transl Med. 2012;10:197. doi:10.1186/1479-5876-10-197
25. Iwasaki Y, Arai K, Katayanagi S, et al. Biomarkers for neoplasmas in digestive organs. Gan to Kagaku Ryoho. 2004;31(7):1015–1020.
26. Yamamoto K, Oka M, Hayashi H, Tangoku A, Gondo T, Suzuki T. CYFRA 21-1 is a useful marker for esophageal squamous cell carcinoma. Cancer Am Cancer Soc. 1997;79(9):1647–1655.
27. Shimada H, Nabeya Y, Okazumi S, et al. Prognostic significance of CYFRA 21-1 in patients with esophageal squamous cell carcinoma. Surgery. 2003;133(5):486–494. doi:10.1067/msy.2003.139
28. Mao YS, Zhang DC, Zhao XH, Wang LJ, Qi J, Li XX. Significance of CEA, SCC and Cyfra21-1 serum test in esophageal cancer. Zhonghua Zhong Liu Za Zhi. 2003;25(5):457–460.
29. Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol. 2003;21(24):4592–4596. doi:10.1200/JCO.2003.12.095
30. Yi Y, Li B, Sun H, et al. Predictors of sensitivity to chemoradiotherapy of esophageal squamous cell carcinoma. Tumour Biol. 2010;31(4):333–340. doi:10.1007/s13277-010-0041-9
31. Kwon D, Yun JY, Keam B, Kim YT, Jeon YK. Prognostic implications of FGFR1 and MYC status in esophageal squamous cell carcinoma. World J Gastroenterol. 2016;22(44):9803–9812. doi:10.3748/wjg.v22.i44.9803
32. Wadhwa R, Wang X, Baladandayuthapani V, et al. Nuclear expression of Gli-1 is predictive of pathologic complete response to chemoradiation in trimodality treated oesophageal cancer patients. Br J Cancer. 2017;117(5):648–655. doi:10.1038/bjc.2017.225
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.