Back to Journals » Cancer Management and Research » Volume 10
Clinical value of carcinoembryonic antigen for predicting the incidence of brain metastases and survival in small cell lung cancer patients treated with prophylactic cranial irradiation
Authors Guo D , Jing W, Zhu H, Li MH, Zou B, Zhang Y, Fu L, Kong L, Yue J, Yu J
Received 23 May 2018
Accepted for publication 1 August 2018
Published 4 September 2018 Volume 2018:10 Pages 3199—3205
DOI https://doi.org/10.2147/CMAR.S175043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Dong Guo,1,2,* Wang Jing,2,3,* Hui Zhu,2 Minghuan Li,2 Bing Zou,2 Yan Zhang,2 Lei Fu,2 Li Kong,2 Jinbo Yue,2,4 Jinming Yu2,4
1Department of Oncology, Clinical College, Weifang Medical University, Weifang, Shandong Province, People’s Republic of China; 2Department of Radiotherapy, Shandong Cancer Hospital Affiliated to Shandong University, Jinan, Shandong Province, People’s Republic of China; 3Department of Radiotherapy, The First Affiliated Hospital to Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China; 4Shandong Academy of Medical Sciences, Jinan, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Background: Although the role of prophylactic cranial irradiation (PCI) in the treatment of small cell lung cancer (SCLC) has been confirmed, the occurrence of brain metastases (BM) in patients remains a major problem. We designed this study to evaluate the clinical value of carcinoembryonic antigen (CEA) for predicting the incidence of BM and survival in SCLC patients who received PCI.
Materials and methods: The records of 128 consecutive SCLC patients, who underwent PCI in our institute between 2005 and 2015, were analyzed. The collected data included clinicopathological features and the levels of CEA, neuron-specific enolase (NSE), cytokeratin 19 fragments (CYFRA21-1), and albumin. Kaplan–Meier and Cox regression analyses were used to determine the factors that affect BM and survival in SCLC patients after PCI.
Results: In total, 128 patients were identified, with a median (range) age of 62 (30–83) years. Thirty-two patients developed BM at some time during follow-up. The median levels of CEA, NSE, CYFRA21-1, and albumin were 7.6 ng/mL, 44 ng/mL, 4.6 ng/mL, and 42.1 g/L, respectively. In the multivariate analysis, CEA level (HR: 2.479, 95% CI: 1.101–5.581; P=0.028), advanced clinical stage (HR: 2.929, 95% CI: 1.338–6.413; P=0.007), and NSE level (HR: 3.021, 95% CI: 1.226–7.442; P=0.016) were significantly correlated with BM. CEA (HR: 1.903, 95% CI: 1.133–3.195; P=0.015) and advanced clinical stage (HR: 2.002, 95% CI: 1.227–3.267; P=0.005) were independently associated with worse overall survival in SCLC patients.
Conclusion: CEA is an independent predictive factor for the incidence of BM after PCI in SCLC and can be used as a predictor of BM in SCLC. In addition, a high level of CEA indicates a poor prognosis in SCLC patients after PCI. Prospective randomized clinical studies are required to confirm these findings.
Keywords: carcinoembryonic antigen, brain metastases, predicting, survival, small cell lung cancer
Introduction
Lung cancer is among the most common malignancies worldwide. Small cell lung cancer (SCLC) represents 15%–20% of all lung cancers.1 SCLC is characterized by rapid growth and a high incidence of metastasis,2 and their biological features are significantly different from those of non-small-cell lung cancer (NSCLC). Although SCLC has a high response rate to both radiation therapy and chemotherapy (ChT), lung cancer metastases and recurrence frequently occur. Moreover, the median survival time in SCLC is approximately 15–20 months for limited-stage (LS) disease and only 8–13 months for extensive-stage (ES) disease.2–4 Brain metastases (BM) are common, especially in SCLC. Approximately, 10% of patients with SCLC have detectable BM at the time of initial diagnosis, and more than 50% of patients are at high risk of developing BM, particularly in the first 2 years.5,6
Aupérin et al conducted a meta-analysis of prophylactic cranial irradiation (PCI) trials in LS-SCLC and showed that the application of PCI can reduce the risk of BM by 25%7; PCI is also offered to patients with ES-SCLC.8 Moreover, PCI was associated with a significant survival benefit for both ES-SCLC and LS-SCLC patients.9 Thus, PCI has become one of the standard treatment options for SCLC patients because of these confirmed advantages. However, SCLC patients still cannot avoid developing BM after PCI, and the survival of these patients with intracranial disease generally remains poor.8,10–12
Several clinical factors, such as lymphovascular invasion, advanced stage, and treatment with hyperfractionated accelerated radiation therapy, have been identified as the risk factors for BM in SCLC patients,13,14 but there is no study that shows the clinical value of carcinoembryonic antigen (CEA) in predicting the development of BM in SCLC patients after PCI. The objectives of the present study were to investigate the effect of CEA on the development of BM and to analyze the influence of CEA on the survival time of SCLC patients who received PCI.
Materials and methods
Patients
We carried out a retrospective study of 128 consecutive patients who received PCI for SCLC in Shandong Cancer Hospital and Institute (Jinan, Shandong, China) between 2005 and 2015. Clinicopathological data and serum tumor marker levels were obtained from the electronic medical record system. The inclusion criteria were as follows: pathological diagnosis of SCLC, either LS-SCLC or ES-SCLC; no history of previous anticancer therapy; no initial diagnosis of BM or other metastasis; and the presence of complete case and follow-up data. Patients were excluded if they had synchronous malignancies or had not been treated with standard therapy for LS-SCLC or ES-SCLC. All patients underwent a systematic evaluation before treatment, which included physical examination, enhanced computed tomography (CT) of the chest and abdomen, a brain CT scan or magnetic resonance imaging (MRI), bone radionuclide imaging, and routine blood tests, including serum tumor marker tests. Finally, 128 patients with SCLC who had received PCI were selected for the analyses.
Ethical approval
The study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients before their participation in this clinical research, and their data are used for future research.
Treatment
Patients with LS-SCLC were treated with sequential chemoradiotherapy (SCRT) or concurrent chemoradiotherapy (CCRT), and ES-SCLC patients were treated with ChT with thoracic radiation therapy (TRT). For radiation therapy, each patient had an individual mask, and the treatment modes for both LS-SCLC and ES-SCLC patients included conventional fractionation and accelerated hyperfractionation. ChT used cisplatin/carboplatin combined with etoposide. PCI was implemented for all patients who had stable disease or a high response rate; PCI was given at 2.5 Gy per fraction to a total dose of 25 Gy.
Data collection and statistical analysis
Variables including age, gender, smoking history, Eastern Cooperative Oncology Group (ECOG) score, loss of body weight (%), clinical stage, number of ChT cycles, radiation mode, and tumor marker levels were recorded. Tumor markers including CEA, neuron-specific enolase (NSE), and cytokeratin 19 fragments (CYFRA21-1) were examined as routine clinical practice before the initiation of treatment. The CEA, NSE, and CYFRA21-1 levels were measured by commercial electrochemiluminescence immunoassays using the Elecsys cobas e601 analyzer and reagent kits (Roche Diagnostics, Mannheim, Germany). Time to BM was measured from the start of the initial standard treatment to either the date that BM were confirmed by a brain CT scan or MRI or the date of last follow-up if no BM occurred. Overall survival (OS) was defined from the date of diagnosis to either the date of death from any cause or the last date that the patient was known to be alive.
SPSS 20.0 statistical software (IBM Corporation, Armonk, NY, USA) was used for data analysis. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values, with serum tumor markers as test variables and positive-BM and negative-BM as state variables. The sensitivity, specificity, and area under the curve (AUC) were calculated. BM development and OS were analyzed with the Kaplan–Meier method and compared with the log-rank test. Variables with statistical significance in the univariate analysis were included in the multivariate analysis (Cox regression model analysis). In general, a two-sided P-value <0.05 was considered statistically significant.
Results
Patient characteristics
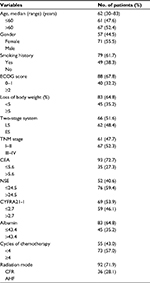
The clinical characteristics of the 128 patients, who underwent PCI included in the study group, are summarized in Table 1. The age of the patients ranged from 30 to 83 years (median: 62 years), and male patients accounted for 55.5% of the study population. The majority of the patients were in good general condition with an ECOG performance status of 0–1. Of the patients with SCLC at the time of diagnosis, 66 (51.6%) had LS disease and 62 (48.4%) had ES disease. The median follow-up time was 39 months (range: 9–84.5 months).
ROC curves for CEA were generated to determine the appropriate cutoff values, and it was found that a value of 5.6 ng/mL resulted in the optimal AUC of 0.672 (95% CI: 0.563–0.781, 56.3% sensitivity and 78.1% specificity). In the 128 patients, the median serum CEA level was 4.63 ng/mL (range: 0.42–237.5 ng/mL); and 35 (27.3%) patients exhibited high CEA levels (>5.6 ng/mL), while the other 93 (72.7%) exhibited low CEA levels (≤5.6 ng/mL). The median serum NSE level was 44 ng/mL (range: 7.4–337.1 ng/mL), and the median serum CYFRA21-1 level was 2.5 ng/mL (range: 0.6–217.5 ng/mL).
Association between serum tumor markers and the development of BM
Of the 128 patients, 32 (25%) developed BM at some time during the course of follow-up. Several clinicopathological risk factors for the development of BM that were identified with the univariate and multivariate analyses are shown in Table 2. The univariate analysis showed that the variables associated with a higher incidence of BM were advanced clinical stage (P=0.016), CEA level (P=0.001), NSE level (P=0.007), and CYFRA21-1 level (P=0.025). Moreover, patients with a high CEA level had a higher incidence of BM compared to those with a low CEA level (P=0.001, Figure 1). In the multivariate analysis, CEA level (HR: 2.479, 95% CI: 1.101–5.581; P=0.028), advanced clinical stage (HR: 2.929, 95% CI: 1.338–6.413; P=0.007), and NSE level (HR: 3.021, 95% CI: 1.226–7.442; P=0.016) were associated with an increased risk of developing BM.
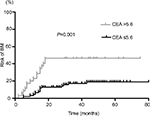  | Figure 1 Risk of BM for SCLC patients according to CEA stratification. Abbreviations: BM, brain metastases; CEA, carcinoembryonic antigen; SCLC, small cell lung cancer. |
Association between serum tumor markers and OS
In the overall population, the median OS was 38.5 months. It was significantly shorter in patients with BM (14.5 months) compared with that in patients without BM (38.5 months). The variables significantly associated with poor OS in the univariate analysis were advanced clinical stage (P=0.024), CEA level (P=0.001), NSE level (P=0.033), and CYFRA21-1 level (P=0.045). In the multivariate analysis, CEA (HR: 1.903, 95% CI: 1.133–3.195; P=0.015) and advanced clinical stage (HR: 2.002, 95% CI: 1.227–3.267; P=0.005) were significant independent predictors of OS (Table 3). Patients with a high CEA level had a worse OS compared to those with a low CEA level (P=0.001, Figure 2).
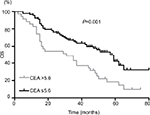  | Figure 2 OS for SCLC patients according to CEA stratification. Abbreviations: CEA, carcinoembryonic antigen; OS, overall survival; SCLC, small cell lung cancer. |
Discussion
The occurrence of BM is often a bottleneck in the treatment of malignant tumors and impairs patients’ quality of life.15,16 Especially in SCLC, patients show a higher risk of suffering from BM. PCI is an effective treatment to decrease the incidence of BM and improve survival in patients with SCLC. However, some patients still develop BM. Moreover, the value of the serum tumor marker CEA for predicting the incidence of BM and survival in SCLC patients has not been confirmed, and further research is warranted. Our study revealed that high CEA levels were associated with a higher risk of BM and decreased survival in patients with SCLC who received PCI.
CEA is a glycoprotein that attaches to the apical membrane of epithelial cells via its C-terminal glycosylphosphatidylinositol anchor. CEA is expressed more abundantly in gastrointestinal epithelium and can be found in other mucosal epithelia, such as breast, colonic, and lung epithelia.17 CEA is closely associated with tumor cell adhesion, immunologic defense, and cell survival,18,19 and tumors with high CEA expression could possess an increased capacity for BM development; this phenomenon could be due to the high ability of CEA to penetrate the blood–brain barrier and vascular-tumoral cell-cell adhesion. CEA is a useful indicator of PFS and OS in patients with colorectal cancer and NSCLC, especially, adenocarcinoma.20–23Although CEA is not specific for SCLC, studies have demonstrated that 32.8%–42.7% of patients with SCLC expressed high serum CEA levels.24–26
In a previous study by Fizazi et al,27 SCLC patients who had no PCI with a normal serum CEA level had significantly better survival than those with a high CEA level. Li et al24 reported that SCLC patients who had no PCI with a serum CEA level of >10 ng/mL have a significantly lower 5-year survival rate than those with a normal serum CEA level (P=0.004). Similar results were shown in another study. Yang et al26 studied the prognostic value of serum CEA level in SCLC without PCI. They also reported that a high serum CEA level was associated with poor clinicopathological characteristics and was an independent prognostic indicator for survival (P<0.001). However, a study conducted by Paesmans et al28 showed that the median survival time did not differ significantly between SCLC patients who had no PCI with a high CEA level and those with a low CEA level (P=0.13). These discrepancies may reflect the heterogeneity of patients in each clinical study.
In NSCLC, high CEA levels have been reported to be correlated with BM. Arrieta et al20 found that high CEA serum level is a risk factor for BM development in patients with advanced NSCLC (HR: 11.4, 95% CI: 1.7–74; P<0.01). A study to identify risk factors for BM in SCLC patients without PCI was conducted by Zeng et al,13 who found that TNM classification (P<0.05) was associated with BM in univariate and multivariate analyses. A similar study conducted in our cancer center14 found that patients with completely resected advanced-stage and lymphovascular invasion are at the highest risk for BM for SCLC without PCI. Nevertheless, they did not evaluate the predictive value of the serum tumor marker CEA for the development of BM in SCLC patients with PCI; they only evaluated risk factors for BM in SCLC patients without PCI. To the best of our knowledge, this is the first report to evaluate the prognostic significance of serum CEA level for BM and OS in SCLC patients treated with PCI. Our results showed that CEA (P=0.028), advanced clinical stage (P=0.007), and NSE level (P=0.016) were significantly related to BM and that patients with a low CEA level (P=0.015) and an early clinical stage (P=0.005) achieved significantly better OS than patients with a high CEA level and an advanced clinical stage. We found that high expression of CEA was also associated with BM (HR: 2.479, 95% CI: 1.101–5.581; P=0.028) and OS (HR: 1.903, 95% CI: 1.133–3.195; P=0.015) in SCLC patients treated with PCI.
Disease stage is a vital clinical factor that is used to predict prognosis in SCLC. Although the seventh edition of the TNM classification for lung cancer has been proposed, the two-stage system is still extensively applied in clinical practice. However, we did not observe that the two-stage system was associated with BM and OS (for all P>0.05) in SCLC in this study. Comparison of the two staging systems shows that TNM classification provides a more accurate assessment of prognostic value because it integrates various biological characteristics of tumors, lymph nodes, and metastases.29
Our study has some limitations. First, it was a retrospective analysis, which rendered it susceptible to bias in data selection and analysis. Second, our study included a small sample of patients with different stages of disease, so we could not control for all possible confounding factors. Therefore, we focused on the relationship between serum CEA level and BM and OS in this study, and our results suggest that CEA is an effective index for predicting the incidence of BM and OS in SCLC patients treated with PCI. We deeply hope that more prospective studies on this topic will be conducted to verify our conclusions.
Conclusion
In summary, this retrospective analysis revealed that a high serum CEA level is an independent prognostic factor for BM development and decreased survival in patients with SCLC after PCI. Based on our results, further investigations regarding the effect of CEA in predicting the incidence of BM and survival in SCLC patients who received PCI are needed.
Acknowledgment
This study was supported by Grant ZR2015HZ004 and Grant 2016GSF201148 from the Key Research and Development Program of Shandong Province.
Disclosure
The authors report no conflicts of interest in this work.
References
Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. | ||
van Meerbeeck JP, Fennell DA, de Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. | ||
Früh M, de Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–vi105. | ||
Morabito A, Carillio G, Daniele G, et al. Treatment of small cell lung cancer. Crit Rev Oncol Hematol. 2014;91(3):257–270. | ||
Hirsch FR, Paulson OB, Hansen HH, Vraa-Jensen J. Intracranial metastases in small cell carcinoma of the lung: correlation of clinical and autopsy findings. Cancer. 1982;50(11):2433–2437. | ||
Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer. 2004;100(4):801–806. | ||
Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–484. | ||
Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. | ||
Schild SE, Foster NR, Meyers JP, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol. 2012;23(11):2919–2924. | ||
Naidoo J, Kehoe M, Sasiadek W, Hacking D, Calvert P. Prophylactic cranial irradiation in small cell lung cancer: a single institution experience. Ir J Med Sci. 2014;183(1):129–132. | ||
Ramlov A, Tietze A, Khalil AA, Knap MM. Prophylactic cranial irradiation in patients with small cell lung cancer. A retrospective study of recurrence, survival and morbidity. Lung Cancer. 2012;77(3):561–566. | ||
Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36–42. | ||
Zeng H, Xie P, Meng X, et al. Risk factors for brain metastases after prophylactic cranial irradiation in small cell lung cancer. Sci Rep. 2017;7:42743. | ||
Zhu H, Bi Y, Han A, et al. Risk factors for brain metastases in completely resected small cell lung cancer: a retrospective study to identify patients most likely to benefit from prophylactic cranial irradiation. Radiat Oncol. 2014;9:216. | ||
Lee JJ, Bekele BN, Zhou X, Cantor SB, Komaki R, Lee JS. Decision analysis for prophylactic cranial irradiation for patients with small-cell lung cancer. J Clin Oncol. 2006;24(22):3597–3603. | ||
Yang GY, Matthews RH. Prophylactic cranial irradiation in small-cell lung cancer. Oncologist. 2000;5(4):293–298. | ||
Kokkonen N, Ulibarri IF, Kauppila A, et al. Hypoxia upregulates carcinoembryonic antigen expression in cancer cells. Int J Cancer. 2007;121(11):2443–2450. | ||
Ilantzis C, DeMarte L, Screaton RA, Stanners CP. Deregulated expression of the human tumor marker CEA and CEA family member CEACAM6 disrupts tissue architecture and blocks colonocyte differentiation. Neoplasia. 2002;4(2):151–163. | ||
Nittka S, Böhm C, Zentgraf H, Neumaier M. The CEACAM1-mediated apoptosis pathway is activated by CEA and triggers dual cleavage of CEACAM1. Oncogene. 2008;27(26):3721–3728. | ||
Arrieta O, Saavedra-Perez D, Kuri R, et al. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: a prospective analysis. BMC Cancer. 2009;9:119. | ||
Kim JY, Kim NK, Sohn SK, et al. Prognostic value of postoperative CEA clearance in rectal cancer patients with high preoperative CEA levels. Ann Surg Oncol. 2009;16(10):2771–2778. | ||
Thirunavukarasu P, Sukumar S, Sathaiah M, et al. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103(8):689–697. | ||
Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Serum carcinoembryonic antigen level in non-small-cell lung cancer patients with preoperative normal serum level. Gen Thorac Cardiovasc Surg. 2009;57(6):303–306. | ||
Li J, Dai CH, Chen P, et al. Survival and prognostic factors in small cell lung cancer. Med Oncol. 2010;27(1):73–81. | ||
Molina R, Auge JM, Escudero JM, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol. 2008;29(6):371–380. | ||
Yang X, Wang D, Yang Z, et al. CEA is an independent prognostic indicator that is associated with reduced survival and liver metastases in SCLC. Cell Biochem Biophys. 2011;59(2):113–119. | ||
Fizazi K, Cojean I, Pignon JP, et al. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: an early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer. 1998;82(6):1049–1055. | ||
Paesmans M, Sculier JP, Lecomte J, et al. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000;89(3):523–533. | ||
Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.