Back to Journals » Cancer Management and Research » Volume 11
Clinical value of ROMA index in diagnosis of ovarian cancer: meta-analysis
Authors Cui RL, Wang YC, Li Y, Li YG
Received 24 December 2018
Accepted for publication 4 February 2019
Published 28 March 2019 Volume 2019:11 Pages 2545—2551
DOI https://doi.org/10.2147/CMAR.S199400
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Ranliang Cui,1,* Yichao Wang,2,* Ying Li,3 Yueguo Li1
1Department of Clinical Laboratory, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, China; 2Department of Clinical Laboratory Medicine, Taizhou Central Hospital (Taizhou University Hospital), Taizhou, Zhejiang Province, China; 3The Third Department of Breast Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin‘s Clinical Research Center for Cancer, Tianjin, China
*These authors contributed equally to this work
Objectives: The role of retrospective analysis has evolved greatly in cancer research. We undertook this network meta-analysis to evaluate retrospectively the diagnostic value of ROMA in ovarian cancer.
Materials and methods: We systematically retrieved 56 relevant articles published about ROMA index from 2009–2018 and about ovarian cancer from China National Knowledge Infrastructure (CNKI), PubMed and EMBASE. Data were comprehensively analyzed by RevMan 5.3 and MetaDisc 12.4 software.
Results: Data of 5,954 cases were retrieved from 23 literatures. Among them, 2,117 cases were in the ovarian cancer group and 3,837 cases in the control group. The pooled estimates for the ROMA index were sensitivity: 0.90 (95% CI: 0.88–0.93), specificity: 0.91 (95% CI: 0.89–0.94), positive predictive: 0.90 (95% CI: 0.88–0.95), negative predictive: 0.93 (95% CI: 0.91–0.95), and area under ROC curve: 0.96, compared to 0.71 (95% CI: 0.56–0.82), 0.87 (95% CI: 0.80–0.92), 0.82 (95% CI: 0.78–0.86), 0.92 (95% CI: 0.90–0.94), and 0.88 of HE4, respectively.
Conclusions: This meta-analysis confirms that the risk of ovarian malignancy algorithm can facilitate the diagnosis of ovarian cancer to some extent.
Keywords: ROMA index, ovarian cancers, meta-analysis
Introduction
Ovarian cancer is one of the most common malignant tumors in the female reproductive system, it has a very high fatality rate because it is too insidious to identify in the early diagnosis. Carbohydrate antigen 125 (CA125) is a traditional marker for ovarian cancer screening. QuNa et al.1 statistically analyzed 13 documents using meta-analysis and showed that serum human epididymis (HE4) was also a common indicator for detecting ovarian cancer. Over the past few decades, with the rapid development of statistical technology, the use of mathematical models makes it possible to combine multiple markers to detect disease. Considering that the combination of multiple tumor markers can improve the diagnostic sensitivity and not reduce the diagnostic specificity, people are working on finding such a mathematical model for the diagnosis of ovarian epithelial carcinoma.2 Therefore, the emergence of the risk of ovarian malignancy algorithm (ROMA) model caused a lot of attention.
The clinical value of the ROMA index in the diagnosis of ovarian cancer has been widely reported in the literature, but most of these studies come from small samples and the results are not consistent with its reliability waiting to be explored. Meta-analysis is also called the gather analysis; it is a kind of statistical method for quantitative synthesis of many studies on the same subject with specific conditions. It improves the credibility of the results by increasing the number of samples to solve the inconsistency among the results of the study. As for evidence-based medicine, this paper systematically evaluates the clinical application value of ROMA index by using meta-analysis method, in order to find a reliable basis for clinical workers to diagnose ovarian cancer and improve the diagnostic efficiency, early detection and treatment to improve the prognosis and quality of life.
Materials and methods
Participants: Patients with ovarian cancer diagnosed by pathological examination were collected venous blood before operation, serum was extracted, serum CA125 and HE4 levels were measured, and the ROMA index was calculated. Search strategy: search CNKI, VIP, PubMed, and EMBASE. Chinese search term: Ovarian malignant tumor risk prediction value, ROMA index, ovarian cancer. English retrieval words: ROMA index, ovarian cancer. Retrieval years: 2009–2018
Inclusion and exclusion criteria
Inclusion criteria
(1) The purpose of this study was to explore the value of ROMA in the diagnosis of epithelial ovarian cancer; (2) the literature included was a case-control study; (3) the experimental group was ovarian cancer group and the control group was pelvic benign mass which could not be distinguished from ovarian malignant tumor. The diagnostic standard was postoperative pathological examination; (4) empirical method, the source of the reagent used is clear; (5) the critical reference value is clear; (6) the results of the study are clear and capable of statistical analysis; (7) document quality score higher than 7.
Exclusion criteria
(1) Literature theme or type inconsistency; (2) non-primary study; (3) literature on failure to calculate ROMA index in patients with ovarian cancer; (4) the sample size is too small to reach the standard of statistical analysis (<15 cases); (5) result indicators cannot be statistically analyzed; (6) primary study on healthy people as control group only; (7) no definite critical reference value. Fifty-six papers were selected and 23 articles were selected according to inclusion and exclusion criteria.
Analysis method and data processing
Review of the quality of the literature was undertaken using diagnostic test accuracy evaluation tool QUADAS (quality assessment of diagnostic accuracy studies). The 14 requirements was evaluated according to the “yes, not clear, or not”, and the corresponding evaluation was carried out. We obtain data directly from the study or compute it to make a four-grid table: true positive and false positive, false negative, and true negative. Statistical analysis was calculated using Revman 5.3 (St Albans, London) and MetaDisc 12.4 software (Clinical Biostatistics Unit, Ramón y Cajal Hospital, Spain). This study used risk difference as a diagnostic effect to calculate the 95% CI. The heterogeneity of sensitivity, specificity, positive predictive value and negative predictive value were analyzed by chi-squared test. When P>0.1, I2 <50, there was no significant heterogeneity among the studies, selection of fixed - effect model analysis, When P<0.1, I2 >50, the heterogeneity between the studies is significant and analyzed with the random effect model.
Results
Basic information for inclusion in the literature
The study included 23 articles that met the requirements between 2009 and 2018, a total of 2,117 patients with ovarian cancer and the control group with a total of 3,837 cases. The pathology types included in the literature quality score and the ovarian cancer group is shown in Table 1
  | Table 1 Literature inclusion basic information |
Meta-analysis results
By chi-squared test, the results of literature heterogeneity analysis showed that there was heterogeneity among the studies (P<0.1/I2>50). Therefore, the statistical analysis of the ROMA index is based on the random effect model, the results show that the sensitivity of the ROMA index is 90% (95% CI: 88–93), as shown in Figure 1. Specific 91% (95% CI: 89–94), as shown in Figure 2. Positive predictive value 90% (95% CI: 88–95), Figure 3. Negative predictive value 93% (95% CI: 91–95), Figure 4. And area under ROC curve (AUC): 0.96, Figure 5. Compared to sensitivity: 0.71 (95% CI: 0.56–0.82), specificity: 0.87 (95% CI: 0.80–0.92), and AUC: 0.88 respectively, of HE4.28
  | Figure 1 Sensitivity forest map of the ROMA index for diagnosis of ovarian cancer (random effect model). |
  | Figure 2 Specific forest map for diagnosis of ovarian cancer with the ROMA index (random effect model). |
  | Figure 3 Positive predictive value of the ROMA index for diagnosis of ovarian cancer forest map (random effect model). |
  | Figure 4 Negative predictive value of the ROMA index for diagnosis of ovarian cancer forest chart (random effect model). |
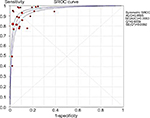  | Figure 5 Area under ROC curve (AUC) of the ROMA index for diagnosis of ovarian cancer forest chart (random effect model). |
Bias analysis (as an example of a sensitive funnel diagram)
Inverted funnel graph is a common method to identify bias. In the case of no bias, the graph is symmetrical inverted funnel shape. Otherwise, it suggests that there is bias in the study. The bias analysis of this study shows that the funnel graph shows asymmetry, indicating the existence of bias (Figure 6).
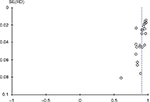  | Figure 6 ROMA index evaluation of ovarian cancer risk bias analysis inverted funnel graph. Abbreviation: RD, risk difference. |
Discussion
The early clinical symptoms of ovarian cancer are not obvious so as to make it easy to transfer and spread, leading to a high death rate. Therefore, it was found in the middle and late stage thus missing the best treatment opportunity, so that the treatment effect is poor. NIH reports20 that about 13% to 21% of ovarian tumor patients were diagnosed with ovarian cancer. The American Cancer Society conducted a statistical analysis of 21,550 (13% of 169,000) women with ovarian tumors diagnosed as ovarian cancer in 2009. Two sets of statistics are consistent. Therefore, improving the accuracy of early diagnosis and diagnosis of ovarian cancer and comprehensive preoperative evaluation of ovarian cancer patients are very important for the treatment and prognosis of ovarian cancer patients.
There may be many cases of elevated CA125 and its diagnostic sensitivity is relatively low, especially when it comes to the early diagnosis of ovarian cancer which is not ideal, just vulnerable to the impact of benign diseases. Besides, the specificity is also poor. HE4 was not expressed in normal ovarian tissues, but it was highly expressed in ovarian cancer, but not expressed or low in most nonmalignant ovarian diseases. So, for the diagnosis of ovarian cancer, HE4 has shown its better specificity. It is the difference in sensitivity and specificity between CA125 and HE4 that leads to a more effective method of detection with two characteristics. Some scholars have proposed the concept of the ROMA index to assess the risk of ovarian cancer by using the results of the study and related statistical analysis. But at present, only a few documents in China refer to the clinical value of the ROMA index for the diagnosis of ovarian cancer and do not systematically analyze it.
As a new literature research method, meta-analysis can play a positive role in enhancing the credibility of the results by quantitative synthesis of many studies of the same subject with specific conditions.21 The purpose of this paper is to further explore the clinical value of ROMA index in the diagnosis of ovarian cancer by means of meta-analysis of evidence-based medicine. A total of 23 domestic and foreign literatures were included in this study, and a statistical analysis was performed using the Revman 5.3 software. Meta-analysis showed that the sensitivity, specificity, positive predictive value and negative predictive value of the ROMA index in ovarian cancer were 90, 91, 90% and 93%, respectively. According to the theory of medical statistics,19 if the ROMA index reaches 79% at the same time, the sensitivity can reach >80%. So we speculate that the ROMA index has the value of clinical differential diagnosis. Obviously, in this study, the specificity of the ROMA index diagnosis was 90%, and the sensitivity was 91%. From the above data, we can see that the ROMA index can provide a reliable basis for clinical diagnosis of ovarian cancer.
But in the bias analysis, the “inverted funnel graph” shows asymmetry, and the system indicates that there is bias. We consider that the cause of bias may be that the baseline of the original: (1) document is inconsistent; (2) different experimental measures; (3) death and loss bias appeared in the course of experiment; (4) some of the literature samples only involved epithelial ovarian cancer with no other types of ovarian cancer, and some of the literature did not describe the borderline tumors; and (5) because the ROMA value needs to be based on the results of serum HE4 and CA125, combined with the calculation of menstrual state, there are many influencing factors in the process, which leads to a decrease of the reliability of the analysis results. Meta-analysis bias: (1) part of the gray literature cannot be retrieved, but the language of the literature is limited. In Chinese and English, it is impossible to obtain complete information; (2) the standard of exclusion and inclusion is not perfect; and (3) few literatures are included in the study and the number of cases in the study sample is relatively small.
According to the statistical principle that the larger the sample size, the more reliable the result will be. The reliability of the results is not consistent due to the differences in sample size among the studies. Therefore, we still need a multi-center, large-scale randomized controlled study. Reasonable selection of large and relatively consistent research, improve the quality of research, in order to improve the reliability of meta-analysis conclusions.
Brief summary
The ROMA index combined serum CA125 HE4 and menopausal state with multiple parameters, which greatly improved the accuracy of single factor diagnosis of ovarian cancer. The preoperative assessment of the patient and the accurate judgment of the pelvic masses will be beneficial to the patient’s further treatment and even the prognosis. Through the statistical analysis of 23 literatures, this study shows that the ROMA index has a higher clinical application value in the diagnosis of ovarian cancer and can be used to guide the clinical work of doctors. The results will be more reliable if we can obtain relatively perfect information and reduce bias as much as possible during the experiment.
Ethics approval and consent to participate
For this type of study, ethics approval is not applicable.
Availability of data and materials
The datasets used during the current study are available from GEO database (https://ncbi.nlm.nih.gov/geo/).
Acknowledgment
This work was supported by research grants from National Scientific Foundation of China (#81871719).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Na Q, Guokojun, Lycke M, Kristjansdottir B, Sundfeldt K. Meta-analysis of expression levels of epididymal protein 4 in ovarian cancer serum. J Chinese Cancer Prevent. 2012;19(16):1258–1262. | ||
Qing L, Xiaoling S, Yang Yuqin. Research progress of ROMA in early diagnosis of ovarian epithelial carcinoma. Adv Mod Biomed Sci. 2011;11(24):4999–5000. | ||
Li D. Gu J, Zhao S. Changes in serum HE4 and CA125 levels in patients with endometrial cancer. Shandong Med. 2017;57(3):73–75. | ||
Chi L, Zhouxiaoying, Huangruiyu, et al. Diagnostic value of serum HE4, CA125, and ROMA index in ovarian cancer. Chinese Maternal and Child Health. 2017:48–50. | ||
Yang S, Lu W, Zheng Y, et al. Analysis of the diagnosis of ovarian cancer by serum CA125, HE4 combined ROMA index. Med Clin. 2016;13(14):1931–1933. | ||
Xie W, Lin X, Chen W. Diagnostic value of serum CA125, HE4 detection and ROMA model in malignant ovarian cancer. Int J Test Med. 2016;24:3503–3504. | ||
Lei Z. Xiao J, Xie H, et al. HE4 and CA125 combined application of the ROMA index to the diagnostic value of pelvic masses. Harbin Pharmaceut. 2015;35(s1):55–56. | ||
Farzaneh F, Honarvar Z, Yaraghi M, et al. Preoperative evaluation of risk of ovarian malignancy algorithm index in prediction of malignancy of adnexal masses. Iran Red Crescent Med J. 2014;16(6):e17185. | ||
He H, Jiang L, Hu R, et al. Application value of serum HE4 level and ROMA index in ovarian cancer diagnosis. J Gannan Med Coll. 2014;6:846–848. | ||
Yang H, Yu J, Wu Y. The significance of ROMA in ovarian cancer diagnosis. Jiangsu Med. 2014;40(16):1905–1907. | ||
Ren Hongying, Huang Weicheng Xiaoyu, et al The value of serum HE4 joint CA125 detection in risk assessment of ovarian cancer. China Health Ind. 2014;21:177–178. | ||
Li J. [Value of serum human epididymis secretory protein 4, CA125 and ROMA [D] (Risk of Ovarian Malignancy Algorithm)in the diagnosis of pelvic mass]. Southern Medical University; 2014. Chinese. | ||
Huo Y. [Discussion of HE4,CA125 and ROMA diagnstic value of serum index in epithelial ovarian cacer[D]]. Xinjiang Medical University; 2014. | ||
Stiekema A, Lok CA, Kenter GG, et al. A predictive model combining human epididymal protein 4 and radiologic features for the diagnosis of ovarian cancer. Gynecol Oncol. 2014;132(3):573–577. Chinese. | ||
Pan Y, Lin X, He X. The application value of serum HE4 and CA125 joint detection in the risk assessment of malignant ovarian cancer in pelvic mass patients. Int J Test Med. 2013;34(5):543–544. | ||
Xie Z, Wang H, Liu Q, et al. Application of serum HE4 and CA125 joint detection in the risk assessment of epithelial ovarian cancer. J Clin Milit Med. 2012;40(2):392–394. | ||
Bandiera E, Romani C, Specchia C, et al. Serum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnostic and prognostic tools for epithelial ovarian cancer management. Cancer Epidemiol Biomarkers Prev. 2011;20(12):2496–2506. | ||
Chen Y, Hermione, Song Z. Secretion of serum human epididymis protein 4 and CA125 in ovarian tumors and ovarian cancer risk model in the differential diagnosis value. J Suzhou University, Med Sci. 2010;30(4):795–798. | ||
Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40–46. | ||
Paulsen T, Kjaerheim K, Kaern J, Tretli S, Tropé C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16(Suppl 1):11–17. | ||
Jing L, Peng Qpeng. A meta-analysis of clinical value in ovarian cancer diagnosis. Practical Obstetrics and Gynecology. 2015;31(3):209–213. | ||
Lycke M, Kristjansdottir B, Sundfeldt K. A multicenter clinical trial validating the performance of HE4, CA125, risk of ovarian malignancy algorithm and risk of malignancy index. Gynecol Oncol. 2018;151(1):159–165. | ||
Huy NVQ, van Khoa V, Tam LM, Tam LM. Standard and optimal cut-off values of serum CA-125, HE4 and ROMA in preoperative prediction of ovarian cancer in Vietnam. Gynecol Oncol Rep. 2018;25:110–114. | ||
Al Musalhi K, Al Kindi M, Al Aisary F, et al. Evaluation of HE4, CA-125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) in the preoperative assessment of patients with adnexal mass. Oman Med J. 2016;31(5):336–344. | ||
Karlsen MA, Sandhu N, Høgdall C, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012;127(2):379–383. | ||
Dikmen ZG, Colak A, Dogan P, Tuncer S, Akbiyik F. Diagnostic performances of CA125, HE4, and ROMA index in ovarian cancer. Eur J Gynaecol Oncol. 2015;36(4):457–462. | ||
Van Gorp T, Veldman J, van Calster B, et al. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur J Cancer. 2012;48(11):1649–1656. | ||
Li J, Wang X, Qu W, Wang J, Jiang SW. Comparison of serum human epididymis protein 4 and CA125 on endometrial cancer detection: a meta-analysis. Clinica Chimica Acta. 2019;488:215–220. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
