Back to Journals » Journal of Inflammation Research » Volume 14
Clinical Value of Prognostic Nutritional Index in Prediction of the Presence and Severity of Neonatal Sepsis
Authors Li T, Qi M, Dong G, Li X, Xu Z, Wei Y, Feng Y, Ren C, Wang Y, Yang J
Received 21 October 2021
Accepted for publication 8 December 2021
Published 21 December 2021 Volume 2021:14 Pages 7181—7190
DOI https://doi.org/10.2147/JIR.S343992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Tiewei Li,1,* Minglu Qi,2,* Geng Dong,1 Xiaojuan Li,1 Zhe Xu,1 Yulei Wei,1 Yichuang Feng,1 Chong Ren,1 Yaguo Wang,3 Junmei Yang1
1Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, Henan Children’s Hospital, Zhengzhou Children’s Hospital, Zhengzhou, People’s Republic of China; 2Department of Emergency Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Institute of Biophysics, Chinese Academy of Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tiewei Li; Junmei Yang Email [email protected]; [email protected]
Purpose: The prognostic nutritional index (PNI) is a common indicator of nutritional and inflammatory status and is associated with various diseases such as cancer, cardiovascular diseases and infectious diseases. However, to date, no study has concentrated on the role of PNI in assessing and predicting the presence and severity of neonatal sepsis. Therefore, the present study aimed to explore the association of the PNI with the presence and severity of neonatal sepsis.
Materials and Methods: A total of 1196 neonates with suspected sepsis were enrolled in this study and their complete clinical and laboratory data were collected. PNI was calculated as serum albumin (g/L) + 5 × total lymphocyte count (109/L). Multivariate logistic regression analysis was performed to identify the risk factors for the presence and severity of neonatal sepsis. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive value of PNI. All statistical analyses were performed using the statistical package SPSS 24.0.
Results: PNI was lower in neonates with sepsis and decreased significantly with the severity of sepsis. The correlation analysis demonstrated that the PNI was negatively correlated with the levels of the inflammatory marker procalcitonin (PCT) and C-reactive protein (CRP), and the length of hospital stay. Multivariate logistic regression analysis revealed that the PNI was independently and inversely associated with the presence and severity of neonatal sepsis. The area under the ROC curve of the PNI was 0.64 (95% confidence interval (CI): 0.61– 0.67, P < 0.001) for severe sepsis and 0.69 (95% CI: 0.60– 0.78, P < 0.001) for septic shock. In addition, our data revealed that PNI was also independently correlated with the length of hospital stay.
Conclusion: PNI is an independent predictor for the presence and severity of neonatal sepsis.
Keywords: neonatal sepsis, predictor, prognostic nutritional index, severity
Introduction
Sepsis is a life-threatening condition caused by a dysregulated host response to infection and remains a major cause of morbidity and mortality worldwide.1–3 Neonates are more prone to infections than adults and tend to develop neonatal sepsis due to their immature immune system.4 Failure to diagnose and manage the bloodstream infection promptly can lead to septic shock, multiple organ failure and death.5 Therefore, a timely diagnosis of neonatal sepsis is very important. Currently, the gold standard for the diagnosis of neonatal sepsis is blood culture.6 However, several factors such as inadequate blood volume, antimicrobial exposure and blood contamination pose challenges in confirming the presence of neonatal sepsis.7 Moreover, the clinical presentation of condition is non-specific.8 Therefore, biomarkers circulating in the blood may be useful in the early diagnosis of neonatal sepsis.
Proper functioning of the body is ensured by adequate nutrition, and an optimal nutritional status aids in preventing infection. This signifies that malnutrition can impair the immune system.9,10 Sepsis leads to dysfunction of the gastrointestinal tract causing nutritional deficiency in the patient, which can subsequently prove life-threatening.11,12 Asiimwe et al13 reported that malnourished patients had a higher risk of developing severe sepsis at admission and that further led to death within 30 days of admission. Therefore, nutrition-related indicators may play an important role in identifying the presence of neonatal sepsis.
The prognostic nutritional index (PNI) is calculated based on the level of serum albumin (ALB) and peripheral lymphocyte count, which can reflect the nutritional and immune status of patients. Several studies have revealed that PNI is a reliable prognostic biomarker in patients with cancer.14–18 In addition, studies have demonstrated that PNI was a prognostic biomarker in patients with acute ischemic stroke19 and patients in the coronary care unit.20 Recently, Shimoyama et al21 revealed that PNI was a predictor of septic acute kidney injury (AKI), renal replacement therapy initiation in adult patient with sepsis, and prognosis in adult patients with septic AKI. As an objective nutritional marker, calculating PNI is easy based on the serum ALB concentration and lymphocyte count. Therefore, laboratory parameters that constitute PNI are routinely evaluated in most clinical settings making PNI a readily available biomarker.
To the best of our knowledge, there are no published studies evaluating the role of PNI to predict the presence and severity of neonatal sepsis. Therefore, our study aimed to evaluate the clinical value of PNI in predicting the presence and severity of neonatal sepsis.
Materials and Methods
Study Design and Population
This was a retrospective single-center study. A total of 1196 neonates with suspected sepsis admitted to the Henan Children’s Hospital (Zhengzhou, China) between January 2016 to December 2019 were enrolled in this study. Neonates with the following conditions were excluded: (1) aged >28 days, (2) presence of haematological system diseases, malignancies or major congenital malformations and (3) incomplete clinical and laboratory data at admission. The study protocol complied with the Declaration of Helsinki and was approved by the ethics review board of the hospital. All procedures performed in this study were undertaken as a part of routine clinical practice, and the data by which the subjects could identify were removed. Therefore, the requirement for informed consent was waived, considering the retrospective nature of the present study.
Clinical Evaluation and Definition
Neonatal sepsis is defined as suspected or confirmed infection accompanied with at least two of the systemic inflammatory response syndrome (SIRS) criteria, one of which must be abnormal body temperature or leukocyte count. The criteria for SIRS are as follows: (1) body temperature of > 38.5°C or < 36°C; (2) tachycardia; (3) mean respiratory rate > 2 standard deviations (SD) above normal for age or mechanical ventilation for an acute process that is not related to an underlying neuromuscular disease or the receipt of general anesthesia; (4) abnormal leukocyte count or >10% immature neutrophils. Severe sepsis was defined as sepsis along with any one of the following conditions: cardiovascular dysfunction, acute respiratory distress syndrome, or dysfunction of two or more other organs. Septic shock was defined as severe sepsis along with cardiovascular dysfunction. Infection was defined as a suspected or proven infection caused by any pathogen or clinical sign associated with a high probability of infection, including abnormal temperature or leukocyte count, cough, chest radiograph consistent with pneumonia, petechial or purpuric rash, or purpura fulminans.22 The diagnosis of clinical neonatal infection and sepsis was made by two study investigators [initials of the investigators here] as per the International Pediatric Sepsis Consensus.23 In addition, the severity of neonatal sepsis was assessed by using the neonatal sequential organ failure assessment (nSOFA) score that consisted respiratory, cardiovascular, and hematological criteria.24
Data Collection and Laboratory Measurements
Clinical and pathological data, such as age, sex, weight, body temperature, respiratory rate, heart rate and systolic and diastolic blood pressure, obtained during the first admission were collected from the medical records. Data of the laboratory indices, such as the procalcitonin (PCT), C-reactive protein (CRP), creatine kinase (CK), CK-MB, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (CREA), uric acid (UA) and ALB levels of the neonates obtained immediately after admission were recorded. The detection methods of these laboratory indices are described in our previous studies.25,26 For our dataset, CRP levels <0.8 mg/L were assigned a value of 0.7 mg/L. PCT level >100 ng/mL or <0.02 ng/mL were assigned, 101 ng/mL and 0.01 ng/mL, respectively.
Statistical Analysis
Variables are presented as mean ± standard deviation (SD), median (interquartile range) or number (percentage), as applicable. The independent t-test or one-way analysis of variance (ANOVA) test was performed for continuous variables, and the Chi-square test was performed for categorical variables for comparison between the groups. Pearson or Spearman correlation test was performed to determine the relationship between the PNI and its clinical parameters. Multivariate logistic regression analysis was performed to determine the association of the PNI with the presence and severity of neonatal sepsis. Variables with a P-value <0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. All data analyses were performed using the software of IBM SPSS 22.0 software (SPSS Inc., Chicago, Illinois, USA). A two-sided P value ≤ 0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 1196 neonates with suspected sepsis were enrolled in this study (median age 9.0 [5.0, 15.0] days), including 709 boys (59.3%) and 487 girls (40.7%). The demographic and laboratory findings are presented in Table 1. Of the total 1196 neonates, 755 neonates were finally clinically diagnosed with sepsis and the remaining 441 neonates were diagnosed with signs of infection that further served as the control group. Compared with the neonates in the control group, neonates with sepsis were older, and had a higher body temperature, respiratory rate, and heart rate. Biochemical analysis revealed that the levels of PCT, CRP, BUN, UA, CK, CK-MB, CREA and ALB were lower, and the lymphocyte count was higher in neonates with sepsis (P < 0.05). Meanwhile, neonates with sepsis also had higher PNI and nSOFA scores, and a longer length of hospital stay (P < 0.001).
 |
Table 1 Comparison of the Baseline Characteristics According to the Presence and Severity of Sepsis |
Based on the severity of sepsis, the neonates with sepsis were further categorised as having mild, severe or septic shock. Of the total 755 neonates with sepsis, 335 had mild sepsis, 379 had severe sepsis and 41 had septic shock. The respiratory rate, heart rate, nSOFA score, the length of hospital stay and the levels of PCT, CRP and UA increased with the severity of sepsis (Table 1). Additionally, our results revealed that the lymphocyte count, ALB level and the PNI decreased gradually in the mild sepsis, severe sepsis and septic shock groups (P < 0.001).
Association of PNI with the Presence and Severity of Neonatal Sepsis
Neonates were classified into three groups based on the PNI tertiles as follows: low PNI (< 44.90), intermediate PNI (44.90–55.10) and high PNI (>55.10). As shown in Table 2, neonates with low PNI had higher PCT and CRP levels and nSOFA scores and longer lengths of hospital stay compared to other groups. The prevalence of neonatal sepsis decreased significantly from 79.3% in the low PNI group to 50.7% in the high PNI group (P < 0.001), whereas the controls were more likely to be in the intermediate PNI and high PNI groups. Further analysis also revealed that the prevalence of severe sepsis and septic shock was significantly higher in the low PNI group than that in the intermediate- and high-PNI groups (P < 0.05).
 |
Table 2 Clinical and Demographic Characteristics Based on the PNI Tertiles |
Correlation Between PNI and Clinical Parameters
In general, PNI was correlated positively with the age (r = 0.201, P < 0.001) and weight (r = 0.172, P < 0.001), and negatively correlated with body temperature (r = −0.058, P = 0.044), respiratory rate (r = −0.117, P < 0.001), PCT (r = −0.425, P < 0.001), CRP (r = −0.248, P < 0.001), CK (r = −0.086, P = 0.003), BUN (r = −0.059, P = 0.042), CREA (r = −0.145, P < 0.001), nSOFA score (r = −0.294, P < 0.001), and the length of hospital stay (r = −0.213, P < 0.001) (Table 3). There was no significance between the PNI and heart rate, CK-MB, AST, and UA levels.
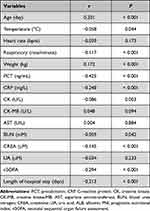 |
Table 3 Correlations Between the PNI and Clinical Parameters |
Ability of the PNI to Predict the Presence and Severity of Neonatal Sepsis
Univariate and multivariable binary logistic regression analyses were performed to evaluate the ability of PNI to predict the presence and severity of neonatal sepsis. Variables, including age, body temperature, heart rate, respiratory rate, the levels of PCT, CRP, AST, ALT, UREA and UA and PNI, with P < 0.05 in the univariate analysis were included in the multivariate analysis. As shown in Table 4, after adjusting the above variables, PNI proved to be an independent risk factor for the presence of sepsis (OR = 0.967, 95% confidence interval [CI]: 0.955–0.979, P < 0.001), severe sepsis (OR = 0.988, 95% CI: 0.997–0.999, P < 0.001) and septic shock (OR = 0.952, 95% CI: 0.920–0.985, P < 0.001). Further analysis revealed that the PNI tertiles were also independently associated with the presence of sepsis, severe sepsis and septic shock.
 |
Table 4 Relative Risks of PNI for the Presence and Severity of Neonatal Sepsis |
Diagnostic Value of PNI in Neonatal Sepsis
The ROC curve analysis was performed to calculate the diagnostic value of the PNI in neonatal sepsis. Based on the analysis, the optimal cut-off value of the PNI to predict the presence of neonatal sepsis was 50.63, with 66% sensitivity and 61% specificity (AUC = 0.66, 95% CI: 0.63–0.70, P < 0.001) (Figure 1A). The optimal cut-off value of the PNI to predict severe sepsis and septic shock was 43.6, with a sensitivity of 79% and specificity of 56% (AUC = 0.63, 95% CI: 0.59–0.66, P < 0.001) (Figure 1B). We separately evaluated the ability of the PNI to predict septic shock. As shown in Figure 1C, the AUC was 0.68 (95% CI: 0.58–0.77), with a sensitivity and specificity of 70% and 61%, respectively, at a cut-off value of 44.2.
Discussion
Sepsis is a SIRS caused by infection and is commonly accompanied by multiple organ dysfunction.27 In comparison with adults, neonates are more susceptible to infections than older children, which can lead to the development of neonatal sepsis, severe sepsis, and septic shock in the future.28 Data published in previous studies reveal that neonatal sepsis remains the third leading cause of neonatal death and is one of the leading causes of death among children under 5 years of age, which has become a public health problem.29–31 Neonatal sepsis can present with subtle signs but can rapidly progress to multisystem organ failure and meningitis.32 Therefore, the rapid identification of neonatal sepsis is the key to successful treatment.
From a pathophysiology perspective, sepsis has often been considered as a state of systemic and hypermetabolic inflammation, wherein inflammatory cells play an important role by secreting multiple pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, tumour necrosis factor-alpha (TNF-α) and neutrophil extracellular traps (NETs).33–35 Those cytokines disrupt the balance between the pro-inflammatory and anti-inflammatory responses. Studies have revealed that the biomarkers of infection and inflammation play an important role in predicting the presence of sepsis.26,36,37 The total and differential leukocyte counts are the less expensive and widely utilised indicators of the inflammatory response. Among leukocytes, neutrophils and lymphocytes are the two most abundant cells in peripheral blood. Commonly, an infection can activate neutrophils for the secretion of pro-inflammatory cytokines and cause damage to the organ; whereas lymphocytes can secrete anti-inflammatory cytokines (such as IL-10 and TGF-β) to inhibit the inflammatory response.38–40 In adult patients with sepsis, the balance between the neutrophils and lymphocytes is disrupted, resulting in an elevated neutrophil count and decreased lymphocyte count.41–44 Several clinical studies have revealed that the neutrophil and lymphocyte counts are a reliable inflammatory marker and prognostic indices for sepsis.25,43
In addition to the immune abnormalities, sepsis is commonly accompanied by metabolic and endocrine abnormalities that lead to life-threatening organ dysfunction.27 Sepsis can easily lead to dysfunction of the gastrointestinal tract and liver, resulting in the dysfunction of synthesis and metabolism, thereby affecting the nutritional status of patients.11,12,45 Nutritional status plays an important role in the maintenance of health and the prevention of infection.46 Inadequate nutrition predisposes acutely ill individuals with compromised immune systems to the development of sepsis.47 Serum ALB level is the simplest and most effective parameter that reflects the nutritional status of the body. ALB is one of the proteins produced by the liver that plays an important role in the maintaining the colloid osmotic pressure, preventing fluid from leaking out of blood vessels; nourishing the tissues and transporting hormones, vitamins, drugs, and calcium throughout the body.48,49 In addition, studies have also revealed that there is a close correlation between ALB and inflammation, wherein lower serum ALB levels were associated with more severe inflammation in adult patients.50–54 In neonates with sepsis, Yang et al55 reported that hypoalbuminemia occurred frequently and that lower ALB levels might be associated with a poorer prognosis.
PNI is calculated based on the serum ALB level and the total number of peripheral blood lymphocytes and is widely used as a marker of both nutritional status and inflammation. In addition, PNI has been widely used to assess the immunonutritional status of patients with cancer and is a useful prognostic marker in adult patients with various malignancies, such as colorectal cancer,18 non-small cell lung cancer,17 liver cancer,56 oesophageal carcinoma15,57 and osteosarcoma.16 In recent years, PNI has attracted significant attention for its convenience and significance in clinical applicability. Studies have reported that PNI is clinically significant in adult patients with other diseases, such as coronavirus disease 2019 (COVID-19),58,59 cardiovascular disease,60,61 and Crohn’s disease62,63 and sepsis. However, to date, no studies have concentrated on the role of the PNI in assessing and predicting the presence and severity of neonatal sepsis so far.
In the present study, we evaluated the clinical value of PNI in predicting the presence and severity of neonatal sepsis in a relatively large population for the first time. Our results revealed that PNI was lower in neonates with sepsis and showed a gradual decrease with the severity of neonatal sepsis. In addition, there was a significant negative correlation between PNI and the markers of infection and inflammation (PCT and CRP). Multivariate regression analysis revealed that PNI was an independent risk factor for the the presence and severity of neonatal sepsis. ROC curve analysis demonstrated that PNI has a favourable discriminatory ability in predicting sepsis and septic shock.
However, there are several limitations to our study as follows: 1) The diagnosis of neonatal sepsis was based on clinical signs and not positive blood culture, which could be associated with underestimation or overestimation of the true prevalence of the condition; 2) we could not obtain the data on the type of feeding and daily intake of protein before the neonates were admitted to the hospital, which may later PNI; 3) this was a retrospective single-center study and multicenter clinical studies are required to confirm our results; 4) PNI was only calculated at admission. Continuous monitoring of PNI and the severity of neonatal sepsis might provide more significant insights.
Conclusions
Our study revealed that PNI was negatively and independently associated with the presence and severity of neonatal sepsis. These findings highlighted the potential clinical value of PNI as a convenient and significant biomarker for clinical application to predict the presence and severity of neonatal sepsis.
Data Sharing Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved the Hospital Ethics Review Board of Henan Children’s Hospital. We confirmed that all the data were anonymized and maintained with confidentiality; therefore, the requirement for informed consent has been waived because of the retrospective nature of the current study.
Acknowledgments
We thank Bullet Edits for its linguistic assistance during the preparation of this manuscript. Tiewei Li and Minglu Qi are co-first authors for this study.
Funding
This work was supported by the Key Research, Development, and Promotion Projects of Henan Province (202102310132), and Medical Science and Technology Project of Henan Province (LHGJ20190970, LHGJ20200607, LHGJ20200651 and LHGJ20210661).
Disclosure
The authors report no conflicts of interest.
References
1. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi:10.1164/rccm.201504-0781OC
2. Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625–631. doi:10.1097/CCM.0000000000000026
3. Berg D, Gerlach H. Recent advances in understanding and managing sepsis. F1000Res. 2018;7:1570. doi:10.12688/f1000research.15758.1
4. Marodi L. Neonatal innate immunity to infectious agents. Infect Immun. 2006;74(4):1999–2006. doi:10.1128/IAI.74.4.1999-2006.2006
5. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
6. Ershad M, Mostafa A, Dela Cruz M, Vearrier D. Neonatal sepsis. Curr Emerg Hosp Med Rep. 2019;7(3):83–90. doi:10.1007/s40138-019-00188-z
7. Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Ped Res. 2017;82(4):574–583. doi:10.1038/pr.2017.134
8. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet (London, England). 2017;390(10104):1770–1780. doi:10.1016/s0140-6736(17)31002-4
9. Felblinger DM. Malnutrition, infection, and sepsis in acute and chronic illness. Crit Care Nurs Clin North Am. 2003;15(1):71–78. doi:10.1016/s0899-5885(02)00040-0
10. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016;37(6):386–398. doi:10.1016/j.it.2016.04.003
11. Chierego M, Verdant C, De Backer D. Microcirculatory alterations in critically ill patients. Minerva Anestesiol. 2006;72(4):199–205.
12. Bardou M, Quenot JP, Barkun A. Stress-related mucosal disease in the critically ill patient. Nat Rev Gastroenterol Hepatol. 2015;12(2):98–107. doi:10.1038/nrgastro.2014.235
13. Asiimwe SBAA, Vittinghoff E, Muzoora CK. Causal impact of malnutrition on mortality among adults hospitalized for medical illness in sub-Saharan Africa: what is the role of severe sepsis? BMC Nutri. 2015;1(1):1–8. doi:10.1186/s40795-015-0023-9
14. Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. doi:10.1097/SLA.0000000000002985
15. Dai Y, Fu X, Li T, et al. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Ann Transl Med. 2019;7(8):175. doi:10.21037/atm.2019.03.60
16. Huang X, Hu H, Zhang W, Shao Z. Prognostic value of prognostic nutritional index and systemic immune-inflammation index in patients with osteosarcoma. J Cell Physiol. 2019;234(10):18408–18414. doi:10.1002/jcp.28476
17. Mori S, Usami N, Fukumoto K, et al. The significance of the prognostic nutritional index in patients with completely resected non-small cell lung cancer. PLoS One. 2015;10(9):e0136897. doi:10.1371/journal.pone.0136897
18. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37(11):2688–2692. doi:10.1007/s00268-013-2156-9
19. Xiang W, Chen X, Ye W, Li J, Zhang X, Xie D. Prognostic nutritional index for predicting 3-month outcomes in ischemic stroke patients undergoing thrombolysis. Front Neurol. 2020;11:599. doi:10.3389/fneur.2020.00599
20. Hu Y, Cao Q, Wang H, et al. Prognostic nutritional index predicts acute kidney injury and mortality of patients in the coronary care unit. Exp Ther Med. 2021;21(2):123. doi:10.3892/etm.2020.9555
21. Shimoyama Y, Umegaki O, Kadono N, Minami T. Presepsin and prognostic nutritional index are predictors of septic acute kidney injury, renal replacement therapy initiation in sepsis patients, and prognosis in septic acute kidney injury patients: a pilot study. BMC Nephrol. 2021;22(1):219. doi:10.1186/s12882-021-02422-x
22. Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37(2):439–479. doi:10.1016/j.clp.2010.04.002
23. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Ped Crit Care Med. 2005;6(1):2–8. doi:10.1097/01.Pcc.0000149131.72248.E6
24. Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Ped Res. 2020;88(1):85–90. doi:10.1038/s41390-019-0517-2
25. Li T, Dong G, Zhang M, et al. Association of neutrophil-lymphocyte ratio and the presence of neonatal sepsis. J Immunol Res. 2020;2020:7650713. doi:10.1155/2020/7650713
26. Li T, Li X, Wei Y, et al. Predictive value of C-reactive protein-to-albumin ratio for neonatal sepsis. J Inflamm Res. 2021;14:3207–3215. doi:10.2147/JIR.S321074
27. De Waele E, Malbrain M, Spapen H. Nutrition in sepsis: a bench-to-bedside review. Nutrients. 2020;12(2). doi:10.3390/nu12020395
28. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. 2013;60(2):367–389. doi:10.1016/j.pcl.2012.12.003
29. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet (London, England). 2015;385(9966):430–440. doi:10.1016/s0140-6736(14)61698-6
30. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 2012;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
31. Wang H, Liddell CA, Coates MM, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2014;384(9947):957–979. doi:10.1016/S0140-6736(14)60497-9
32. Kim F, Polin RA, Hooven TA. Neonatal sepsis. BMJ (Clinical Research Ed). 2020;371:m3672. doi:10.1136/bmj.m3672
33. Schulte W, Bernhagen J, Bucala R. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets–an updated view. Mediators Inflamm. 2013;2013:165974. doi:10.1155/2013/165974
34. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. Vivo. 2013;27(6):669–684.
35. Sae-Khow K, Tachaboon S, Wright HL, et al. Defective neutrophil function in patients with sepsis is mostly restored by ex vivo ascorbate incubation. J Inflamm Res. 2020;13:263–274. doi:10.2147/JIR.S252433
36. Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Mater Fetal Neonatal Med. 2018;31(12):1646–1659. doi:10.1080/14767058.2017.1322060
37. Li T, Zhang Z, Li X, et al. Neutrophil extracellular traps: signaling properties and disease relevance. Mediators Inflamm. 2020;2020:9254087. doi:10.1155/2020/9254087
38. Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi:10.3389/fphys.2018.00113
39. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi:10.1038/nri3399
40. Zhang Y, Zhang Y, Gu W, He L, Sun B. Th1/Th2 cell’s function in immune system. Adv Exp Med Biol. 2014;841:45–65. doi:10.1007/978-94-017-9487-9_3
41. Tomar B, Anders HJ, Desai J, Mulay SR. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9(6):1383. doi:10.3390/cells9061383
42. Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during sepsis: an overview and update. J Cell Mol Med. 2017;21(9):1687–1697. doi:10.1111/jcmm.13112
43. de Pablo R, Monserrat J, Prieto A, Alvarez-Mon M. Role of circulating lymphocytes in patients with sepsis. BioMed Res Int. 2014;2014:671087. doi:10.1155/2014/671087
44. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock (Augusta, Ga). 2014;42(5):383–391. doi:10.1097/shk.0000000000000234
45. Nesseler N, Launey Y, Aninat C, Morel F, Malledant Y, Seguin P. Clinical review: the liver in sepsis. Crit Care. 2012;16(5):235. doi:10.1186/cc11381
46. Krehl WA. The role of nutrition in maintaining health and preventing disease. Health Values. 1983;7(2):9–13.
47. Dipasquale V, Cucinotta U, Romano C. Acute malnutrition in children: pathophysiology, clinical effects and treatment. Nutrients. 2020;12(8):2413. doi:10.3390/nu12082413
48. Fanali G, Di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209–290. doi:10.1016/j.mam.2011.12.002
49. Sun L, Yin H, Liu M, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med. 2019;51(7–8):333–344. doi:10.1080/07853890.2019.1693056
50. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi:10.1111/j.0894-0959.2004.17603.x
51. Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–722.e7. doi:10.1016/j.amjmed.2019.10.031
52. Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396–407. doi:10.1016/j.jhep.2014.04.012
53. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–193. doi:10.1002/jpen.1451
54. Kaysen GA. Biochemistry and biomarkers of inflamed patients: why look, what to assess. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S56–63. doi:10.2215/CJN.03090509
55. Yang C, Liu Z, Tian M, et al. Relationship between serum albumin levels and infections in newborn late preterm infants. Med Sci Monit. 2016;22:92–98. doi:10.12659/msm.895435
56. Caputo F, Dadduzio V, Tovoli F, et al. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PLoS One. 2020;15(5):e0232449. doi:10.1371/journal.pone.0232449
57. Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–1802. doi:10.1002/jcp.27052
58. Wang ZH, Lin YW, Wei XB, et al. Predictive value of prognostic nutritional index on COVID-19 severity. Front Nutr. 2020;7:582736. doi:10.3389/fnut.2020.582736
59. Hu X, Deng H, Wang Y, Chen L, Gu X, Wang X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition (Burbank, Los Angeles County, Calif). 2021;84:111123. doi:10.1016/j.nut.2020.111123
60. Hayashi J, Uchida T, Ri S, et al. Clinical significance of the prognostic nutritional index in patients undergoing cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2020;68(8):774–779. doi:10.1007/s11748-020-01300-x
61. Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6). doi:10.1161/JAHA.116.004876
62. Zhou W, Cao Q, Qi W, et al. Prognostic nutritional index predicts short-term postoperative outcomes after bowel resection for Crohn’s disease. Nutr Clin Pract. 2017;32(1):92–97. doi:10.1177/0884533616661844
63. Maeda K, Nagahara H, Shibutani M, et al. A preoperative low nutritional prognostic index correlates with the incidence of incisional surgical site infections after bowel resection in patients with Crohn’s disease. Surg Today. 2015;45(11):1366–1372. doi:10.1007/s00595-014-1044-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

