Back to Journals » Infection and Drug Resistance » Volume 7
Clinical utility of telavancin for treatment of hospital-acquired pneumonia: focus on non-ventilator-associated pneumonia
Authors Rubinstein E, Stryjewski ME, Barriere SL
Received 15 February 2014
Accepted for publication 3 April 2014
Published 20 May 2014 Volume 2014:7 Pages 129—135
DOI https://doi.org/10.2147/IDR.S25930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Ethan Rubinstein,1 Martin E Stryjewski,2 Steven L Barriere3
1University of Manitoba, Winnipeg, MB, Canada; 2Department of Medicine, Section of Infectious Diseases, Centro de Educación Médica e Investigaciones Clínicas (CEMIC), Buenos Aires, Argentina; 3Theravance, Inc., South San Francisco, CA, USA
Background: Hospital-acquired pneumonia (HAP) is the most common health care-associated infection contributing to death. Studies have indicated that there may be differences in the causative pathogens and outcomes of ventilator-associated pneumonia (VAP) and non-ventilator-associated pneumonia (NV-HAP). However, with limited NV-HAP-specific data available, treatment is generally based on data from studies of VAP. The Phase 3 Assessment of Telavancin for Treatment of Hospital-Acquired Pneumonia (ATTAIN) studies were two double-blind randomized controlled trials that demonstrated the non-inferiority of telavancin to vancomycin for treatment of Gram-positive HAP. We conducted a post hoc subgroup analysis of patients enrolled in the ATTAIN studies who had NV-HAP.
Methods: Data from the two ATTAIN studies were pooled, and patients with NV-HAP were analyzed. The all-treated (AT) population consisted of all randomized patients who received ≥1 dose of study medication, and the clinically evaluable (CE) population consisted of AT patients who were protocol-adherent or who died on or after study day 3, where death was attributable to the HAP episode under study. The primary endpoint was clinical response (cure, failure, or indeterminate) at the follow-up/test of cure visit, conducted 7–14 days after the end of therapy.
Results: A total of 1,076 patients (71.6% of overall ATTAIN AT population) had NV-HAP (533 and 543 patients in the telavancin and vancomycin treatment groups, respectively). Clinical cure rates in the CE population were similar for patients with NV-HAP treated with telavancin and vancomycin (83.1% [201/242] and 84.1% [233/277], respectively). In patients with methicillin-resistant Staphylococcus aureus isolated at baseline, cure rates in the CE population were 74.8% (77/103) for telavancin and 79.3% (96/121) for vancomycin. The incidence of adverse events, serious adverse events, and deaths in patients with NV-HAP was similar whether patients received telavancin or vancomycin.
Conclusion: This post hoc subgroup analysis of the ATTAIN studies demonstrated similar cure rates for telavancin and vancomycin for treatment of NV-HAP.
Keywords: nosocomial pneumonia, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus
Introduction
Hospital-acquired pneumonia (HAP), defined as pneumonia occurring at least 48 hours after admission to a health care facility (which was not incubating at admission), is the most common health care-associated infection contributing to death.1–3 The treatment of HAP is complicated by the frequent involvement of multidrug-resistant (MDR) pathogens, including methicillin-resistant Staphylococcus aureus (MRSA).1,2 Mechanical ventilation is the primary risk factor for HAP, with mechanically ventilated patients up to 21 times more likely to develop HAP than non-ventilated patients.1,4 As such, ventilator-associated pneumonia (VAP), defined as pneumonia occurring at least 48–72 hours after endotracheal intubation, has been the focus of the majority of HAP research to date.1,5
Although there is a paucity of data on patients with non-ventilator-associated pneumonia (NV-HAP), recent reports suggest that it may be worthy of greater attention. Certain studies have suggested that mortality in patients with NV-HAP may be similar to, or even higher than, those with VAP.6,7 Furthermore, studies have indicated that the underlying pathogens, and the antimicrobial susceptibility thereof (including incidence of MDR strains), of VAP and NV-HAP may be different.7–11 It is well recognized that early, appropriate antimicrobial therapy is critical in reducing HAP mortality.1 Successful empiric therapy hinges on knowledge of the likely pathogens involved, and in particular, on recognizing patients at risk of infection with MDR pathogens.1
Despite these potential differences, treatment decisions concerning NV-HAP are most often made based on extrapolation of VAP data, which may prove to be suboptimal.
Telavancin is a lipoglycopeptide antibiotic with bactericidal activity against clinically relevant Gram-positive bacteria, mediated by a dual mechanism of action involving inhibition of cell-wall biosynthesis and disruption of bacterial membrane barrier function.12–14 Telavancin has been shown to adequately penetrate into the epithelial lining fluid and alveolar macrophages and remain active in the presence of pulmonary surfactant.15,16 Telavancin is approved in the United States and Europe for the treatment of hospital-acquired bacterial pneumonia (HABP), including ventilator-associated bacterial pneumonia (VABP) due to susceptible isolates of S. aureus (methicillin-resistant strains [MRSA] only in Europe), when alternative medicines are unsuitable.
The Assessment of Telavancin for Treatment of Hospital-Acquired Pneumonia (ATTAIN) studies were two identical double-blind randomized controlled trials that demonstrated the non-inferiority of telavancin to vancomycin for treatment of Gram-positive HAP (NCT00107952 and NCT00124020).17
To contribute to the understanding of NV-HAP, we conducted a post hoc subgroup analysis of patients with NV-HAP enrolled in the ATTAIN studies.
Methods
Study design
The methodology of the ATTAIN studies has been described in detail previously17 and is summarized here. Patients were eligible for enrollment if they had pneumonia acquired after 48 hours in an inpatient acute or chronic care facility, or that developed within 7 days after being discharged. Patients were randomized (1:1) to receive either telavancin 10 mg/kg intravenously every 24 hours or vancomycin 1 g intravenously every 12 hours for 7–21 days. Vancomycin dosage could be modified per site-specific guidelines based on weight, renal function, and/or vancomycin serum level determinations as long as the study blind was maintained. Respiratory samples (invasive or noninvasive) and two blood culture specimens were obtained at baseline for Gram stain and culture in accordance with clinical practice guidelines.1 Isolated pathogens were submitted to a central laboratory for confirmation of identity and susceptibility testing, per Clinical and Laboratory Standards Institute guidance.18
Statistical analysis
For the present analysis, data from the two ATTAIN studies were pooled. Patients with NV-HAP were patients with HAP who had never been ventilated, plus those who developed pneumonia after at least 48 hours of hospitalization (HAP) and then went on to require mechanical ventilator support (and so were deemed to have developed their pneumonia prior to being ventilated).
The analysis populations were defined as follows: the safety population included all patients who received ≥1 dose of study medication (according to the treatment received). The all-treated (AT) population included all randomized patients who received ≥1 dose of study medication (according to the randomized treatment). The “modified AT” population consisted of patients in the AT population who had a respiratory pathogen identified from baseline samples, or from blood cultures if no respiratory sample was positive. The clinically evaluable (CE) population consisted of patients in the AT population who were protocol-adherent or who died on or after study day 3, where death was attributable to the HAP episode under study. The microbiologically evaluable (ME) population consisted of CE patients who had a Gram-positive respiratory pathogen recovered from baseline respiratory specimens or blood cultures.
One of the stratification factors at randomization was ventilatory status (in addition to geographic region and the presence or absence of diabetes). Patient demographics, pathogens, clinical response, adverse events (AEs), and mortality were evaluated by treatment group (telavancin or vancomycin). The primary endpoint was clinical response in the AT and CE populations at the follow-up/test of cure (FU/TOC) visit, which was conducted 7–14 days after the end of therapy (EOT). Clinical response was classified as cure, failure, or indeterminate. “Cure” was defined as improvement or lack of progression of baseline radiographic findings at EOT and resolution of signs and symptoms of pneumonia at FU/TOC. “Failure” was defined as at least one of: persistence or progression of signs and symptoms, or progression of radiological signs of pneumonia at EOT; termination of study medications due to “lack of efficacy” and initiation within 2 calendar days of a different potentially effective antistaphylococcal medication; death on or after day 3 attributable to primary infection; or relapsed infection at TOC after termination of study medications. “Indeterminate” was defined as inability to determine outcome. Failure at EOT was carried forward to FU/TOC. Two-sided 95% confidence intervals (CIs) were calculated on the difference in response rate; pooled-study CIs were stratified by study.
Survival over the 28-day period was summarized using Kaplan–Meier estimates.
Results
Demographics and patient disposition
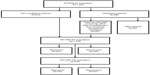
The NV-HAP AT population constituted 71.6% (1,076/1,503) of the overall ATTAIN AT population (533 and 543 patients in the telavancin and vancomycin treatment groups, respectively) (Figure 1).
The demographic and baseline characteristics of the telavancin- and vancomycin-treated patients in the NV-HAP AT population were generally similar (Table 1). The number of patients who received telavancin and vancomycin who had creatinine clearance ≤50 mL/min or who had acute renal failure at baseline was comparable (Table 1).
Approximately half of modified AT patients had solely Gram-positive pathogens isolated at baseline (Table 2). Approximately two-thirds of the S. aureus identified at baseline was MRSA (Table 2). A greater number of telavancin-treated than vancomycin-treated patients with NV-HAP had mixed Gram-positive/Gram-negative infections at baseline (Table 2).
Reasons for study drug discontinuation were similar between the treatment groups. The five most common reasons for discontinuation in telavancin-treated patients were death (9%), unsatisfactory therapeutic response (8%), Gram-positive coverage no longer clinically indicated (8%), AEs (4%), and withdrawal of consent (4%); the corresponding frequencies in the vancomycin group were 8%, 8%, 7%, 4%, and 3%, respectively.
Clinical response
Clinical cure rates in the CE population were similar for patients with NV-HAP treated with telavancin (83.1%) and vancomycin (84.1% [95% CI for the difference: −7.5% to 5.3%]) (Table 3). Likewise, the rates of clinical failure and the reasons thereof were similar between the treatment groups (Table 3).
Cure rates were similar for NV-HAP patients treated with telavancin and vancomycin who had S. aureus isolated at baseline (78.8% and 79.9%, respectively), including methicillin-sensitive S. aureus (85.0% and 80.0%, respectively) and MRSA baseline (74.8% and 79.3%, respectively) (Table 3).
Safety
The incidence of AEs and serious AEs (SAEs) in patients with NV-HAP was similar between the treatment groups (Table 4). There was little difference between the treatment groups in the incidence of common (at least 5% in either treatment group) gastrointestinal AEs (constipation, diarrhea, and nausea). The incidence of any renal events was higher in the vancomycin group (11.8%) than the telavancin group (8.4%), but the incidences of acute renal failure, renal insufficiency, hematuria, and oliguria were similar for patients treated with telavancin and vancomycin (Table 4).
Figure 2 shows the Kaplan–Meier 28-day survival curves by ventilator status in the NV-HAP AT population. The 28-day survival estimates for NV-HAP patients who did not receive mechanical ventilation were 80.1% (95% CI: 76.3%–84.0%) and 80.7% (95% CI: 76.8%–84.6%) for patients treated with telavancin and vancomycin, respectively (95% CI for the difference: −6.0% to 4.9%). The 28-day survival estimates for NV-HAP patients who developed pneumonia after at least 48 hours of hospitalization and then went on to require mechanical ventilator support were lower, at 73.3% (95% CI: 65.4%–81.2%) and 68.6% (95% CI: 60.7%–76.5%) for patients treated with telavancin and vancomycin, respectively (95% CI for the difference: −6.5% to 15.9%).
Discussion
This post hoc subgroup analysis of the ATTAIN studies sought to evaluate telavancin versus vancomycin in the subgroup of patients with NV-HAP, including those patients who were ventilated, but did not meet the definition for VAP.
Whether a hospitalized patient acquires pneumonia before or during ventilation may have a significant effect on the characteristics of the illness and its outcomes, although the vast majority of studies to date have focused on patients with VAP.
Clinical cure rates were similar for patients with NV-HAP treated with telavancin or vancomycin. These cure rates were consistent with those observed in the overall ATTAIN population (82.4% for telavancin and 80.7% for vancomycin).17
The incidence of AEs, SAEs, and deaths was similar in patients with NV-HAP, regardless of the treatment received, although renal AEs were more frequently observed in patients with NV-VAP who were treated with vancomycin compared with telavancin. Compared with the overall ATTAIN population, the incidence of AEs, SAEs, and deaths in the NV-HAP subgroup was similar to the overall ATTAIN population.17
Kaplan–Meier analysis demonstrated that survival rates were consistently higher for NV-HAP patients who were not mechanically ventilated compared with those who were deemed to have developed pneumonia prior to being mechanically ventilated in both treatment groups.
These results indicate that telavancin is a useful treatment option for patients with NV-HAP (consistent with the available prescribing information in the United States and Europe). Use of telavancin may be particularly suitable for treatment of infections caused by strains of S. aureus with reduced susceptibility to vancomycin (eg, vancomycin-intermediate or heterogeneously vancomycin-intermediate), linezolid, or daptomycin, against which telavancin has demonstrated in vitro activity owing to its dual mechanism of action.12–14,19
The major limitation of this study is that it is a post hoc analysis, and as such, the ATTAIN studies were not designed to study the differences between telavancin and vancomycin in the subgroup of patients with NV-HAP. However, one of the randomization stratification factors was ventilatory status at baseline. A further limitation is that adjustment of vancomycin dosing using serum levels was not mandatory or part of the hospital policies for all participating sites. That said, the majority of patients for whom vancomycin levels were determined had mean trough levels that were considered adequate (5–15 μg/mL) at the time of the studies.17
Conclusion
The results of this post hoc subgroup analysis of the Phase 3 ATTAIN studies demonstrate that NV-HAP clinical cure and mortality rates for telavancin and vancomycin were similar. Patients with NV-HAP who were deemed to have developed pneumonia prior to be being mechanically ventilated had lower survival rates than those who were not mechanically ventilated.
Acknowledgments
The ATTAIN studies were sponsored by Theravance, Inc. Medical writing support was provided by Emily Howard of Envision Scientific Solutions, funded by Theravance, Inc.
Disclosure
Ethan Rubinstein has served on advisory boards for Astellas, Atox Bio, Bayer, BiondVax, Pfizer, and Theravance, has served as a consultant for Roche and Theravance, and has received payment for lectures/speaker bureaus from Astellas, Bayer, and Pfizer. Martin E Stryjewski has served as a consultant for Astellas, Cempra, Cerexa, Furiex, Nabriva, PRA International, The Medicines Company, Theravance, and Trius, has received grants from Duke University (NIH), and has received other financial support (including reimbursement for travel expenses and/or manuscript preparation) from Cempra, JMI Laboratories, and Theravance. Steven L Barriere is an employee of, and holds equity securities of, Theravance, Inc.
References
American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. | |
Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128(6):3854–3862. | |
Masterton RG, Galloway A, French G, et al. Guidelines for the management of hospital-acquired pneumonia in the UK: report of the working party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother. 2008;62(1):5–34. | |
Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165(7):867–903. | |
Davis J, Finley E. Focus on Infection Prevention. The breadth of hospital-acquired pneumonia: nonventilated versus ventilated patients in Pennsylvania. Pennsylvania Patient Safety Advisory. 2012;9(3):99–106. | |
Esperatti M, Ferrer M, Theessen A, et al. Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med. 2010;182(12):1533–1539. | |
Yang YS, Lee YT, Huang TW, et al. Acinetobacter baumannii nosocomial pneumonia: is the outcome more favorable in non-ventilated than ventilated patients? BMC Infect Dis. 2013;13:142. | |
Bousbia S, Papazian L, Saux P, et al. Repertoire of intensive care unit pneumonia microbiota. PLoS One. 2012;7(2):e32486. | |
Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28(7):825–831. | |
Bhavnani SM, Rubino CM, Hammel JP, et al. Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother. 2012;56(2):1065–1072. | |
Sopena N, Sabrià M. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest. 2005;127(1):213–219. | |
Lunde CS, Hartouni SR, Janc JW, Mammen M, Humphrey PP,Benton BM. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob Agents Chemother. 2009;53(8):3375–3383. | |
Lunde CS, Rexer CH, Hartouni SR, Axt S, Benton BM. Fluorescence microscopy demonstrates enhanced targeting of telavancin to the division septum of Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54(5):2198–2200. | |
Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49(3):1127–1134. | |
Gotfried MH, Shaw JP, Benton BM, et al. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob Agents Chemother. 2008;52(1):92–97. | |
Lodise TP Jr, Gotfried M, Barriere S, Drusano GL. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother. 2008;52(7):2300–2304. | |
Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to Gram-positive pathogens. Clin Infect Dis. 2011;52(1):31–40. | |
CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Seventh Edition. M7-A7. Wayne, PA: CLSI; 2006. Available from: http://isoforlab.com/phocadownload/csli/M7-A7.pdf. Accessed April 3, 2014. | |
Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis. 2009;49(12):1908–1914. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.