Back to Journals » Journal of Inflammation Research » Volume 15
Clinical Value of Serum Interleukin-18 in Neonatal Sepsis Diagnosis and Mortality Prediction
Authors Li X, Li T, Dong G, Wei Y, Xu Z, Yang J
Received 25 October 2022
Accepted for publication 24 December 2022
Published 30 December 2022 Volume 2022:15 Pages 6923—6930
DOI https://doi.org/10.2147/JIR.S393506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Xiaojuan Li, Tiewei Li, Geng Dong, Yulei Wei, Zhe Xu, Junmei Yang
Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, People’s Republic of China
Correspondence: Tiewei Li, Email [email protected]
Purpose: Previous studies have demonstrated that interleukin-18 (IL-18) levels were elevated in adult patients with sepsis. However, its role in neonatal sepsis remains unknown. The current research was conducted to assess the clinical value of serum IL-18 level as a candidate biomarker in neonatal sepsis diagnosis and prediction of mortality.
Patients and Methods: From July 2022 to September 2022, we prospectively enrolled 91 septic neonates and 31 non-sepsis neonates in the intensive care unit of neonates at Henan Children’s Hospital in Zhengzhou, China. Neonatal peripheral blood serum was collected at admission and levels of serum IL-18 were assessed. Employing multivariate logistic regression analysis, the evaluation of the potential of IL-18 as an independent biomarker for sepsis was executed. Furthermore, employing the receiver operating characteristic (ROC) curve analysis, the diagnostic value of IL-18 in sepsis and the ability of IL-18 in predicting the mortality of neonatal sepsis was measured. The statistical package SPSS 24.0 was employed to conduct all statistical analyses.
Results: Serum IL-18 levels in neonates in the sepsis group were elevated compared to the control group, reaching the highest levels in the non-survival sepsis group (P < 0.001). Correlation analysis exhibited a positive relationship between IL-18 levels and age, body temperature, respiratory rate, and C-reactive protein levels. IL-18 was identified as an independent biomarker in identifying sepsis (OR = 4.747, 95% CI 1.493– 15.092, P = 0.008) by multiple logistic regression. ROC curve analysis exhibited that IL-18 was good in identifying neonatal sepsis (area under curve (AUC) = 0.77, 95% CI = 0.68– 0.85, P < 0.001) and predicting neonatal mortality (AUC = 0.80, 95% CI = 0.63– 0.96, P = 0.003).
Conclusion: IL-18 was a potential biomarker for identifying neonatal sepsis and neonatal mortality prediction.
Keywords: interleukin-18, neonatal sepsis, mortality
Introduction
Neonatal sepsis is a severe bloodstream infection associated with a systemic inflammatory response that seriously threatens the health and life of neonates worldwide.1 A population-level estimate of neonatal sepsis, with a death rate in the range of 11% and 19%, was found in an epidemiological study to be 2202 per 100000 live births.2 Neonatal sepsis remains a leading cause of morbidity and mortality in infants worldwide.3–5 Surviving Sepsis Campaign Physician’s management guidelines promote early sepsis identification, as well as treatment.6 Traditionally, confirmed neonatal sepsis is diagnosed by isolating pathogens from sterile body fluids, such as blood, cerebrospinal fluid, and urine. However, the isolation of pathogens from sterile body fluids usually has a long waiting time and the accuracy is easily affected by the volume of body fluids and the pre-hospital antibiotic use.7 Therefore, it is critical to identify new biomarkers for neonatal sepsis.
Uncontrolled inflammatory response play an important role in sepsis-induced multiple organ dysfunction.8 As a pro-inflammatory cytokine, Interleukin-18 (IL-18) is an important effector during sepsis, which is crucial for host defense against bacteria, viruses, and fungi.9,10 Upon stimulation, several cell types produce IL-18.11 Excessive IL-18 can aggravate the body’s inflammatory response and induce myocardial and kidney injury.12,13 Okuhara Y et al14 demonstrated that deletion of IL-18 could improve cardiac dysfunction and survival during sepsis. Clinical studies showed that serum IL-18 levels were up-regulated in adult patients with sepsis.15,16 In terms of neonates, only one study reported that salivary IL-18 level was not a viable biomarker for the early neonatal sepsis diagnosis,17 while there has not been any research on the relationship between serum IL-18 levels and neonatal sepsis. Accordingly, we envisioned in the present research to evaluate the clinical value of serum IL-18 level for its application as a potential biomarker in neonatal sepsis diagnosis and predicting mortality.
Materials and Methods
Study Population
During July 2022 to September 2022, the prospective case-control research was performed in the intensive care unit of neonates at Henan Children’s Hospital in Zhengzhou, China, which included 122 neonates with probable sepsis. The inclusion criteria for this study were (1) neonates aged ≤ 28 days, and (2) neonates with complete clinical and laboratory data comprised in this study. The exclusion criteria were neonates with cancers, hematological system diseases, auto-immune diseases, or major congenital malformations.
Clinical Definition
The systemic inflammatory response syndrome (SIRS) criteria for defining neonatal sepsis include a suspected or confirmed infection. In neonates, SIRS is characterized as having at least two or more of the subsequent four situations, one of which must be aberrant body temperature or leukocyte count: (1) core body temperature of > 38.5°C or < 36°C; (2) tachycardia or bradycardia, (3) average respiratory rate > 2 SD above normal for age or in the presence of mechanical ventilation, and (4) abnormal leukocyte count or >10% immature neutrophils. Both pneumonia and sepsis were diagnosed by two research investigators, as per the issued International Pediatric Sepsis Consensus.18
Laboratory Measurements
Upon hospital admission, blood samples were obtained, and the separation of serum was completed by centrifugation, which was kept at −80°C until analysis. Utilizing a commercial enzyme-linked immunosorbent assay kit (Human IL-18 Valukine enzyme linked immunosorbent assay Kit, Bio-Techne, China), the assessment of IL-18 serum levels was done in accordance with the instructions provided by the manufacturer. IL-18 has <3.0% intra-assay and <8.0% inter-assay coefficients of variation. Using the UPPER analyzer (Ultrasensitive C-reactive protein kit, Upper Bio-Tech, Shanghai, China), C-reactive protein (CRP) was quantified. A routine clinical analytical method and an automatic biochemistry analyzer (AU5800 Clinical Chemistry Analyzers, Beckman Coulter, California) were employed to measure the levels of serum albumin (ALB), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). In this study, CRP levels <0.8 mg/L were defined as a value of 0.7 mg/L. In this study, all data were obtained from electronic records, except for IL-18.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) for variables conforming to the normal distribution or expressed as medians (interquartile range) for non-normally distributed variables, which were analyzed using independent t-tests, one-way analysis of variance (ANOVA) or the Mann–Whitney U-test. The normality of the distribution of variables was tested with the Shapiro–Wilk test. Examination of categorical variables was achieved utilizing Chi-square tests and articulated as percentages. Employing Spearman correlation test, the associations between IL-18 levels and other continuous variables were investigated. For neonatal sepsis, to determine whether IL-18 was an independent risk factor, multiple logistic regression analysis was employed. Previously published literature and univariate P-values below 0.01 served as the basis for prespecified risk aspects. In addition, employing the receiver operating characteristic (ROC) curve analysis, the diagnostic value of IL-18 in sepsis and the ability of IL-18 in predicting the mortality of neonatal sepsis were assessed. Youden’s index (sensitivity + specificity − 1) was employed to discover the optimal cut-off point of IL-18 in the diagnosis of neonatal sepsis and in predicting mortality.19 All data were analyzed employing SPSS version 24.0 (Chicago, Illinois, USA). Significant results were defined as a two-sided P-value <0.05.
Results
Study Population Characteristics
According to the diagnosis criteria for sepsis, the 122 neonates who participated in this study were classified into two groups. Specifically, 91 of the 122 neonates were identified as having sepsis, and the other 31 neonates without sepsis were severed as the control group. Of the 91 septic neonates, nine died during hospitalization. For the three groups, Table 1 provides an overview of the baseline clinical and laboratory information. In comparison with neonates belonging to the control group, those in the sepsis group had lower body weight and an elevated heart rate, respiratory rate, and body temperature. Biochemical analysis exhibited that septic neonates had elevated levels of CRP, ALT, and AST and lower levels of ALB. Furthermore, neonates with sepsis also had elevated levels of IL-18. Further analysis exhibited that IL-18 level was elevated steadily in the control, survivor sepsis, and non-survivor sepsis groups.
 |
Table 1 Basic Characteristics of Study Subjects by Groups |
Correlation Between Clinical Parameters and Levels of Serum IL-18
As showed in Table 2, IL-18 was positive correlated with age (r = 0.239, P = 0.008), body temperature (r = 0.214, P = 0.018), respiratory rate (r = 0.301, P < 0.001), and CRP (r = 0.280, P = 0.002). There was no correlation between IL-18 and heart rate, body weight, neutrophil count, and lymphocyte count.
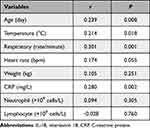 |
Table 2 Correlations Between the IL-18 and Clinical Parameters |
Independence of IL-18 in Identifying Neonatal Sepsis
The multiple logistic regression analysis incorporated variables with a P-value <0.01 from the univariate logistic analysis, including respiratory rate, ALB, total neutrophil count, ALT, and IL-18. In addition, age, gestational age, body weight, body temperature and heart rate, as traditional risk factors for neonatal sepsis, were also incorporated in the multiple logistic regression analysis. After adjusting these variables, IL-18 still served as an independent indicator for neonatal sepsis (OR = 4.747, 95% CI 1.493–15.092, P = 0.008). Furthermore, our data also showed that ALB (OR = 0.724, 95% CI 0.607–0.865, P < 0.001) and neutrophil count (OR = 1.791, 95% CI 1.299–2.470, P < 0.001) were independent biomarkers for neonatal sepsis (Table 3).
 |
Table 3 Relative Risk of IL-18 for Neonatal Sepsis |
Clinical Value of IL-18 in Neonatal Sepsis Diagnosis and Predicting Mortality
Employing the ROC curve analysis, the diagnostic value of IL-18 in sepsis and the ability of IL-18 in anticipating the mortality of neonatal sepsis were measured. Figure 1A depicts the area under curve (AUC) for IL-18 in identifying neonatal sepsis was 0.77 (95% CI, 0.68–0.85, P < 0.001). The optimal cut-off value of IL-18 was 0.85 ng/mL with 60% sensitivity and 84% specificity, in neonatal sepsis diagnosis. Based on the cutoff value of IL-18 in neonatal sepsis diagnosis, neonates were classified into the following two groups: the group with low IL-18 (IL-18 ≤ 0.85) and the group with high IL-18 (IL-18 > 0.85). As shown in Table 4, there were 55 (91.7%) neonates with sepsis and 5 (8.3%) neonates without sepsis in the group with high IL-18. As depicted in Figure 2, the prevalence of neonatal sepsis in the group with high IL-18 was substantially higher in comparison with the group with low IL-18 (91.7% vs 58.1%, P < 0.001) (Figure 2). Meanwhile, the AUC for IL-18 in predicting neonatal mortality was 0.80 (95% CI, 0.63–0.96, P < 0.001). The optimal cut-off value of IL-18 was 1.49 ng/mL with 78% sensitivity and 79% specificity, in predicting neonatal mortality (Figure 1B).
 |
Table 4 Distribution of Neonates With/Without Sepsis Based on the Optimal Cutoff Point of IL-18 |
 |
Figure 2 Distribution of neonates in high or low IL-18 groups. |
Discussion
Newborns’ underdeveloped immune systems make them more vulnerable to pathogenic infections. Uncontrolled initiation of inflammatory cascades induced by the infections aids in the progression of neonatal sepsis.20 In the neonatal period, neonatal sepsis is linked to severe morbidity and mortality.1 A timely and accurate neonatal sepsis diagnosis is essential for an appropriate treatment, which can avoid the occurrence of severe and life-threatening complications and death. Presently, blood culture. which is a gold standard of neonatal sepsis diagnosis, goes through an extended waiting time as well as a low positive pathogenic detection rate.21 Furthermore, the clinical manifestations of neonatal sepsis are non-specific. Thus, the early identification of pathogenic organisms causing sepsis is difficult and identifying new biomarkers for neonatal sepsis diagnosis and mortality prediction is critical.
IL-18 was a member of the IL-1 superfamily and has an important role in inflammatory processes.22 Sun et al23 reported that serum IL-18 level is elevated in adult patients with sepsis and had a positively correlate with disease severity. Neutralization of IL-18 or deletion in IL-18 can protect against sepsis-induced injuries, including myocardial dysfunction, and acute kidney and lung injury.12,14,24–26 Wynn et al27 demonstrated that IL-18 administration could enhance the murine neonatal systemic inflammatory response to sepsis and increase the mortality of neonatal sepsis mice. In contrast, IL-18 deletion has been shown to significantly improve the survival rate of septic mice.27 To date, only one research has presented that the serum IL-18 levels were substantially improved in premature neonates with infection.28 However, a few studies have evaluated the clinical value of IL-18 as a candidate biomarker for neonatal sepsis diagnosis and mortality prediction.
The link between neonatal sepsis and the levels of serum IL-18 was first explored in this study, and we discovered that neonates with sepsis had elevated levels of IL-18. Furthermore, compared with the survivor septic neonates, serum IL-18 level was further elevated in non-survivor septic neonates. Multivariate analysis exhibited that IL-18 served as a risk variable for neonatal sepsis. ROC curve analysis confirmed that IL-18 with a good discriminatory capability in neonatal sepsis diagnosis. The optimal cutoff value of IL-18 in neonatal sepsis diagnosis was 0.85 ng/mL, with 60% sensitivity and 84% specificity. Based on the IL-18 cutoff value, neonates were divided into the groups with high or low IL-18 groups and the majority of neonates (91.7%) that had sepsis were in the group with high IL-18 group. Furthermore, it was also found that IL-18 has a good value for mortality prediction.
This research has several limitations. First, the current study population size was limited, and to confirm the findings, its necessities more research with a larger sample size. Second, based on clinical features, neonatal sepsis was diagnosed rather than a positive blood culture test result. Consequently, the manifestation rate of neonatal sepsis could be overestimated or underestimated. Lastly, we did not follow the clinical course of the survivor septic neonates. Therefore, we cannot use the serum IL-18 levels to anticipate future events.
Conclusions
In conclusion, our research revealed that serum IL-18 is an independent risk factor for the presence of neonatal sepsis and may be a potential early biomarker for identifying neonatal sepsis and neonatal mortality prediction.
Data Sharing Statement
Owing to ethical constraints, the data cannot be made freely available in the manuscript or a public repository because the study involved human participants. The corresponding author can provide the data used to support the study’s findings upon request.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by Hospital Ethics Review Board of Henan Children’s Hospital in June 2022 (NO. 2022-K-105). All procedures performed in this study were undertaken as a part of routine clinical practice. Informed written consent was obtained from the parents of neonates.
Acknowledgments
We appreciate Bullet Edits helping with the linguistic editing of this work.
Funding
This work received funding from the Key Research, Development, and Promotion Projects of Henan Province (202102310132 and 222102310171), and the Medical Science and Technology Project of Henan Province (LHGJ20200669, LHGJ20210665, LHGJ20210672 and LHGJ20220774).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–1780. doi:10.1016/S0140-6736(17)31002-4
2. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi:10.1016/s2213-2600(18)30063-8
3. Procianoy RS, Silveira RC. The challenges of neonatal sepsis management. J Pediatr. 2020;96(Suppl 1):80–86. doi:10.1016/j.jped.2019.10.004
4. Dong Y, Basmaci R, Titomanlio L, Sun B, Mercier JC. Neonatal sepsis: within and beyond China. Chin Med J. 2020;133(18):2219–2228. doi:10.1097/CM9.0000000000000935
5. Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–140. doi:10.1097/MOP.0000000000000315
6. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(Suppl 1):10–67. doi:10.1007/s00134-019-05878-6
7. Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. 2015;61(1):1–13. doi:10.1093/tropej/fmu079
8. Molloy EJ, Bearer CF. Paediatric and neonatal sepsis and inflammation. Pediatric Res. 2022;91(2):267–269. doi:10.1038/s41390-021-01918-4
9. Vecchie A, Bonaventura A, Toldo S, Dagna L, Dinarello CA. Abbate A. IL-18 and infections: is there a role for targeted therapies? J Cell Physiol. 2021;236(3):1638–1657. doi:10.1002/jcp.30008
10. Nanda JD, Ho TS, Satria RD, Jhan MK, Wang YT. Lin CF. IL-18: the forgotten cytokine in dengue immunopathogenesis. J Immunol Res. 2021;2021:8214656. doi:10.1155/2021/8214656
11. Yasuda K, Nakanishi K, Tsutsui H. Interleukin-18 in health and disease. Int J Mol Sci. 2019;20(3):649. doi:10.3390/ijms20030649
12. Nozaki Y, Hino S, Ri J, et al. Lipopolysaccharide-induced acute kidney injury is dependent on an IL-18 receptor signaling pathway. Int J Mol Sci. 2017;18(12):2777. doi:10.3390/ijms18122777
13. Wang M, Markel TA, Meldrum DR. Interleukin 18 in the heart. Shock. 2008;30(1):3–10. doi:10.1097/SHK.0b013e318160f215
14. Okuhara Y, Yokoe S, Iwasaku T, et al. Interleukin-18 gene deletion protects against sepsis-induced cardiac dysfunction by inhibiting PP2A activity. Int J Cardiol. 2017;243:396–403. doi:10.1016/j.ijcard.2017.04.082
15. Zaki Mel S, Elgendy MY, El-Mashad NB. Farahat ME. IL-18 level correlates with development of sepsis in surgical patients. Immunol Invest. 2007;36(4):403–411. doi:10.1080/08820130701244275
16. Feng M, Sun T, Zhao Y, Zhang H. Detection of serum interleukin-6/10/18 levels in sepsis and its clinical significance. J Clin Lab Anal. 2016;30(6):1037–1043. doi:10.1002/jcla.21977
17. Barekatain B, HasanGhalyaei N, Mohammadizadeh M, Tavakolifard N. Investigation of salivary C-reactive protein and interleukin-18 for the diagnosis of neonatal sepsis. J Res Med Sci. 2021;26(1):131. doi:10.4103/jrms.JRMS_1256_20
18. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatric Critical Care Med. 2005;6(1):2–8. doi:10.1097/01.Pcc.0000149131.72248.E6
19. Hughes G. Youden’s index and the weight of evidence. Methods Inf Med. 2015;54(2):198–199. doi:10.3414/ME14-04-0003
20. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60(2):367–389. doi:10.1016/j.pcl.2012.12.003
21. Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatric Res. 2017;82(4):574–583. doi:10.1038/pr.2017.134
22. Kaplanski G. Interleukin-18: biological properties and role in disease pathogenesis. Immunol Rev. 2018;281(1):138–153. doi:10.1111/imr.12616
23. Sun RQ, Zhang SL. 脓毒症早期血清白细胞介素-18和10在疾病严重程度及预后评估中的价值研究 [The value of serum interleukin-18 and 10 in the evaluation of severity and prognosis in the early stage of sepsis]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2011;23(5):299–301. Chinese.
24. Zhang LM, Zhang J, Zhang Y, et al. Interleukin-18 binding protein attenuates lipopolysaccharide-induced acute lung injury in mice via suppression NF-kappaB and activation Nrf2 pathway. Biochem Biophys Res Commun. 2018;505(3):837–842. doi:10.1016/j.bbrc.2018.09.193
25. Netea MG, Fantuzzi G, Kullberg BJ, et al. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol. 2000;164(5):2644–2649. doi:10.4049/jimmunol.164.5.2644
26. Raeburn CD, Dinarello CA, Zimmerman MA, et al. Neutralization of IL-18 attenuates lipopolysaccharide-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2002;283(2):H650–H657. doi:10.1152/ajpheart.00043.2002
27. Wynn JL, Wilson CS, Hawiger J, et al. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A. 2016;113(19):E2627–E2635. doi:10.1073/pnas.1515793113
28. Kingsmore SF, Kennedy N, Halliday HL, et al. Identification of diagnostic biomarkers for infection in premature neonates. Mol Cell Proteomics. 2008;7(10):1863–1875. doi:10.1074/mcp.M800175-MCP200
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

