Back to Journals » Journal of Asthma and Allergy » Volume 13
Clinical Utility of Rush Venom Immunotherapy: Current Status
Authors Gruzelle V , Mailhol C, Waters DW, Guilleminault L
Received 19 October 2019
Accepted for publication 3 December 2019
Published 7 January 2020 Volume 2020:13 Pages 1—10
DOI https://doi.org/10.2147/JAA.S200917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Vianney Gruzelle,1 Claire Mailhol,2,3 David W Waters,4 Laurent Guilleminault2,4
1Department of Paediatric Pneumology and Allergology, University Hospital Centre of Toulouse, Toulouse, France; 2Department of Respiratory Medicine and Allergic Diseases, University Hospital Centre of Toulouse, Toulouse, France; 3Mastocytosis Reference Centre and Dermatology Department, University Hospital Centre of Toulouse, Toulouse, France; 4Center for Pathophysiology Toulouse Purpan, INSERM U1043, CNRS UMR 5282, Toulouse III University, Toulouse, France
Correspondence: Laurent Guilleminault
Department of Respiratory Medicine and Allergic Diseases Hôpital Larrey, 24 Chemin De Pouvourville, Toulouse TSA 30030, France
Tel +335 67 77 18 50
Fax +335 67 77 14 72
Email [email protected]
Abstract: Hymenoptera venom allergy (HVA) is the leading cause of anaphylactic reactions in adults and the second most common cause in children. Venom immunotherapy (VIT) is used to elicit an immune tolerance against hymenoptera venom in allergic patients and is based on the administration of purified venom extracts regularly for defined periods. The protocols of administration include 2 phases: an up-dosing phase that incrementally reaches the final dose resulting in a protective effect, and a maintenance phase in order to obtain the sustained effect. The goal of this review is to detail the efficacy and the safety of the up-dosing phase also named rush. Pathophysiological mechanisms, indications of VIT and technical aspects of up-dosing protocol are also covered.
Keywords: hymenoptera, allergy, immunotherapy
Introduction
Hymenoptera venom allergy (HVA) is the leading cause of anaphylactic reactions in adults.1 In children, it is acknowledged as the second most common cause, after food-related anaphylaxis.2 Such reactions are primarily due to stings by honeybees (Apis) and social wasps (Vespula vulgaris, Vespula germanica, and Polistes dominula, in particular). Insect stings by hymenoptera species are frequent with 56.6% to 94.5% of the general population stung at least once in their lifetime.3 Insect stings also affect young children, in an Irish prospective longitudinal study, 6.8% of children had been stung by the age of 2 years, and 21.9% had been stung by the age of 5 years.4
Sensitization to hymenoptera venom (HV) (defined by the positivity of skin prick-tests and/or specific IgE) is widely observed in the general population. The prevalence ranges from 27% to 40% of adults, and up to 50% of children.5 The prevalence of large local reactions (LLR), usually defined as swelling exceeding 10 cm of diameter that lasts >24 hrs, varies considerably from one study to another due to a non-uniform definition of LLR, different methodology of surveys, and different populations surveyed. In the general population, LLR ranges from 2.4% to 26.4%,6 in children a prevalence of 19% has been reported.7 In beekeepers, a high-risk group, prevalence ranges from 31% to 38%.8,9 Currently, the risk of evolution of LLR to a systemic sting reactions (SSR) after re-sting is considered low, ranging from 5% to 15% in adults, and 2% to 4% in children.10–12 The rate of self-reported SSR in European studies ranges from 0.3% to 7.5% in adults of the general population.13 There is less reported SSR in children, rates range from 0.15% to 3.4%.13–15 Systemic sting reactions can be life threatening, with an estimated death rate of 0.03 to 0.48/1,000,000 inhabitants per year.3
History of Venom Immunotherapy
Reports of HVA can be found in the annals of history, the description of an anaphylactic response by Pharaoh Menes (2600 BC) to a wasp sting is thought to be the first documented anaphylactic death to HV.16 For centuries the management of HVA was rudimentary, with no noticeable progress for millennia. Detailed descriptions of HV stings started to appear in publications of the eighteenth and nineteenth century, describing what is now referred to as anaphylaxis.17
The first notable date regarding therapy was in 1925, when Dr Braun reported the effective use of immunotherapy in a bee allergic patient. In the report, Dr Braun described snipping off the posterior 1/8 inch of the Bee’s body and grinding it up, extracting it in saline, and filtering the solution, demonstrating a positive skin test response and then using the same material to perform immunotherapy. In the following 40 years, a plethora of publication reinforced the efficacy of whole-body extract (WBE) for immunotherapy to Hymenoptera.18
In the 1970s, a second revolution took place, with the first purified venoms for desensitization. A publication from 1974 reports a successful case of immunotherapy using purified bee venom to treat a 4-year-old child with HVA.19–21
Pathophysiological Mechanism of Venom Immunotherapy
Initial sensitization to HV involves priming of allergen-specific T helper type 2 (Th2) cells resulting in the production of interlukin-4 (IL-4) and IL-13, and also CD40 ligand signaling. This cytokine signaling and ligand binding drives immunoglobulin-E (IgE) production by B cells.22 The resulting IgE binds mast cells and basophils via high-affinity FcεRI receptors expressed on their surface.23 Upon subsequent HV exposure, antigen mediated IgE/FcεRI crosslinking on the surface of sensitized cells triggers the release of pre-formed mediators contained in cytoplasmic granules, such as histamine, resulting in the development of a type I hypersensitivity reaction.24–26
The goal of venom immunotherapy (VIT) is to elicit a healthy immune response in HV allergic patients. Acquired tolerance is characterized by several mechanisms including changes to allergen-specific T and B cell responses and higher mast cell and basophil activation thresholds.27,28 The early response to VIT is characterized by desensitization and subsequent decreased mediator release from mast cells and basophils.28–30 The rapid up-regulation of the histamine type 2 receptor, which has a suppressive effect on FcεRI-mediated activation and degranulation, has been proposed to contribute to early desensitization,31 as have changes in FcεRI expression;32 however, underlying mechanisms need to be further elucidated in order to better explain early desensitization events.
As allergen dosing increases, the next key event in the development of a healthy immune response is a change in T cell subset ratios.33 Research into beekeepers has shown that continuous exposure to high doses of HV suppresses allergen-specific T cell subsets and drives the switch towards IL-10 and Transforming growth factor beta (TGFβ) secreting regulatory T cells (Tregs).31 These soluble mediators are key regulators of immunological processes in peripheral tolerance to allergens.34 Interluken-10 and TGF- β drive the switch in B cell antibody (Ab) production away from IgE towards the non-inflammatory isotypes IgA and IgG.35,36 This switch in Ab production from IgE to IgA to IgG4 is a key feature of long-term tolerance. Immunoglobulin-G4 competes with IgE for allergen, blocking allergen-IgE complexes to prevent the degranulation of mast cells and basophils.37 Interlukin-10 also plays a direct role in inhibiting mast cell degranulation through signaling inhibition and also reducing FcεRI expression and function38 (Figure 1).
Indications of VIT
Reactions following hymenoptera stings include physiological local reactions, related to the composition of venoms (histamine, serotonin, bradykinin, etc.), and allergic reactions. The latter can be divided into LLR at the sting site, which are the most common manifestation of HVA, and systemic reactions, ranging from isolated cutaneous reaction (e.g., urticaria) to SSR, which are the most severe (Classifications of reactions are detailed in Table 1).
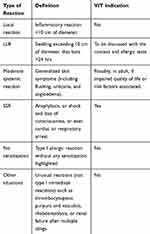 |
Table 1 Definitions and VIT Indications for Each Type of Reaction |
Venom immunotherapy is indicated in children and adults following a SSR, exceeding generalized skin symptoms, with a documented sensitization to the venom of the culprit insect via skin prick tests and/or specific serum IgE tests and/or the basophil activation test.39–43 Concerning moderate SSR (i.e., SSR confined to generalized skin symptoms), VIT is recommended in adult patients if the quality of life is impaired.40,44,45 When faced with this clinical situation it is also necessary to identify risk factors for relapse, and factors aggravating the severity of a possible recurrence (e.g., mastocytosis).
Generally, patients with repeated LLR are reported as having minimal risk of progressing to SSR. VIT is not typically recommended for these patients.46,47 Nevertheless, a recent prospective study indicates a risk of 24% SSR in adults and children after an initial LLR.48 The risk of SSR was reported to be higher in cases of skin test reactivity to Apis mellifera or Vespula species (OR 2.1 and 3.8, respectively), if positive at 0.001 µg/mL concentration (OR 13.4 and 16.5, respectively). For the authors, an accurate diagnostic workup may be considered in LLR, particularly skin tests. If skin tests are positive at low dilutions, VIT may be performed because of a high risk (24%) of developing SSR if re-sting occurs particularly in at-risk population. However, more studies are warranted to clarify the place of VIT in LLR.
Scenarios when VIT should not be considered include: the lack of sensitization to insect venom during skin prick tests or the absence of specific IgE. In patients that are sensitized to insect venom but have not experienced a SSR, VIT should not be considered. Additionally, VIT should also not be considered in the context of unusual reactions that are not attributed to type I hypersensitivity reactions such as thrombocytopenic purpura and vasculitis, rhabdomyolysis, or renal failure after multiple stings3 (see Table 1).
Typically, the diagnosis of HVA has been based on skin tests and specific IgE to WBE. However, recently component-resolved diagnosis (CRD) has been developed in which venom allergen proteins, instead of WBE, are now used to characterize sensitization in patients.49 Venom allergen proteins are produced by genetic engineering using insect cells.50 The available CRD data on HV in clinical practice are presented in Table 2. There are limited benefits of adding recombinant allergens to improve the detection of Hymenoptera venom-allergic patients.49 One of the most important disadvantages of WBE is the underrepresentation of allergens that are present in low abundance. This may lead to the failure to detect sensitization in some cases.51 Regarding the high amount of Api m 1 and Api m 4 in WBE, which represent together 62% of the dry weight, CRD does not improve detection rates. However, the use of WBE allergens in low abundance, such as Api m 3 or Api m 10, may lead to underdiagnosing the severity of venom allergy.
 |
Table 2 Available Component-Resolved Diagnosis of Venom Hymenptera in Clinical Practice |
Venom Immunotherapy: Technical Aspects and Up-Dosing Protocols
Although early case studies carried out VIT using WBE, VIT is now practiced with industrially produced venom extracts with the specific allergen composition standardized. When the culprit hymenoptera has been identified and allergy diagnosis confirmed, venom extract can be administered, subcutaneously, following standardized protocols. These protocols always include 2 phases: an up-dosing phase that incrementally reaches the final dose resulting in a protective effect, and a maintenance phase in order to obtain the sustained effect.
This final dose may be reached within a few weeks to months (in outpatient clinics), days or hours (ultra-rush or cluster protocols including several injections a day) depending on the protocol. These protocols include administrated doses and cumulative doses (Table 3 describes some published administration protocols).52–57 In the published literature describing VIT protocols, clinical characteristics of the patients are disparate, and the number of patients is sometimes low. Considering its brevity and simplicity, Birnbaum’s protocol is used as a reference in our department. The data in Table 3 may help to determine the most appropriate protocol for other teams to adopt. In case of toxicity or reaction to an ultra-rush protocol, our department utilizes a protocol with a slow administration among those presented in Table 3.
 |
Table 3 Examples of Injection Schedule Published for VIT |
The data suggest that sustained tolerance is achieved more effectively using long-way protocols compared to ultra-rush protocols.39
At-Risk Populations
Recent data suggest that 5 to 15% of anaphylactic reactions to venom hymenopthera show signs of potential mastocytosis. Systemic mastocytosis may be evoked and diagnosed during hymenoptera allergy care by serum tryptase dosage and cutaneous examination.58 These patients often show a very low specific IgE level. Mastocytosis and serum tryptase level over 20 µg/L are associated with higher risk of adverse events in VIT in vespid allergy.39 The REMA score can help to identify, with a 90% predictive value, patients with a clonal mast cell disorder when at least 2 criteria are met.59 Efficacy is potentially lower with relapses after stopping VIT. Life-long therapy is recommended in patients with mastocytosis. Up-dosing is not necessary except in adverse events during VIT. Ultra-rush protocols seem to be well tolerated in patients with mastocytosis60 and VIT is considered safe.39
In beekeepers, treatment with bee venom is the most important risk factor for systemic adverse events with VIT. Upgrading maintenance dose and long-life treatment should be considered in the bee-keeping population.39
Regarding current guidelines, VIT should not be initiated during pregnancy; however, if ongoing VIT is well tolerated this may be continued.39 In children below 5 years of age, VIT should only be considered in the event of a SSR and when the child is likely to be co-operative. VIT is not recommended in patients with active multi-system autoimmune disorders. Nevertheless, VIT is recommended in patients with organ-specific autoimmune disorders and patients with cardiovascular disease providing that the underlying disease is stabilized, and in high-risk venom-allergic patients with malignant disease as long as the disease is stable or in remission.
Effectiveness of Venom Immunotherapy
The main goal of VIT is to prevent a relapse of systemic reaction in a context of hymenoptera re-sting. This treatment is mainly considered as a preventive treatment. Evaluation of efficacy can be challenging especially when the patient undergoing VIT has not been exposed to HV since the last clinical visit.
Two meta-analysis studies have evaluated the effect of VIT on the risk of systemic reaction to a sting. Meta-analysis conducted by the Cochrane database pooled the results from 6 studies (392 patients).41 A subsequent systemic allergic reaction to a sting was observed in 3 out of 113 (2.7%) participants treated with VIT compared to 37 out of 93 (39.8%) untreated patients. The risk ratio was established at 0.10 [95% confidence interval (CI) 0.03 to 0.28]. Suggesting the risk of a systemic reaction to a sting decreases by 90% with VIT. In a more recent meta-analysis, 17 studies were retained for the final analysis.40 The authors concluded that VIT was beneficial with a substantial reduction of the risk of subsequent SSR (OR=0.08, 95% CI 0.03 to 0.26).
Venom immunotherapy is recognized as the only treatment that can potentially prevent further SSR. Its effectiveness ranges from 77% to 84% of patients treated with honeybee venom, and in 91–96% of patients receiving vespid venom.61,62 The difference between honeybee and vespid has not been fully elucidated. It has been speculated that the amount of venom delivered by a honeybee sting is more important and some patients allergic to honeybee venom are sensitized to protein present in a low amount of immunotherapy solution.63,64
In the European Academy of Allergy and Clinical Immunology (EAACI) guidelines, beyond the benefit of VIT in preventing SSR, the authors highlight VIT also improves quality of life.39 In patients with an allergy to HV, 80% declared a significant improvement in health-related quality of life after a sting challenge test.65 Disease-specific quality of life was also shown to be improved in a meta-analysis study with a risk difference of 1.41 (95% CI 1.04–1.79).40
These data take into account the whole treatment with immunotherapy and do not focus on the build-up phase. As explained above, several schedules are used in the build-up phase to reach the maintained dose. The efficacy of the build-up phase in terms of efficacy has not been extensively studied. After rush immunotherapy, a decrease in skin-test sensitivity has been observed.66 However, no study has yet identified a relevant difference in skin test reactivity between tolerant subjects and patients with relapses.67,68 Sting challenges are considered the gold standard for the assessment of VIT efficacy, but the challenges are feasible only in specialized centers. Only one study to date had focused on the efficacy of rush VIT, the authors report interesting data on response to sting challenge after the build-up phase.69 Bee venom-allergic patients who underwent conventional or rush VIT were challenged with a live bee sting within 1 week after reaching the 100 µg maintenance dose. Among the 107 patients, 79 (73.8%) agreed to be challenge, 70 (88.6%) tolerated the challenge with no reaction. Only 4 (5.1%) patients developed a transient rash. Five (6.3%) presented a mild-to-moderate systemic reaction and 4 were given an increased dose of VIT (200–250µg) and tolerate further challenges.
To our knowledge, the effectiveness of the different schedules used in the build-up phase have never been compared. Studies have only focused on the safety of VIT schedules.
Safety of Venom Immunotherapy
The most important objective of the build-up phase is to reach the maintenance dose with no reactions. As described above, several schedules can be employed in the build-up phase. The safety of the build-up phase is high as the maintenance dose is reached in 96.6% of patients with a rush protocol70 and 97.5% of patients with an ultra-rush protocol.56 A systemic reaction was shown to occur in patients receiving a 3-day protocol with a frequency ranging from 10.9% to 29.6%.70–72 A local reaction has been described in 42.3% of patients receiving a 3-day protocol. Regarding the ultra-rush protocol, systemic reactions range from 7% to 22%.56,73–75 The vast majority of systemic reactions are mild and do not need medication.76 However, life-threatening anaphylaxis and fatal anaphylaxis have been described,77 and the build-up phase should be managed by trained health care professionals able to start resuscitation if needed. Uncontrolled asthma, a prior history of SRs, administration of SCIT injections during peak pollen season, and delay in administration of epinephrine are recognized factors of fatal reaction to VIT.78,79 Reactions to immunotherapy are not restricted to the build-up phase and may appear during the maintenance phase. An increase in observed reactions during the build-up phase compared to the maintenance phase has been described (1.5% vs 2.4%).72 However, this is still debated and some authors described an increased risk of fatal reactions during the maintenance phase.78
Potential adverse reactions from VIT are difficult to predict. In a prospective study carried out on 680 patients, index sting reaction grade III or IV, and female sex were not identified as risk factors of a reaction needing an emergency intervention.80 Bee VIT clearly increases the risk of reactions compared to vespid venom.70,74,80 Before VIT, measurement of baseline serum tryptase concentration should be used to identify patients with a high risk for side effects. Moreover, mastocytosis is a significant determinant for VIT failure.62 Conventional protocols are less likely to result in a reaction needing an emergency intervention80 compared to ultra-rush protocols which have been shown to increase the frequency of adverse reactions.80 It should be acknowledged that several studies have suggested that an ultra-rush protocol may be associated with less adverse reactions compared to rush or conventional protocols; however, the cumulative effect was not comparable between the schedules.52,81
Beta-blockers had been considered to increase the risk of a systemic reaction during VIT particularly in patients with cardiovascular diseases.82 However, two large studies,80,83 and a position paper published by the EAACI84 suggest beta-blockers are no longer to be considered as contra-indicated in VIT. Although angiotensin conversion enzyme inhibitors (ACEI) were previously associated with systemic reactions during VIT, the EAACI has now recommended that ACEI therapy can be continued during VIT as long as the patient is informed about possible risks.39 Any use of anti-hypertensive drugs has been identified as a significant risk factor of systemic reactions to VIT.80 However, in a retrospective study that focused on patients with cardiovascular diseases, cardiovascular medication did not impair the safety and/or the efficacy of VIT.85 Surprisingly, reaction rates were lower in patients taking any kind of cardiovascular medication or an ACEI. Patients taking anti-hypertensive drugs should be evaluated carefully before starting VIT, based on an individual risk/benefit assessment.
Pre-treatment with antihistamines reduces large local reactions and systemic reactions and should be used in clinical practice 1 or 2 hrs before starting VIT.86 In rare cases, VIT is associated with severe systemic reactions with conventional protocols. Pre-treatment with omalizumab has been used in case reports and case series with interesting effects.87–89 In patients with severe systemic reactions to VIT, omalizumab was not associated with a reaction when administered concomitantly with VIT. The regimen of omalizumab administration varies between studies and so a typical regimen of administration cannot be referenced.
Maintenance Dosing and Long-Term Evaluation of Venom Immunotherapy
Once the maintenance dose has been reached, venom extract must be injected regularly in order to obtain long-term effects.
The maintenance dose is typically 100 µg of the culprit venom (equivalent to 2 honeybee stings or 5 wasp stings). Some specific cases require an increase to 200 µg: patients who experience anaphylaxis under ongoing VIT, beekeepers and other high-risk populations. Intervals of 4 to 6 weeks between injections are recommended in the EAACI guidelines: 4 weeks during the first year, 6 during the second year and 8 weeks between 3 and 5 years, intervals may be extended to 3 months however no effect has been demonstrated when intervals are extended to 6 months.
Venom immunotherapy duration of 3 years may be sufficient in most cases with mild reactions; however, 5 years of VIT are associated with more successful long-term effectiveness. The EAACI guidelines also recommend considering life-long treatment in patients that experience a severe initial systemic reaction or systemic adverse events during VIT. Honeybee venom-allergic patients with a high risk of stings, such as beekeepers should also carry out lifelong VIT to prevent relapse.
Effectiveness may reach a 97 to 98% protection with a better score in vespid VIT than in honeybee.39
Conclusion
Venom immunotherapy is effective for patients with HVA. It is the only causal and preventive treatment with respect to potential future sting reactions. Hymenoptera venom allergy indications are well codified. The build-up phase relies mainly on the ultra-rush protocol. Venom immunotherapy should be carried out for 3 to 5 years, or life-long in high-risk populations.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
LG declares financial and non-financial support from ALK, during the conduct of this work. CM declares non-financial support from ALK. The authors report no other conflicts of interest in this work.
References
1. Worm M, Moneret‐Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy. 2014;69(10):1397–1404. doi:10.1111/all.12475
2. Grabenhenrich LB, Dölle S, Moneret-Vautrin A, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137(4):1128–1137. e1121. doi:10.1016/j.jaci.2015.11.015
3. Antonicelli L, Bilò MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2(4):341–346. doi:10.1097/00130832-200208000-00008
4. Clifford D, Ni Chaoimh C, Stanley E, O’B Hourihane J. A longitudinal study of hymenoptera stings in preschool children. Pediatr Allergy Immunol. 2019;30(1):93–98. doi:10.1111/pai.2019.30.issue-1
5. Jakob T, Rafei-Shamsabadi D, Spillner E, Müller S. Diagnostics in Hymenoptera venom allergy: current concepts and developments with special focus on molecular allergy diagnostics. Allergo J Int. 2017;26(3):93–105. doi:10.1007/s40629-017-0014-2
6. Bilo M, Bonifazi F. The natural history and epidemiology of insect venom allergy: clinical implications. Clin Exp Allergy. 2009;39(10):1467–1476. doi:10.1111/cea.2009.39.issue-10
7. Novembre E, Cianferoni A, Bernardini R, et al. Epidemiology of insect venom sensitivity in children and its correlation to clinical and atopic features. Clin Exp Allergy. 1998;28(7):834–838. doi:10.1046/j.1365-2222.1998.00313.x
8. García-Robaina J, Vázquez-Moncholí C, Fierro J, Bonnet-Moreno C. Epidemiology of allergic reactions in beekeepers: a lower prevalence in subjects with more than 5 years exposure. Allergol Immunopathol. 1995;23(3):127–132.
9. Annila IT, Karjalainen ES, Annila PA, Kuusisto PA. Bee and wasp sting reactions in current beekeepers. Ann Allergy Asthma Immunol. 1996;77(5):423–427. doi:10.1016/S1081-1206(10)63342-X
10. Severino M, Bonadonna P, Passalacqua G. Large local reactions from stinging insects: from epidemiology to management. Curr Opin Allergy Clin Immunol. 2009;9(4):334–337. doi:10.1097/ACI.0b013e32832d0668
11. Graft DF, Schuberth KC, Kagey-Sobotka A, et al. A prospective study of the natural history of large local reactions after Hymenoptera stings in children. J Pediatr. 1984;104(5):664–668. doi:10.1016/S0022-3476(84)80940-3
12. Müller UR. Insect sting allergy: clinical picture, diagnosis and treatment. Insect Sting Allergy. 1990.
13. Bilo BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8(4):330–337. doi:10.1097/ACI.0b013e32830638c5
14. Bilo B, Rueff F, Mosbech H, Bonifazi F, Oude‐Elberink J, Hypersensitivity EIGoIV. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60(11):1339–1349. doi:10.1111/j.1398-9995.2005.00963.x
15. Jennings A, Duggan E, Perry IJ, Hourihane JOB. Epidemiology of allergic reactions to hymenoptera stings in Irish school children. Pediatr Allergy Immunol. 2010;21(8):1166–1170. doi:10.1111/pai.2010.21.issue-8
16. Vetter RS. Wasp or hippopotamus? J Allergy Clin Immunol. 2000;106(1):196. doi:10.1067/mai.2000.106547
17. Ring J. History of allergy in antiquity. Chem Immunol Allergy. 2014;100:2–14.
18. Oppenheimer J, Golden DB. Hymenoptera venom immunotherapy: past, present, and future. Ann Allergy Asthma Immunol. 2018;121(3):276–277. doi:10.1016/j.anai.2018.06.006
19. Lichtenstein LM, Valentine MD, Sobotka AK. A case for venom treatment in anaphylactic sensitivity to Hymenoptera sting. N Engl J Med. 1974;290(22):1223–1227. doi:10.1056/NEJM197405302902204
20. Galli SJ. Mary Hewitt Loveless, MD: the origin of venom immunotherapy. Ann Allergy Asthma Immunol. 2018;121(3):268–271. doi:10.1016/j.anai.2018.06.020
21. Loveless MH, Nall TM. Use of polistes venom in petrolatum: arlacel repositories to immunize against yellow jacket wasp-sting allergy. J Immunol. 1965;94(5):785–793.
22. Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med. 2007;39(6):440–456. doi:10.1080/07853890701449354
23. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi:10.1038/nm.2755
24. Bradding P, Walls AF, Holgate ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. 2006;117(6):1277–1284. doi:10.1016/j.jaci.2006.02.039
25. Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S161–181. doi:10.1016/j.jaci.2009.12.981
26. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9(11):1215–1223. doi:10.1038/ni.f.216
27. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119(4):780–791. doi:10.1016/j.jaci.2007.01.022
28. Eberlein-Konig B, Ullmann S, Thomas P, Przybilla B. Tryptase and histamine release due to a sting challenge in bee venom allergic patients treated successfully or unsuccessfully with hyposensitization. Clin Exp Allergy. 1995;25(8):704–712. doi:10.1111/cea.1995.25.issue-8
29. Jutel M, Muller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996;26(10):1112–1118. doi:10.1111/j.1365-2222.1996.tb00496.x
30. Celesnik Smodis N, Silar M, Erzen R, Rijavec M, Kosnik M, Korosec P. Down-regulation of FcepsilonRI-mediated CD63 basophil response during short-term VIT determined venom-nonspecific desensitization. PLoS One. 2014;9(4):e94762. doi:10.1371/journal.pone.0094762
31. Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205(12):2887–2898. doi:10.1084/jem.20080193
32. Celesnik N, Vesel T, Rijavec M, et al. Short-term venom immunotherapy induces desensitization of FcepsilonRI-mediated basophil response. Allergy. 2012;67(12):1594–1600. doi:10.1111/all.12044
33. Braga M, Quecchia C, Cavallucci E, et al. T regulatory cells in allergy. Int J Immunopathol Pharmacol. 2011;24(1 Suppl):55S–64S.
34. Calzada D, Baos S, Cremades-Jimeno L, Cardaba B. Immunological mechanisms in allergic diseases and allergen tolerance: the role of treg cells. J Immunol Res. 2018;2018:6012053. doi:10.1155/2018/6012053
35. Malisan F, Briere F, Bridon JM, et al. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183(3):937–947. doi:10.1084/jem.183.3.937
36. Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175(3):671–682. doi:10.1084/jem.175.3.671
37. Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310–1317 e1316. doi:10.1016/j.jaci.2014.05.042
38. Kennedy Norton S, Barnstein B, Brenzovich J, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180(5):2848–2854. doi:10.4049/jimmunol.180.5.2848
39. Sturm GJ, Varga EM, Roberts G, et al. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018;73(4):744–764. doi:10.1111/all.13262
40. Dhami S, Zaman H, Varga EM, et al. Allergen immunotherapy for insect venom allergy: a systematic review and meta‐analysis. Allergy. 2017;72(3):342–365. doi:10.1111/all.13077
41. Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012; 10. doi:10.1002/14651858.CD008838.pub2
42. Golden DB, Kagey-Sobotka A, Norman PS, Hamilton RG, Lichtenstein LM. Outcomes of allergy to insect stings in children, with and without venom immunotherapy. N Engl J Med. 2004;351(7):668–674. doi:10.1056/NEJMoa022952
43. Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. N Engl J Med. 1978;299(4):157–161. doi:10.1056/NEJM197807272990401
44. Oude JE, De JM, Van SDH, Guyatt GH, Dubois A. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol. 2002;110(1):174–182. doi:10.1067/mai.2002.125827
45. Oude Elberink J, Van Der Heide S, Guyatt G, Dubois A. Immunotherapy improves health‐related quality of life of adult patients with dermal reactions following yellow jacket stings. Clin Exp Allergy. 2009;39(6):883–889. doi:10.1111/cea.2009.39.issue-6
46. Pucci S, D’Alò S, De Pasquale T, Illuminati I, Makri E, Incorvaia C. Risk of anaphylaxis in patients with large local reactions to hymenoptera stings: a retrospective and prospective study. Clin Mol Allergy. 2015;13(1):21. doi:10.1186/s12948-015-0030-z
47. Sturm GJ, Kranzelbinder B, Schuster C, et al. Sensitization to Hymenoptera venoms is common, but systemic sting reactions are rare. J Allergy Clin Immunol. 2014;133(6):1635–1643. e1631. doi:10.1016/j.jaci.2013.10.046
48. Bilò MB, Martini M, Pravettoni V, et al. Large local reactions to Hymenoptera stings: outcome of re‐stings in real life. Allergy. 2019. doi:10.1111/all.13863
49. Bilò MB, Ollert M, Blank S. The role of component-resolved diagnosis in Hymenoptera venom allergy. Curr Opin Allergy Clin Immunol. 2019;19(6):614–622. doi:10.1097/ACI.0000000000000574
50. Spillner E, Blank S, Jakob T. Hymenoptera allergens: from venom to “venome”. Front Immunol. 2014;5:77. doi:10.3389/fimmu.2014.00077
51. Cifuentes L, Vosseler S, Blank S, et al. Identification of Hymenoptera venom–allergic patients with negative specific IgE to venom extract by using recombinant allergens. J Allergy Clin Immunol. 2014;133(3):909–910. doi:10.1016/j.jaci.2013.09.047
52. Birnbaum J, Charpin D, Vervloet D. Rapid Hymenoptera venom immunotherapy: comparative safety of three protocols. Clin Exp Allergy. 1993;23(3):226–230. doi:10.1111/cea.1993.23.issue-3
53. Laurent J, Smiejan J, Bloch‐Morot E, Herman D. Safety of Hymenoptera venom rush immunotherapy. Allergy. 1997;52(1):94–96. doi:10.1111/j.1398-9995.1997.tb02551.x
54. Sturm G, Kränke B, Rudolph C, Aberer W. Rush Hymenoptera venom immunotherapy: a safe and practical protocol for high-risk patients. J Allergy Clin Immunol. 2002;110(6):928–933. doi:10.1067/mai.2002.129124
55. Van der Zwan J, Flinterman J, Jankowski I, Kerckhaert J. Hyposensitisation to wasp venom in six hours. Br Med J (Clin Res Ed). 1983;287(6402):1329–1331. doi:10.1136/bmj.287.6402.1329
56. Roll A, Hofbauer G, Ballmer-Weber B, Schmid-Grendelmeier P. Safety of specific immunotherapy using a four-hour ultra-rush induction scheme in bee and wasp allergy. J Invest Allerg Clin Immunol. 2006;16(2):79.
57. Malling HJ, Djurup R, Søndergaard I, Weeke B. Clustered immunotherapy with yellow jacket venom: evaluation of the influence of time interval on in vivo and in vitro parameters. Allergy. 1985;40(5):373–383. doi:10.1111/j.1398-9995.1985.tb00250.x
58. Schuch A, Brockow K. Mastocytosis and anaphylaxis. Immunol Allergy Clin. 2017;37(1):153–164. doi:10.1016/j.iac.2016.08.017
59. González-de-Olano D, Matito A, Orfao A, Escribano L. Advances in the understanding and clinical management of mastocytosis and clonal mast cell activation syndromes. F1000Research. 2016;5. doi:10.12688/f1000research.9565.1
60. Gruzelle V, Ramassamy M, Bulai Lidiveanu C, Didier A, Mailhol C, Guilleminault L. Safety of ultra‐rush protocols for hymenoptera venom immunotherapy in systemic mastocytosis. Allergy. 2018;73(11):2260–2263. doi:10.1111/all.2018.73.issue-11
61. Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992;89(2):529–535. doi:10.1016/0091-6749(92)90319-W
62. Rueff F, Vos B, Oude Elberink J, et al. Predictors of clinical effectiveness of Hymenoptera venom immunotherapy. Clin Exp Allergy. 2014;44(5):736–746. doi:10.1111/cea.2014.44.issue-5
63. Hoffman DR, Jacobson RS. Allergens in hymenoptera venom XII: how much protein is in a sting. Ann Allergy. 1984;52(4):276–278.
64. Blank S, Seismann H, Michel Y, et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66(10):1322–1329. doi:10.1111/all.2011.66.issue-10
65. Fischer J, Teufel M, Feidt A, Giel KE, Zipfel S, Biedermann T. Tolerated wasp sting challenge improves health-related quality of life in patients allergic to wasp venom. J Allergy Clin Immunol. 2013;132(2):489–490. doi:10.1016/j.jaci.2013.03.010
66. TSICOPOULOS A, Tonnel A, Wallaert B, Ramon P, Joseph M, Capron A. Short‐term decrease of skin‐test sensitivity after rush desensitization in Hymenoptera venom hypersensitivity. Clin Exp Allergy. 1990;20(3):289–294. doi:10.1111/j.1365-2222.1990.tb02686.x
67. Lerch E, Müller UR. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol. 1998;101(5):606–612. doi:10.1016/S0091-6749(98)70167-8
68. Keating M, Kagey-Sobotka A, Hamilton RG, Yunginger J. Clinical and immunologic follow-up of patients who stop venom immunotherapy. J Allergy Clin Immunol. 1991;88(3):339–348. doi:10.1016/0091-6749(91)90095-6
69. Goldberg A, Confino‐Cohen R. Bee venom immunotherapy – how early is it effective? Allergy. 2010;65(3):391–395. doi:10.1111/j.1398-9995.2009.02198.x
70. Goldberg A, Yogev A, Confino-Cohen R. Three days rush venom immunotherapy in bee allergy: safe, inexpensive and instantaneously effective. Int Arch Allergy Immunol. 2011;156(1):90–98. doi:10.1159/000322258
71. Bernkopf K, Rönsch H, Spornraft-Ragaller P, Neumeister V, Bauer A. Safety and tolerability during build-up phase of a rush venom immunotherapy. Ann Allergy Asthma Immunol. 2016;116(4):360–365. doi:10.1016/j.anai.2016.02.014
72. Kalogeromitros D, Makris M, Koti I, et al. A simple 3-day “rush” venom immunotherapy protocol: documentation of safety. Allergol Immunopathol (Madr). 2010;38(2):69–73. doi:10.1016/j.aller.2009.08.002
73. Schiavino D, Nucera E, Pollastrini E, et al. Specific ultrarush desensitization in Hymenoptera venom-allergic patients. Ann Allergy Asthma Immunol. 2004;92(4):409–413. doi:10.1016/S1081-1206(10)61775-9
74. Cosme J, Spínola-Santos A, Pereira-Santos M, Pereira-Barbosa M. Venom Immunotherapy: a 20-year experience with an ultra-rush protocol (210-min). Eur Ann Allergy Clin Immunol. 2019;51(3):122–128. doi:10.23822/EurAnnACI.1764-1489.85
75. Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra‐rush venom immunotherapy (210 min): a safety study and risk factors. Clin Exp Allergy. 2003;33(1):58–64. doi:10.1046/j.1365-2222.2003.01564.x
76. Mosbech H, Müller U, Behalf Of The Study Group* O. Side‐effects of insect venom immunotherapy: results from an EAACI multicenter study. Allergy. 2000;55(11):1005–1010. doi:10.1034/j.1398-9995.2000.00587.x
77. Bernstein DI, Epstein T. Systemic reactions to subcutaneous allergen immunotherapy. Immunol Allergy Clin. 2011;31(2):241–249. doi:10.1016/j.iac.2011.02.007
78. Bernstein DI, Wanner M, Borish L, Liss GM. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113(6):1129–1136. doi:10.1016/j.jaci.2004.02.006
79. Reid MJ, Lockey RF, Turkeltaub PC, Platts-Mills TA. Survey of fatalities from skin testing and immunotherapy 1985–1989. J Allergy Clin Immunol. 1993;92(1):6–15. doi:10.1016/0091-6749(93)90030-J
80. Ruëff F, Przybilla B, Biló MB, et al. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126(1):105–111. e105. doi:10.1016/j.jaci.2010.04.025
81. Patella V, Florio G, Giuliano A, et al. Hymenoptera venom immunotherapy: tolerance and efficacy of an ultrarush protocol versus a rush and a slow conventional protocol. J Allergy (Cairo). 2012;2012. doi:10.1155/2012/192192
82. Müller UR, Haeberli G. Use of β-blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115(3):606–610. doi:10.1016/j.jaci.2004.11.012
83. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. doi:10.1016/j.jaci.2004.04.029
84. Pitsios C, Demoly P, Bilò M, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy. 2015;70(8):897–909. doi:10.1111/all.2015.70.issue-8
85. Stoevesandt J, Hosp C, Kerstan A, Trautmann A. Hymenoptera venom immunotherapy while maintaining cardiovascular medication: safe and effective. Ann Allergy Asthma Immunol. 2015;114(5):411–416. doi:10.1016/j.anai.2015.03.001
86. Brockow K, Kiehn M, Riethmüllerb C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1997;100(4):458–463. doi:10.1016/S0091-6749(97)70135-0
87. Kontou‐Fili K. ALLERGY Net: high omalizumab dose controls recurrent reactions to venom immunotherapy in indolent systemic mastocytosis. Allergy. 2008;63(3):376–378. doi:10.1111/j.1398-9995.2007.01604.x
88. Schulze J, Rose M, Zielen S. Beekeepers anaphylaxis: successful immunotherapy covered by omalizumab. Allergy. 2007;62(8):963–964. doi:10.1111/all.2007.62.issue-8
89. Stretz E, Oppel E, Räwer HC, et al. Overcoming severe adverse reactions to venom immunotherapy using anti‐Ig E antibodies in combination with a high maintenance dose. Clin Exp Allergy. 2017;47(12):1631–1639. doi:10.1111/cea.12997
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.