Back to Journals » Blood and Lymphatic Cancer: Targets and Therapy » Volume 11
Clinical Utility of Pegaspargase in Children, Adolescents and Young Adult Patients with Acute Lymphoblastic Leukemia: A Review
Authors Bender C, Maese L, Carter-Febres M, Verma A
Received 12 December 2020
Accepted for publication 12 March 2021
Published 19 April 2021 Volume 2021:11 Pages 25—40
DOI https://doi.org/10.2147/BLCTT.S245210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Wilson Gonsalves
Cynthia Bender,1 Luke Maese,2 Maria Carter-Febres,2 Anupam Verma2
1Department of Pharmacy, Primary Children’s Hospital, Salt Lake City, UT, USA; 2Division of Hematology/Oncology, Department of Pediatrics, University of Utah and Primary Children’s Hospital, Salt Lake City, UT, USA
Correspondence: Anupam Verma
Pediatric Hematology/Oncology, University of Utah, Primary Children’s Hospital, 100 N Mario Capecchi Drive, Salt Lake City, UT, 84113, USA
Tel +1 801 662 4700
Fax +1 801 662 4707
Email [email protected]
Abstract: Acute lymphoblastic leukemia (ALL) is a heterogenous hematological malignancy representing 25% of all cancers in children less than 15 years of age. Significant improvements in survival and cure rates have been made over the past four decades in pediatric ALL treatment. Asparaginases, derived from Escherichia coli and Erwinia chrysanthemi, have become a critical component of ALL therapy since the 1960s. Asparaginases cause depletion of serum asparagine, leading to deprivation of this critical amino acid for protein synthesis, and hence limit survival of lymphoblasts. Pegaspargase, a conjugate of monomethoxypolyethylene glycol (mPEG) and L-asparaginase, has become an integral component of pediatric upfront and relapsed ALL protocols due to its longer half-life and improved immunogenicity profile compared to native asparaginase preparations. Over the past two decades great strides have been made in outcomes for pediatric ALL due to risk stratification, incorporation of multiagent chemotherapy protocols, and central nervous system prophylaxis with pegaspargase having played an important role in this success. However, adolescents and young adults (AYA) with ALL when treated on contemporaneous trials using adult ALL regimens, continue to have poor outcomes. There is increasing realization of adapting pediatric trial regimens for treating AYAs, especially those incorporating higher intensity of chemotherapeutic agents with pegaspargase being one such agent. Dose or treatment-limiting toxicity is observed in 25– 30% of patients, most notable being hypersensitivity reactions. Other toxicities include asparaginase-associated pancreatitis, thrombosis, liver dysfunction, osteonecrosis, and dyslipidemia. Discontinuation or subtherapeutic levels of asparaginase are associated with inferior disease-free survival leading to higher risk of relapse, and in cases of relapse, a higher risk for remission failure. This article provides an overview of available evidence for use of pegaspargase in pediatric acute lymphoblastic leukemia.
Keywords: asparagine, asparaginase, serum asparaginase activity, toxicity
Introduction
Acute lymphoblastic leukemia (ALL) is the most commonly encountered cancer of childhood with peak prevalence between ages 2–5 years. Two main subtypes, B-cell and T-cell, comprise the majority of ALL, at 85–90% and 10–15% of cases, respectively.1 Outcomes within pediatric ALL vary by subtype and risk category, but are generally favorable. While within standard-risk (SR) B-cell ALL overall survival (OS) is as high as 96%, high risk (HR) B-ALL and T-ALL continue to have lower event-free survival (EFS) and OS.2–6 Over the past several decades, significant progress has been made with risk stratification and chemotherapy dose intensification. Asparaginase, introduced as a component of the chemotherapeutic backbone for ALL in the 1970s, has been of significant importance in advancing the treatment of childhood leukemia.3,7,8 Its antileukemic activity is accomplished by converting asparagine to aspartic acid and ammonia in the extracellular fluid. Lymphoblasts are very sensitive to the lack of asparagine and this shortage reduces the synthesis of asparagine-dependent proteins causing cell death.9 There are extensive clinical data supporting the use and improved survival benefits of asparaginase therapy in pediatric ALL.2,3,10–17 Omission or low therapeutic levels of asparaginase may lead to higher risk of relapse, and in cases of relapse, a higher risk for remission failure.3,18–20 Pegaspargase (Oncaspar®), a pegylated form of native Escherichia coli (E. coli) derived L-asparaginase, is the preferred first-line asparaginase preparation in the multiagent chemotherapy regimens for treatment of childhood ALL in the United States (US) and European Union (EU).21–23 We provide an overview on the use of pegaspargase in pediatric ALL.
Pharmacological Characteristics of Pegaspargase
In comparison to healthy cells, leukemic cells have low levels of asparagine synthetase and hence, reduced ability to manufacture the essential amino acid asparagine needed for DNA, RNA and protein synthesis.24,25 Asparaginase hydrolyzes asparagine into aspartic acid and ammonia in the plasma, thus denying the lymphoblast an essential element needed for cell survival.26 Asparaginase was identified as a potential chemotherapeutic in 1961 when it was first isolated as an anti-lymphoma component of guinea pig serum.27 Studies in the 1960s used bacteria as alternate sources of asparaginase, leading to clinically available asparaginase derived from E. coli and Erwinia chrysanthemi.28,29
Pegaspargase is a conjugate of monomethoxypolyethylene glycol (mPEG) and L-asparaginase produced endogenously by E. coli. These mPEG molecules increase solubility and stability of the protein conjugate in the plasma and prevent proteolytic cleavage of the enzyme, consequently increasing its half-life.22,30 They also provide an additional benefit of reduction in the risk of anaphylaxis and hypersensitivity reactions.
Dosage and Administration
Pegaspargase is supplied as a sterile, preservative-free solution in vials containing 3750 International Units (IU) of pegaspargase per 5 mL solution.21,22 The FDA-approved dose for pediatric leukemia is 2500 IU/m2 given either intramuscularly (IM) or intravenously (IV) over 1–2 hours, not more often than every 14 days.21,22 Increased toxicity due to higher doses of pegaspargase is controversial. Lebovic et al reported a retrospective chart review of patients 1–21 years of age with B-ALL on pegaspargase and found that doses higher than 3750 IU were associated with higher rates of venous thromboembolism (VTE), pancreatitis, and hyperglycemia.31 Dose capping is frequently employed in the adult population, particularly in obese patients; however, it has not been standard practice in pediatrics and further studies should investigate the potential for toxicity in this subset of patients.
Pharmacokinetic (PK) Comparison Between IV and IM Administration
The goal of asparaginase therapy is to achieve complete asparagine depletion. Quantification of asparagine directly is biologically and logistically challenging, particularly in the clinical setting, so serum asparaginase activity (SAA) levels are accepted as the most reliable modality to measure asparaginase efficacy.32 While there has been recent debate regarding optimal SAA levels for adequate asparagine depletion, an SAA of >0.1 IU/mL is generally considered optimal.24,32–35 Both Children’s Cancer Group (CCG) 1962 and Children’s Oncology Group (COG) AALL07P4 studies have provided important PK data on IM and IV administration, respectively.24,34 After an IM dose of 2500 IU/m2, the maximum concentration of asparaginase activity peaked at 1 IU/mL compared to 1.6 IU/mL for IV on day 5 with a half-life absorption estimated at 1.7 days.21,22,24 The replacement of native asparaginase with pegaspargase in the treatment of pediatric ALL has come about due to its prolonged terminal half-life. The average half-life is estimated at 5.5 days for both IM and IV administration and is 4–8 times longer than those of the native and Erwinia-derived products.22,36 Silverman et al also confirmed that enzyme activity is maintained for a minimum of 2 weeks after a single IV dose, thus supporting the recommendation that dosing not occur more frequently than 2-week intervals.22,37
Comparison with Other Pegylated Products
Calaspargase (CALASP) was developed using the same asparaginase enzyme and PEG molecule as pegaspargase. However, whereas pegaspargase has a succinimidyl succinate (SS-PEG) molecule which combines the two, CALASP consists instead of a succinimidyl carbonate (SC-PEG) linkage which enables greater biologic stability. This stability is realized in the much longer shelf life of CALASP, 36 months, versus pegaspargase at 8 months. COG AALL07P4 investigated the PK and pharmacodynamic (PD) properties of calaspargase pegol (Asparlas®).38 The results demonstrated a potential advantage to its use with CALASP having a more prolonged SAA compared with pegaspargase through both induction and consolidation.38 The mean half-life SAA was 2.5 times longer with CALASP than with pegaspargase, representing a more prolonged suppression of asparagine in the plasma.38 Although not statistically significant, adverse effects were similar between both groups other than CALASP had increased hyperglycemia in induction and hyperbilirubinemia in delayed intensification (DI).38 Dana-Farber Cancer Institute (DFCI) 11–001 randomized patients to either pegaspargase every 2 weeks or CALASP every 3 weeks during intensification (Table 1). There were no significant differences in treatment outcomes or toxicities between the two arms, but PK data showed more prolonged SAA after CALASP in the induction phase.39 As the DFCI protocols have 3-week cycles during intensification, CALASP is an ideal fit for the asparagine depletion prescribed in their chemotherapeutic backbone. This new pegylated product, as well as the yet unproven pegylated Erwinia asparaginase (pegcrisantaspase), may offer future alternate treatment options to pegaspargase.38,40,41
 |
Table 1 Pediatric Acute Lymphoblastic Leukemia Trials with Pegaspargase Associated Objectives |
Pegaspargase in Newly Diagnosed ALL
In the US there are different types of commercially available asparaginase formulations, an E. coli-derived pegylated asparaginase (pegaspargase) and an Erwinia derived asparaginase. The native E. coli derived L-asparaginase was removed from the US market by the manufacturer in 2012 but still plays an important historical role. The pegylated form of the drug, approved in 1994 for use in patients with ALL, increases the plasma retention time, decreases proteolysis and renal excretion, and protects from immune detection.
Standard Risk (SR) ALL
CCG 1962 was a randomized trial to evaluate safety, efficacy, and PK of a single IM dose of pegaspargase versus multiple IM doses of native E. coli asparaginase in each of 3 phases: a 4-week induction and 2, 8-week DI phases as part of a multiagent chemotherapeutic regimen.24 One hundred eighteen children in 8 CCG centers with SR ALL were enrolled between 1997–1998. Patients were randomly assigned to receive either 2500 IU/m2 of pegaspargase IM on day 3 of induction and each DI phase, or 6000 IU/m2 of native asparaginase IM 3 times per week for 9 doses in induction and 6 doses in each DI phase (Table 1). Patients assigned to the pegaspargase arm had significantly more rapid clearance of lymphoblasts from days 7 and 14 bone marrow aspirates (p ≤ 0.05). Twice (n = 16) as many patients in the native asparaginase arm had M3 bone marrow (>25% blasts) on day 7 than in the pegaspargase arm (n = 8). Four patients had M3 marrow on day 14, all were in the native asparaginase arm. The study postulated that the difference could be from more persistent, higher SAA in the pegaspargase patients.24 Half-lives of asparaginase were 5.5 days and 26 hours for pegaspargase and native asparaginase, respectively. Studies have shown when antibody titers are high, the asparaginase activity is often low.42 Low SAA in the native arm (26%) compared to the pegaspargase arm (2%) during the first DI phase established the primary endpoint of this study that incidence of high anti-asparaginase antibody titers in children treated with pegaspargase would be decreased by at least 50% in the first DI compared with those treated with native asparaginase. Three-year EFS was similar for both arms, 85% for pegaspargase and 78% for native asparaginase.24
High Risk (HR) ALL
DCFI 91–01 protocol was designed to improve the outcome of children with newly diagnosed ALL while minimizing toxicity.3 Three hundred and seventy-seven patients (137 SR and 240 HR), aged 0–18 years, were enrolled between 1991–1995.3 Apart from substituting dexamethasone for prednisone, the duration of high dose asparaginase intensification was extended from 20 weeks (in prior protocols) to 30 weeks, and patients were randomized to receive either native or pegylated E. coli asparaginase (Table 1). Patients were administered either 2500 IU/m2 pegaspargase IM every other week for 15 doses or 25,000 IU/m2 native E. coli asparaginase IM every week for 30 doses during the intensification phase of therapy. Though the study did not find a significant difference in 5-year EFS based upon risk group (87% ± 3% for SR and 81% ± 3% for HR, p = 0.24), asparaginase intolerance (failure to receive at least 26 weeks of asparaginase) was an independent predictor of adverse outcome in multivariate analysis (p < 0.01). This finding suggested that the prolongation of asparaginase therapy may have contributed to the improved overall outcome of 5-year EFS (83% ± 2%) compared to previous protocols.3
DFCI 05–001, conducted between 2005–2010, enrolled 551 newly diagnosed ALL patients aged 1–18 years, with the aim to compare the relative toxicity and efficacy of IV pegaspargase and IM native E. coli L-asparaginase in both SR and HR ALL patients.33 Those patients who achieved complete remission (CR) after induction therapy were assigned to a final risk group and randomized to IV pegaspargase (15 doses of 2500 IU/m2 every 2 weeks) or IM native L-asparaginase (30 doses of 25,000 IU/m2 weekly), beginning at week 7 after study entry (Table 1). The primary endpoint of the study was to evaluate asparaginase-related toxicities, while EFS, SAA, and quality of life during therapy as assessed by PedsQL surveys were secondary endpoints. The two treatme7nt groups did not have statistically significant difference in overall frequency of asparaginase-related toxicities; 28% in the IV pegaspargase group versus 26% in IM native L-asparaginase group (p = 0.60). A significantly higher proportion of pegaspargase than native L-asparaginase recipients completed all 30 weeks of treatment (82 vs. 74%; p = 0.015). The median nadir SAA of ≥0.1 IU/mL was significantly higher in patients who received IV pegaspargase than in those who received IM native L-asparaginase (99 vs. 71%; p < 0.0001).33 Significantly more anxiety was reported by both patients and parent-proxy in the IM native L-asparaginase group than in the IV pegaspargase group (p ≤ 0.03). With a median follow-up of 6 years, 5-year EFS was 90% for IV pegaspargase versus 89% for IM native L-asparaginase (p = 0.58).33
Pediatric Oncology Group (POG) study POG 8704 (T-3) established in 357 patients with T ALL and 195 patients with advanced-stage T lymphoblastic lymphoma (LLy) that high dose asparaginase consolidation therapy improves survival in pediatric patients.13
Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) ALL 2000 protocol, utilizing an intensive multiagent Berlin-Frankfurt-Munster (BFM) chemotherapy backbone, studied the pharmacological effects of pegaspargase as the first-line product in children with ALL.43 Twenty patients with SR, intermediate-risk (IR), and HR ALL on the protocol were given 2 doses and 1 dose of pegaspargase during induction and reinduction phases, respectively (Table 1). The investigators studied the asparagine depletion and clinical outcomes against a comparison cohort of 37 patients treated with the same chemotherapy schedule but with native asparaginase. This study demonstrated pegaspargase effectively substituted for the native asparaginase product in regard to serum activity levels and asparagine depletion. Additionally, allergic reactions and immunologically mediated silent inactivation were drastically minimized despite multiple exposures to the drug.43
Adolescents and Young Adults (AYA) ALL
Survival rates in AYA ALL are poor compared with those in younger children. Surveillance epidemiology and end results (SEER) data from 2000–2004 in ALL, reported a 10-year OS of 80% in children <15 years, falling to 60% in adolescents aged 15–20 years, and 30% in young adults aged 20–30 years.44,45 Multiple studies have looked at adopting pediatric protocols for AYA with improved overall outcomes. Asparaginase, as it does in younger children, plays an important role in AYA ALL therapy. Ram et al conducted a systematic review and meta-analysis of all comparative trials on AYA patients given chemotherapy with either pediatric-inspired regimens or conventional adult protocols, reviewing 11 trials and 2489 patients.46 AYA patients given pediatric inspired regimens had a statistically significant lower mortality rate at 3 years (RR 0.58; 95% CI 0.51–0.67), lower relapse rate (RR 0.51; 95% CI 0.39–0.66), superior CR (RR 1.05; 95% CI 1.01–1.10) and EFS (RR 1.66; 95% CI 1.39–1.99).46 The German Multicenter Study Group For Adult ALL (GMALL) protocols were originally based on pediatric BFM protocols and designed for AYA ALL.47 GMALL 07/03 enrolled 877 patients, utilized intensified shortened induction with dexamethasone rather than with prednisone, pegaspargase rather than native asparaginase in induction and consolidation, plus incorporated changes in the management of minimal residual disease with stem cell transplant. GMALL 07/03 had significant improvements in 5-year OS compared with previous protocol GMALL 05/93 (65% in GMALL 07/03 vs 46% in GMALL 05/93).47 A French study examined the outcomes of 177 AYAs aged 15–20 years comparing those who were treated on a pediatric inspired protocol FRALLE-93, or an adult protocol LALA-94A.48 FRALLE-93 showed significant better CR (98% v 81%; p =0.002) and EFS (p =0.0002) in B-ALL, and EFS (p =0.05) in T-ALL. The cumulative doses of chemotherapies, most notably asparaginase, were higher in the pediatric protocol: 180,000 IU/m2 in the pediatric regimen versus 9000 IU/m2 in the adult regimen.48 Italian and Dutch retrospective studies compared outcomes in adolescents aged 14–18 years treated on pediatric AIEOP ALL 95 and 2000 protocols with those treated on the adult Gruppo Italiano per le Malattie Ematologiche dell’Adulto (GIMEMA) ALL 0496 and 2000 protocols, and Dutch Children’s Oncology Group (DCOG) pediatric regimen with the Hemato-Oncologie voor Volwassenen Nederland (HOVON) adult protocols in AYAs. Both comparisons showed improved outcomes in patients treated on pediatric protocols. The primary differences between the regimens were shorter intervals between courses (≤1 week versus ≤4 weeks) and more asparaginase (mean cumulative dosage: 101,000 IU/m2 vs. 70,000 IU/m2) in the pediatric regimens.49,50
Pediatric inspired regimens use higher doses of chemotherapeutic agents at shorter intervals, including pegaspargase, which have demonstrated superiority to conventional adult regimens in AYA ALL patients. Dosing scheme is not the only reason for improved outcomes as there are biological differences in AYAs that also play a role. Not only do AYAs have more toxicity to the intensive chemotherapies, they also have reduced prevalence of genetic subtypes associated with favorable outcome and simultaneously an increase in subtypes associated with poor outcome.51 There is near-universal consensus from prior studies supporting the use of pegaspargase as the front-line asparaginase product in children and AYAs with newly diagnosed ALL (Tables 2 and 3).
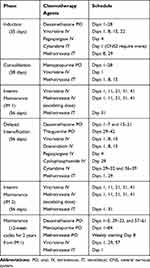 |
Table 2 Standard Risk B-ALL Regimen (Adapted from Children’s Oncology Group AALL0932) |
 |
Table 3 High-Risk B and T-ALL Regimens (Adapted from Children’s Oncology Group AALL1131, AALL0431) |
Relapsed ALL
Apart from becoming an integral component of therapy regimens for first-line treatment of pediatric ALL, pegaspargase has also established a critical role in treatment regimens for relapsed ALL. CHMC 1001 enrolled 28 pediatric patients with relapsed ALL at 3 pediatric institutions to study PK after pegaspargase therapy.52 Patients received induction therapy (including pegaspargase 2500 IU/m2 IM weekly on days 2, 9, 16, and 23) and intensification therapy (including pegaspargase 2500 IU/m2 IM once on day 7) (Table 1). Adequate serum and CSF asparagine depletion with SAA of >0.1 IU/mL was observed and maintained during induction and intensification in the majority of samples. CCG 1941 utilized 2 doses of pegaspargase on days 2 and 16, in combination with multiagent chemotherapy during re-induction, to explore possible relationships among SAA, asparagine depletion, anti-asparaginase antibody titers, and response to re-induction therapy in children and adolescents with early first bone marrow ALL relapse (Table 1).18 The study enrolled 214 patients between 1995–1998. The difference in asparagine levels between M1 and M3 response at end of re-induction was statistically significant (p = 0.01). Lesser asparagine depletion was associated with failure to achieve second remissions.18 ALLR3 enrolled 216 patients aged 1–18 years in centers throughout Europe, Australia and New Zealand, on an open-label randomized trial for first ALL relapse, with a conventional four-drug induction and continuous asparagine depletion throughout the first 3 months. ALLR3 has since become the standard chemotherapy regimen for many relapsed ALL re-induction protocols.53
Pegaspargase is an important chemotherapy for treating pediatric ALL relapse and is included in the majority of US and European re-induction protocols with the ALLR3 or vincristine, dexamethasone, pegaspargase and doxorubicin (VXLD) inspired backbones (Tables 4 and 5).
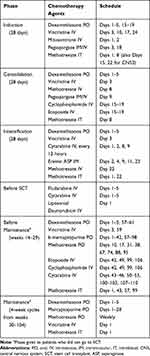 |
Table 4 Relapse ALL Regimen (Adapted from UKALLR3) |
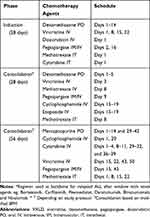 |
Table 5 Relapse ALL Regimen* (Adapted from VXLD Re-Induction) |
Toxicity of Pegaspargase
In addition to its multiple clinical benefits, pegaspargase is notable for significant toxicity in up to 20–25% of all patients.54,55 These toxicities include hypersensitivity, pancreatitis, hyperglycemia, hyperlipidemia, liver dysfunction, hyperbilirubinemia, thrombosis, bleeding, and osteonecrosis (Figure 1).
 |
Figure 1 Pegaspargase toxicity. *Due to lack of reported percentages for specific toxicities, hyperglycemia is not included. |
Hypersensitivity to Pegaspargase
Allergic and infusion-related reactions in patients receiving asparaginase therapy are among the most common toxicities, ranging between 3–71% (Figure 1).56 Asparaginase hypersensitivity is mediated by antigen-specific IgG and/or IgE through the immunoglobulin receptors FcγRIII and FcεRI, respectively.57 Hypersensitivity reactions to E. coli derived asparaginase, necessitate a switch to an alternative preparation such as Erwinia asparaginase.8,56,58 The incidence of hypersensitivity reactions depends on the number of prior exposures, interval between doses, type of asparaginase, use of premedication, and concomitant corticosteroid therapy. There are two types of true hypersensitivity reactions, antibody production with clinical manifestations and neutralizing antibody production reflected by a day 7 SAA below 0.1 IU/mL and/or a day 14 level below the lower limit of quantification with an absence of a clinical reaction, referred to as silent inactivation or subclinical hypersensitivity.32 The antibody formed to the naïve asparaginase or polyethylene glycol portion of the pegaspargase alters the PK of the drug.59,60 The presence of these antibodies can inhibit asparaginase from depleting asparagine and ultimately decrease efficacy. Several studies suggest that pegaspargase is less immunogenic than the native E. coli formulation.3,60–62
The above referenced DFCI 91–01 study reported allergic reaction in 15% of patients; however, pegaspargase was associated with a lower incidence of allergic reactions (p = 0.02).3
The Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL2008 protocol enrolled 106 pediatric patients from 2008–2012. Six patients experienced a clinical allergic reaction to pegaspargase, a cumulative risk of 13.2%. Reactions usually occurred 2 hours after IM administration with symptoms ranging from mild to severe anaphylaxis.63 Additionally, NOPHO ALL2008, through a genome-wide association study (GWAS), correlated several genetic variants to an increased incidence of pegaspargase hypersensitivity.64
The Dutch Childhood Oncology Group (DCOG) ALL-10 study enrolled children ages 1–18 years with newly diagnosed ALL from 2009–2012.65 Patients were stratified into 3 risk groups after induction therapy (standard, medium and high) and all received 8 doses of native E. coli asparaginase (5000 IU/m2 per dose) every 3 days in induction. Eighty-nine patients considered medium risk were given pegaspargase as first-line agent (2500 IU/m2 per dose every other week) for a total of 15 doses during first 30 weeks of intensification. In the setting of allergy or silent inactivation, pegaspargase was replaced with Erwinia asparaginase. The investigators found a high incidence of inactivation of pegaspargase (22% clinical allergy and 8% silent inactivation) in the intensification phase due to antibody development against native E. coli asparaginase used in induction. They concluded that if a second pegaspargase course was administered, the allergy rate of pegaspargase would increase.65 DCOG ALL-11 study reported 14 allergic-like reactions or infusion reactions, 5 were to pegaspargase and 9 to Erwinia asparaginase. Allergic-like reactions occurred relatively late after the start of infusion when compared to real allergies. They also were distinguished from real allergies by the absence of antibodies in all but one patient with an allergic-like reaction, while antibodies were detected in all patients with a real allergy. The study recommended if clinically tolerated, formulations should not be switched in case of allergic-like reactions.66
POG 8866 enrolled 76 patients <21 years with ALL in second bone marrow relapse from 1988–1992, with the aim of comparing efficacy and toxicity of pegaspargase to native E. coli asparaginase in the standard re-induction regimen.67 It was noted that all patients hypersensitive to native asparaginase tolerated at least 2 doses of pegaspargase given during induction phase. In those with known hypersensitivity to native asparaginase, increased clearance and decreased efficacy of pegaspargase were observed.67 Recently multiple studies have shown monitoring of ASNase activity ≥0.1 IU/mL, measured on days 7 and 14 post pegaspargase administration, during treatment of children with ALL is feasible and informative, demonstrating median trough PEG-ASNase activity being high in all patients without hypersensitivity.32,68,69
COG AALL1421 results suggest pre-existing anti-PEG antibodies with the increased use of pegylated products in food processing and cosmetics may also play a role in hypersensitivity reactions.40,41,70
Pegaspargase is a critical component of the chemotherapeutic backbone in upfront ALL protocols (Tables 2 and 3), and those patients who experience hypersensitivity to pegaspargase should discontinue any further dosing and switch to Erwinia asparaginase.71 However, during an Erwinia asparaginase shortage, Verma et al conducted a successful prospective study of pegaspargase desensitization in 10 patients with ALL/LLy who had experienced prior grade 3 or higher hypersensitivity reactions to either pegaspargase (6) or both pegaspargase and Erwinia asparaginase (4). A 13-step rapid desensitization protocol was utilized and SAA levels were measured between days 4–7 and days 10–14.72 Seven patients had sustained SAA activity, one achieved therapeutic levels but showed accelerated clearance, while two did not achieve adequate levels consistent with neutralizing antibodies. Although three of the 10 patients had mild to moderate hypersensitivity reactions, all were able to receive full protocol doses after clinical intervention. Others have since duplicated the safe administration of pegaspargase, while maintaining sustained SAA levels, utilizing a desensitization protocol in patients experiencing prior hypersensitivity reactions.72–74
An additional area of investigation for patients who experience hypersensitivity is the COG protocol AALL1931 (NCT04145531) an open-label, multicenter study for patients with ALL/LLy following hypersensitivity to E. coli derived asparaginase with the objective to determine the efficacy, safety, and tolerability of IM recombinant crisantaspase Pseudomonas fluorescens.
Pancreatitis
Asparaginase-associated pancreatitis (AAP) was first reported in patients receiving the native L-asparaginase formulation. The origin, formulation, dosage and method of administration do not seem to influence the risk of pancreatitis.75 The exact pathophysiology of AAP is unknown, but it is believed to be caused by a systemic depletion of asparagine and thus reduction of protein synthesis. Organs with increased protein turnover such as the pancreas and liver are most at risk. Incidence of AAP is between 7–18% with it being more frequent in the AYA ALL population (Figure 1) and is one of the most common causes of truncation of asparaginase therapy, mostly due to the high chances of recurrence of AAP after rechallenge.76–78
Kearney et al reviewed the clinical course of all children with ALL diagnosed with pancreatitis at DFCI from 1987–2003.79 A total of 1583 patients aged 0–18 years were enrolled in DFCI ALL consortium protocols (87–01, 91–01, 95–01, 00–01). Twenty-eight (7%) out of 403 patients treated at Boston Children’s Hospital and 74 (6.4%) of 1155 patients in 10 other centers were diagnosed with at least one episode of AAP. Those patients with AAP had a higher median age, indicating patients 10–18 years of age have 2.4 times increased risk of developing pancreatitis. There was a non-statistically significant trend towards inferior 5-year EFS, with 29% of patients with history of AAP subsequently relapsing compared to only 14% without AAP.79 UKALL 2003 enrolled 3101 patients aged 1–25 years on 3 risk-stratified arms.80 SR and IR patients were exposed to a relatively small amount of pegaspargase (3000–4000 IU/m2) and HR patients received 12,000 IU/m2. Pancreatitis was more common in asparaginase-containing blocks versus non-asparaginase containing blocks (83% vs. 17%; p < 0.0001). The median interval between receiving pegaspargase dose and developing AAP was 10 days. There was significant morbidity in the setting of AAP evidenced by increased readmission rates (29%), pseudocysts (25%), diabetes mellitus (13%), pleural effusions (8%), and renal failure (2%). Further pegaspargase was omitted in patients with higher grade AAP. The risk for pancreatitis was associated with increasing intensity of asparaginase treatment and dose, as well as increasing age.80 Similar results were observed by the NOPHO ALL2008 study with incidences of AAP higher in AYAs (10.1%) compared to in children (7%, p = 0.03) and 44% developed recurrence of AAP on rechallenge with asparaginase.81 An observational Ponte di Legno Toxicity Working Group reviewed patients on 26 trials conducted by 18 trial groups with children (1–18 years) between 1996–2016, to investigate the risk of complications and risk of re-exposing patients with AAP.82 Complications noted in the 465 patients with AAP included mechanical ventilation (8%), pseudocysts (26%), acute insulin need (21%), and death (2%). Older age was associated with more complications (10.5 years vs. 6.1 years without complications; p < 0.0001). One year after diagnosis of AAP, 11% of patients continued to need insulin, had recurrent abdominal pain, or both. Ninety-six patients were re-exposed to asparaginase, including 59 after severe AAP, and 44 (46%) patients developed a second episode, 22 (52%) being severe, suggesting a high risk of recurrence.82
Patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 1/2 AAP can be re-challenged after resolution, whereas it is recommended to omit further pegaspargase therapy in severe grades 3/4 AAP, as 44–63% have risk of recurrence on re-exposure.75,79,81,82
Hyperglycemia
Pegaspargase-induced hyperglycemia is believed to be due to decreased insulin production and modification or deficiency of insulin receptors.83 The use of concomitant steroids and pegaspargase is believed to cause potentiation of hepatic gluconeogenesis and insulin resistance, thereby worsening hyperglycemia.84,85
POG 9203 enrolled 34 patients between the ages of 1–21 years from 1992–1993. This study’s aims included feasibility determination of 29 biweekly doses of pegaspargase on a backbone of intense multiagent antimetabolite consolidation and maintenance in HR B-ALL. Excessive toxicities attributed to pegaspargase and myelosuppression were encountered during consolidation, causing the early closure of this study. Six patients developed hyperglycemia. The greatest risk factor appeared to be concomitant use of steroid therapy (Figure 1).86
Although insulin therapy may be required in severe cases, severe hyperglycemia is usually transient and does not require truncation of pegaspargase therapy.87
Hyperlipidemia
Asparaginase and steroids during ALL therapy lead to abnormalities in lipid metabolism, most notably resulting in hypertriglyceridemia and/or hypercholesterolemia.88 The exact mechanism for lipid dysregulation with asparaginase therapy is not known, but it is postulated to be due to an increase in the endogenous hepatic synthesis of very low‐density lipoproteins. Another reason may be decreased activity in lipoprotein lipase, an enzyme involved in the removal of triglyceride‐rich lipoproteins from the plasma.89 St. Jude Children’s Research Hospital evaluated different asparaginase formulations in patients ≤18 years of age with ALL on two trials; Total XV conducted from 2000–2007 and Total XVI from 2007–2017, enrolling 498 and 598 patients, respectively. SR/HR patients treated with pegaspargase had more severe hypertriglyceridemia compared to native L-asparaginase, and the highest increase occurred when pegaspargase was given in combination with dexamethasone.90 DCOG ALL 10 reported 47% of patients receiving pegaspargase developed hypertriglyceridemia compared to 0% receiving Erwinia asparaginase, thereby suggesting prolonged exposure to asparaginase therapy was associated with hypertriglyceridemia (Figure 1).91
Most patients have transient, asymptomatic hypertriglyceridemia. Omission or alteration of pegaspargase therapy is not recommended, though hypertriglyceridemia greater than 2000 mg/dL can enhance risk of AAP due to severe chylomicronemia.89 Preventive measures like dietary restrictions, fibrates, insulin infusions, heparin infusions, and plasmapheresis have been attempted, but their success in preventing AAP or other morbidities is not established.92,93
Hepatotoxicity
Hepatic toxicities encountered with pegaspargase include direct damage to hepatocytes (elevated hepatic transferases), cholestatic injury (elevated alkaline phosphatase and bilirubin), and impaired synthetic function (decreases in antithrombin, fibrinogen, and albumin with increases in cholesterol, phospholipids and triglycerides). The hepatotoxicity caused by asparaginase is believed to be due to abnormal mitochondrial function in the liver, alterations in lipoprotein metabolism, and secretion.94
Though 75% patients had grade 1/2 elevation in bilirubin, sinusoidal obstructive syndrome (SOS) was noted in 27%, during NOPHO ALL2008 in patients undergoing maintenance treatment for ALL after the introduction of extended pegaspargase treatment. These findings prompted the hypothesis that pegaspargase in combination with other medications, such as mercaptopurine used during maintenance, might trigger SOS.95 DCOG ALL-11 study noted more patients developed grade 1–2 increases in ALT and AST (91%) during asparaginase with high dose methotrexate courses, but not grade 3–4 hepatotoxicity (9%) (Figure 1).96
Most protocols do not recommend alteration in pegaspargase therapy due to hepatotoxicity, although this is another reason to consider dose capping in AYA patients. There are case reports of improvement in hepatic function with use of levocarnitine, a mitochondrial co-factor and vitamin B therapy.97
Thrombosis and Bleeding
Asparaginase has simultaneous effects on procoagulant and thrombolytic proteins causing an increase in both thrombosis and bleeding. Asparaginase-induced depletion of asparagine in the serum leads to decreased synthesis of fibrinogen, plasminogen, anti-coagulation factor antithrombin III (ATIII), protein C, and protein S leading to increased thrombin formation.98 Additional factors contributing to the risk include concomitant steroid therapy particularly prednisone, indwelling central venous catheter, genetic thrombophilia, and underlying ALL itself.99,100
DFCI studied 501 patients between 1991–2008 with newly diagnosed ALL. These patients were enrolled in four pediatric ALL protocols (91–01, 95–01, 00–01, and 05–01) and adult protocol 01–175.101 Five percent of pediatric patients and 34% of adult patients experienced thrombosis during treatment (Figure 1). The risk of thrombosis was noted to increase with age, with further risk stratification indicating a high risk for those >10 years of age and extremely high risk for those >30 years of age. CCG 1962 studied efficacy and PK of a single IM dose of pegaspargase instead of multiple IM doses of native E. coli asparaginase in 3 phases of SR ALL therapy. An incidence of cerebral venous sinus thrombosis (CVST) of 2–3% was observed for both regimens.24 DCOG ALL-10 enrolled 778 ALL patients between 2004–2013 and demonstrated VTE in 7.6% of patients with CVST in 44.1% of these VTE patients.102 Age >7 years, T-ALL, steroids, and length of exposure to pegaspargase were the main risk factors. The presence of VTE did not impact EFS or OS.101,102
Patients with non-catheter-associated VTE should have asparaginase therapy withheld until imaging shows VTE and CVST resolution. Patients should be treated with anticoagulation, preferably low-molecular-weight heparin, and upon resolution or stabilization of thrombus, consideration may be given to rechallenging with pegaspargase.100,101
Osteonecrosis
Osteonecrosis is a known complication in 15–45% of patients, especially AYA patients, undergoing treatment for ALL.103,104 Glucocorticoids, notably dexamethasone, are believed to induce inhibition of angiogenesis, bone marrow adipogenesis, hypercoagulation, and apoptosis of endothelial cells and osteocytes.105 Asparaginase decreases the clearance of dexamethasone due to its hypoproteinemia effect. Additionally, the immunosuppressive effects of glucocorticoids inhibit the antibody response against asparaginase and prevent a neutralizing effect causing higher SAA.105 The potentiated effect of asparaginase on glucocorticoid-induced osteonecrosis is believed to be in the setting of a hypercoagulable state which can lead to impaired circulation, vascular damage, and subsequent osteonecrosis.106
Liu et al were the first to study the effects of asparaginase treatment on dexamethasone-induced osteonecrosis utilizing a mouse model.105 St. Jude’s Total XV protocol noted that patients with antibodies against asparaginase had a lower risk of developing osteonecrosis than those without the antibodies.107
Osteonecrosis 5-year cumulative incidence (CI) was 6.3% on NOPHO ALL2008 and the patients in the experimental arm who did not receive pegaspargase during dexamethasone containing DI had reduced frequency of osteonecrosis (Figure 1).108 NOPHO ALL2008 also correlated hyperlipidemia with osteonecrosis.109 Prolonged hyperlipidemia was associated with a significantly higher osteonecrosis specific hazard ratio (HR) per mmol/L for hypertriglyceridemia (HR=1.08, p=0.038) as well as for cholesterol levels (HR=1.26, p=0.039), and a tendency towards an inverse association with higher HDL (HR=0.22, p=0.053).109 The COG AALL0331 SR trial enrolled 5261 patients between 2005–2010.110 Osteonecrosis 5-year overall CI was 2.7%. Extended exposure to pegaspargase was a likely contributing factor, possibly by potentiating dexamethasone exposure during DI. The study concluded the risk for osteonecrosis can be significantly reduced by using alternate week dexamethasone during DI, which is now standard on all COG ALL protocols.110
Further studies are needed to elucidate whether lowering triglycerides and cholesterol during combined pegaspargase and dexamethasone therapy can reduce the risk of osteonecrosis.
Conclusion
Pegaspargase is an essential chemotherapeutic agent in all newly diagnosed pediatric and AYA ALL protocols, as well as most re-induction protocols for relapsed ALL. It is imperative to be aware of its toxicities and their management, so as to improve survival and decrease long-term morbidities in pediatric and AYA ALL. Areas of focus for future research should include asparaginase PK/PD of newer preparations, dosing regimens, interventions to mitigate toxicity, and role of genetics as it relates to asparaginase dosing and toxicity.
Disclosure
Anupam Verma, Cynthia Bender and Maria Carter have no conflicts of interest to report in this work. Luke Maese has served as consultant and has received honoraria from Jazz Pharmaceuticals.
References
1. Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043. doi:10.1016/S0140-6736(08)60457-2
2. Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber cancer institute ALL consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000). Leukemia. 2010;24(2):320–334. doi:10.1038/leu.2009.253
3. Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber consortium protocol 91-01. Blood. 2001;97(5):1211–1218. doi:10.1182/blood.v97.5.1211
4. Pui CH, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: results of total therapy study XIIIB at St jude children’s research hospital. Blood. 2004;104(9):2690–2696. doi:10.1182/blood-2004-04-1616
5. Brown P, Inaba H, Annesley C, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(1):81–112. doi:10.6004/jnccn.2020.0001
6. Mattano LA, Devidas M, Friedmann AM, et al. Outstanding outcome for children with Standard Risk-Low (SR-Low) Acute Lymphoblastic Leukemia (ALL) and No Benefit to Intensified Peg-Asparaginase (PEG-ASNase) Therapy: results of Children’s Oncology Group (COG) Study AALL0331. Blood. 2014;124(21):793. doi:10.1182/blood.V124.21.793.793
7. Sallan SE, Hitchcock-Bryan S, Gelber R, Cassady JR, Frei E
8. Egler RA, Ahuja SP, Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother. 2016;7(2):62–71. doi:10.4103/0976-500X.184769
9. Kawedia JD, Rytting ME. Asparaginase in acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S14–S17. doi:10.1016/j.clml.2014.06.017
10. Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi:10.1056/NEJMoa0900386
11. Pieters R, Carroll WL. Biology and treatment of acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2010;24(1):1–18. doi:10.1016/j.hoc.2009.11.014
12. Clavell LA, Gelber RD, Cohen HJ, et al. Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med. 1986;315(11):657–663. doi:10.1056/NEJM198609113151101
13. Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13(3):335–342. doi:10.1038/sj.leu.2401310
14. Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group Phase 3 trial. Blood. 2002;99(8):2734–2739. doi:10.1182/blood.v99.8.2734
15. Pession A, Valsecchi MG, Masera G, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(28):7161–7167. doi:10.1200/jco.2005.11.411
16. Hefazi M, Litzow MR. Recent advances in the biology and treatment of T cell acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(4):265–274. doi:10.1007/s11899-018-0455-9
17. Schrappe M, Möricke A, Reiter A, et al. Key treatment questions in childhood acute lymphoblastic leukemia: results in 5 consecutive trials performed by the ALL-BFM study group from 1981 to 2000. Klin Padiatr. 2013;225(Suppl S 01):S62–S72. doi:10.1055/s-0033-1337966
18. Jarrar M, Gaynon PS, Periclou AP, et al. Asparagine depletion after pegylated E. coli asparaginase treatment and induction outcome in children with acute lymphoblastic leukemia in first bone marrow relapse: a Children’s Oncology Group study (CCG-1941). Pediatr Blood Cancer. 2006;47(2):141–146. doi:10.1002/pbc.20713
19. Avramis VI, Tiwari PN. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomedicine. 2006;1(3):241–254.
20. Gupta S, Wang C, Raetz EA, et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the children’s Oncology Group. J Clin Oncol. 2020;38(17):1897–1905. doi:10.1200/JCO.19.03024
21. European Medicines Agency. Oncaspar (pegaspargase): summary of product characteristics. 2018. Available from: http://www.ema.europa.eu/.
22. Shire Pharmaceuticals. Oncaspar (pegaspargase): US prescribing information. 2019. Available from: http://www.fda.gov.
23. Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist. 2007;12(8):991–998. doi:10.1634/theoncologist.12-8-991
24. Avramis V, Sencer S, Periclou A, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99:1986–1994. doi:10.1182/blood.V99.6.1986
25. Heo YA, Syed YY, Keam SJ. Pegaspargase: a review in acute lymphoblastic leukaemia. Drugs. 2019;79(7):767–777. doi:10.1007/s40265-019-01120-1
26. Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol. 1999;457:621–629.
27. Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med. 1963;118(1):99–120. doi:10.1084/jem.118.1.99
28. Schwartz JH, Reeves JY, Broome JD. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966;56(5):1516–1519. doi:10.1073/pnas.56.5.1516
29. Boyse EA, Old LJ, Campbell HA, Mashburn LT. Suppression of murine leukemias by L-asparaginase. Incidence of sensitivity among leukemias of various types: comparative inhibitory activities of guinea pig serum L-asparaginase and Escherichia coli L-asparaginase. J Exp Med. 1967;125(1):17–31. doi:10.1084/jem.125.1.17
30. Meneguetti GP, Santos JHPM, Obreque KMT, et al. Novel site-specific PEGylated L-asparaginase. PLoS One. 2019;14(2):e0211951. doi:10.1371/journal.pone.0211951
31. Lebovic R, Pearce N, Lacey L, Xenakis J, Faircloth CB, Thompson P. Adverse effects of pegaspargase in pediatric patients receiving doses greater than 3750 IU. Pediatr Blood Cancer. 2017;64(10):e26555. doi:10.1002/pbc.26555
32. van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279–285. doi:10.3324/haematol.2015.137380
33. Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 2015;16(16):1677–1690. doi:10.1016/S1470-2045(15)00363-0
34. Schore RJ, Devidas M, Bleyer A, et al. Plasma asparaginase activity and asparagine depletion in acute lymphoblastic leukemia patients treated with pegaspargase on Children’s Oncology Group AALL07P4. Leuk Lymphoma. 2019;60(7):1740–1748. doi:10.1080/10428194.2018.1542146
35. Vrooman LM, Blonquist TM, Supko JG, et al. Efficacy and toxicity of pegaspargase and calaspargase pegol in childhood acute lymphoblastic leukemia/lymphoma: results of DFCI 11-001. J Clin Oncol. 2019;37(15_suppl):10006. doi:10.1200/JCO.2019.37.15_suppl.10006
36. Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet. 2005;44(4):367–393. doi:10.2165/00003088-200544040-00003
37. Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115(7):1351–1353. doi:10.1182/blood-2009-09-245951
38. Angiolillo AL, Schore RJ, Devidas M, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol. 2014;32(34):3874–3882. doi:10.1200/JCO.2014.55.5763
39. Lew G. Space for calaspargase? A new asparaginase for acute lymphoblastic leukemia. Clin Cancer Res. 2020;26(2):325–327. doi:10.1158/1078-0432.CCR-19-2975
40. Pui C-H, Liu Y, Relling MV. How to solve the problem of hypersensitivity to asparaginase? Pediatr Blood Cancer. 2018;65(3):e26884. doi:10.1002/pbc.26884
41. Rau RE, Dreyer Z, Choi MR, et al. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2018;65(3):e26873. doi:10.1002/pbc.26873
42. Zalewska-Szewczyk B, Andrzejewski W, Młynarski W, Jedrychowska-Dańska K, Witas H, Bodalski J. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48(5):931–936. doi:10.1080/10428190701292049
43. Rizzari C, Citterio M, Zucchetti M, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91(1):24–31.
44. Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–1654. doi:10.1182/blood-2008-01-130237
45. Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113(7):1408–1411. doi:10.1182/blood-2008-06-164863
46. Ram R, Wolach O, Vidal L, Gafter-Gvili A, Shpilberg O, Raanani P. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87(5):472–478. doi:10.1002/ajh.23149
47. Gökbuget N, Beck J, Brandt K, et al. Significant Improvement of outcome in Adolescents and Young adults (AYAs) aged 15-35 years with Acute Lymphoblastic Leukemia (ALL) with a pediatric derived adult ALL protocol; results of 1529 AYAs in 2 consecutive trials of the German Multicenter Study Group For Adult ALL (GMALL). Blood. 2013;122(21):839.
48. Boissel N, Auclerc MF, Lhéritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21(5):774–780. doi:10.1200/JCO.2003.02.053
49. de Bont JM, Holt B, Dekker AW, et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18(12):2032–2035. doi:10.1038/sj.leu.2403538
50. Testi AM, Attarbaschi A, Valsecchi MG, et al. Outcome of adolescent patients with acute lymphoblastic leukaemia aged 10-14 years as compared with those aged 15-17 years: long-term results of 1094 patients of the AIEOP-BFM ALL 2000 study. Eur J Cancer. 2019;122:61–71. doi:10.1016/j.ejca.2019.09.004
51. Roberts KG. Genetics and prognosis of ALL in children vs adults. Hematology Am Soc Hematol Educ Program. 2018;2018(1):137–145. doi:10.1182/asheducation-2018.1.137
52. Hawkins DS, Park JR, Thomson BG, et al. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res. 2004;10(16):5335–5341. doi:10.1158/1078-0432.Ccr-04-0222
53. Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009–2017. doi:10.1016/S0140-6736(10)62002-8
54. Frandsen TL, Heyman M, Abrahamsson J, et al. Complying with the European Clinical Trials directive while surviving the administrative pressure - an alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50(2):251–259. doi:10.1016/j.ejca.2013.09.027
55. Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231–e239. doi:10.1016/S1470-2045(16)30035-3
56. Burke MJ, Devidas M, Maloney K, et al. Severe pegaspargase hypersensitivity reaction rates (grade >/=3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children’s oncology group (COG) clinical trials. Leuk Lymphoma. 2018;59(7):1624–1633. doi:10.1080/10428194.2017.1397658
57. Rathod S, Ramsey M, Relling MV, Finkelman FD, Fernandez CA. Hypersensitivity reactions to asparaginase in mice are mediated by anti-asparaginase IgE and IgG and the immunoglobulin receptors FcεRI and FcγRIII. Haematologica. 2019;104(2):319–329. doi:10.3324/haematol.2018.199448
58. Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. doi:10.1002/cncr.25489
59. Strullu M, Corradini N, Audrain M, et al. Silent hypersensitivity to Escherichia coli asparaginase in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2010;51(8):1464–1472. doi:10.3109/10428194.2010.494316
60. Zalewska-Szewczyk B, Gach A, Wyka K, Bodalski J, Młynarski W. The cross-reactivity of anti-asparaginase antibodies against different L-asparaginase preparations. Clin Exp Med. 2009;9(2):113–116. doi:10.1007/s10238-008-0026-9
61. Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol. 2000;18(7):1525–1532. doi:10.1200/jco.2000.18.7.1525
62. Müller H-J, Beier R, Löning L, et al. Pharmacokinetics of native Escherichia coli asparaginase (Asparaginase medac) and hypersensitivity reactions in ALL-BFM 95 reinduction treatment. Br J Haematol. 2001;114(4):794–799. doi:10.1046/j.1365-2141.2001.03009.x
63. Henriksen LT, Harila-Saari A, Ruud E, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr Blood Cancer. 2015;62(3):427–433. doi:10.1002/pbc.25319
64. Hojfeldt SG, Wolthers BO, Tulstrup M, et al. Genetic predisposition to PEG-asparaginase hypersensitivity in children treated according to NOPHO ALL2008. Br J Haematol. 2019;184(3):405–417. doi:10.1111/bjh.15660
65. Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–2033. doi:10.1182/blood-2013-10-534347
66. Kloos RQ, Pieters R, Escherich G, van der Sluis IM. Allergic-like reactions to asparaginase: atypical allergies without asparaginase inactivation. Pediatr Blood Cancer. 2016;63(11):1928–1934. doi:10.1002/pbc.26123
67. Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–616. doi:10.1097/MPH.0b013e31822d4d4e
68. Würthwein G, Lanvers-Kaminsky C, Gerss J, et al. Therapeutic drug monitoring of asparaginase: intra-individual variability and predictivity in children with acute lymphoblastic leukemia treated with PEG-Asparaginase in the AIEOP-BFM acute lymphoblastic leukemia 2009 Study. Ther Drug Monit. 2020;42(3):435–444. doi:10.1097/FTD.0000000000000727
69. Mondelaers V, Ferster A, Uyttebroeck A, et al. Prospective, real-time monitoring of pegylated Escherichia coli and Erwinia asparaginase therapy in childhood acute lymphoblastic leukaemia and non-hodgkin lymphoma in Belgium. Br J Haematol. 2020;190(1):105–114. doi:10.1111/bjh.16495
70. Yang Q, Jacobs TM, McCallen JD, et al. Analysis of pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the general population. Anal Chem. 2016;88(23):11804–11812. doi:10.1021/acs.analchem.6b03437
71. Salzer WL, Asselin B, Supko JG, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children’s Oncology Group. Blood. 2013;122(4):507–514. doi:10.1182/blood-2013-01-480822
72. Verma A, Chen K, Bender C, Gorney N, Leonard W, Barnette P. PEGylated E. coli asparaginase desensitization: an effective and feasible option for pediatric patients with acute lymphoblastic leukemia who have developed hypersensitivity to pegaspargase in the absence of asparaginase Erwinia chrysanthemi availability. Pediatr Hematol Oncol. 2019;36(5):277–286. doi:10.1080/08880018.2019.1634778
73. August KJ, Farooki S, Fulbright JM, et al. Desensitization to pegaspargase in children with acute lymphoblastic leukemia and lymphoblastic lymphoma. Pediatr Blood Cancer. 2020;67(1):e28021. doi:10.1002/pbc.28021
74. Marini BL, Brown J, Benitez L, et al. A single-center multidisciplinary approach to managing the global Erwinia asparaginase shortage. Leuk Lymphoma. 2019;60(12):2854–2868. doi:10.1080/10428194.2019.1608530
75. Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012;159(1):18–27. doi:10.1111/bjh.12016
76. Alvarez OA, Zimmerman G. Pegaspargase-induced pancreatitis. Med Pediatr Oncol. 2000;34(3):200–205. doi:10.1002/(sici)1096-911x(200003)34:3<200::aid-mpo7>3.0.co;2-t
77. Flores-Calderón J, Exiga-Gonzaléz E, Morán-Villota S, Martín-Trejo J, Yamamoto-Nagano A. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol. 2009;31(10):790–793. doi:10.1097/MPH.0b013e3181b794e8
78. Knoderer HM, Robarge J, Flockhart DA. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007;49(5):634–639. doi:10.1002/pbc.21037
79. Kearney SL, Dahlberg SE, Levy DE, Voss SD, Sallan SE, Silverman LB. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009;53(2):162–167. doi:10.1002/pbc.22076
80. Samarasinghe S, Dhir S, Slack J, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013;162(5):710–713. doi:10.1111/bjh.12407
81. Rank CU, Wolthers BO, Grell K, et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL2008 treatment of patients 1-45 years of age. J Clin Oncol. 2020;38(2):145–154. doi:10.1200/JCO.19.02208
82. Wolthers BO, Frandsen TL, Baruchel A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18(9):1238–1248. doi:10.1016/S1470-2045(17)30424-2
83. Carpentieri U, Balch MT. Hyperglycemia associated with the therapeutic use of L-asparaginase: possible role of insulin receptors. J Pediatr. 1978;93(5):775–778. doi:10.1016/s0022-3476(78)81075-0
84. Khan A, Adachi M, Hill JM. Potentiation of diabetogenic effect of L-asparaginase by prednisolone. Horm Metab Res. 1970;2(5):275–276. doi:10.1055/s-0028-1095058
85. Chan JC, Cockram CS, Critchley JA. Drug-induced disorders of glucose metabolism. Mechanisms and management. Drug Saf. 1996;15(2):135–157. doi:10.2165/00002018-199615020-00005
86. Salzer WL, Devidas M, Shuster JJ, et al. Intensified PEG-L-asparaginase and antimetabolite-based therapy for treatment of higher risk precursor-B acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2007;29(6):369–375. doi:10.1097/MPH.0b013e3180640d54
87. Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leuk Lymphoma. 2011;52(12):2237–2253. doi:10.3109/10428194.2011.596963
88. Bhojwani D, Darbandi R, Pei D, et al. Severe hypertriglyceridaemia during therapy for childhood acute lymphoblastic leukaemia. Eur J Cancer. 2014;50(15):2685–2694. doi:10.1016/j.ejca.2014.06.023
89. Parsons SK, Skapek SX, Neufeld EJ, et al. Asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Blood. 1997;89(6):1886–1895. doi:10.1182/blood.V89.6.1886
90. Finch ER, Smith CA, Yang W, et al. Asparaginase formulation impacts hypertriglyceridemia during therapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67(1):e28040. doi:10.1002/pbc.28040
91. Tong WH, Pieters R, de Groot-kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99(11):1716–1721. doi:10.3324/haematol.2014.109413
92. Cohen H, Bielorai B, Harats D, Toren A, Pinhas-Hamiel O. Conservative treatment of L-asparaginase-associated lipid abnormalities in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54(5):703–706. doi:10.1002/pbc.22305
93. Zawitkowska J, Lejman M, Zaucha-Prażmo A, Sekuła N, Greczkowska-Chmiel T, Drabko K. Severe drug-induced hypertriglyceridemia treated with plasmapheresis in children with acute lymphoblastic leukemia. Transfus Apher Sci. 2019;58(5):634–637. doi:10.1016/j.transci.2019.08.025
94. Sahoo S, Hart J. Histopathological features of L-asparaginase-induced liver disease. Semin Liver Dis. 2003;23(3):295–299. doi:10.1055/s-2003-42647
95. Toksvang LN, De Pietri S, Nielsen SN, et al. Hepatic sinusoidal obstruction syndrome during maintenance therapy of childhood acute lymphoblastic leukemia is associated with continuous asparaginase therapy and mercaptopurine metabolites. Pediatr Blood Cancer. 2017;64(9). doi:10.1002/pbc.26519
96. Kloos RQH, Pieters R, van den Bos C, van Eijkelenburg NKA, de Jonge R, van der Sluis IM. The effect of asparaginase therapy on methotrexate toxicity and efficacy in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60(12):3002–3010. doi:10.1080/10428194.2019.1613537
97. Schulte RR, Madiwale MV, Flower A, et al. Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature. Leuk Lymphoma. 2018;59(10):2360–2368. doi:10.1080/10428194.2018.1435873
98. Bushman JE, Palmieri D, Whinna HC, Church FC. Insight into the mechanism of asparaginase-induced depletion of antithrombin III in treatment of childhood acute lymphoblastic leukemia. Leuk Res. 2000;24(7):559–565. doi:10.1016/s0145-2126(00)00017-5
99. Caruso V, Iacoviello L, Di Castelnuovo A, et al. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. doi:10.1182/blood-2006-04-015511
100. Nowak-Göttl U, Kenet G, Mitchell LG. Thrombosis in childhood acute lymphoblastic leukaemia: epidemiology, aetiology, diagnosis, prevention and treatment. Best Pract Res Clin Haematol. 2009;22(1):103–114. doi:10.1016/j.beha.2009.01.003
101. Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452–459. doi:10.1111/j.1365-2141.2010.08524.x
102. Klaassen ILM, Lauw MN, Fiocco M, et al. Venous thromboembolism in a large cohort of children with acute lymphoblastic leukemia: risk factors and effect on prognosis. Res Pract Thromb Haemost. 2019;3(2):234–241. doi:10.1002/rth2.12182
103. Mogensen SS, Harila-Saari A, Mäkitie O, et al. Comparing osteonecrosis clinical phenotype, timing, and risk factors in children and young adults treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65(10):e27300. doi:10.1002/pbc.27300
104. Patel B, Richards SM, Rowe JM, Goldstone AH, Fielding AK. High incidence of avascular necrosis in adolescents with acute lymphoblastic leukaemia: a UKALL XII analysis. Leukemia. 2008;22(2):308–312. doi:10.1038/sj.leu.2405032
105. Liu C, Janke LJ, Kawedia JD, et al. Asparaginase potentiates glucocorticoid-induced osteonecrosis in a mouse model. PLoS One. 2016;11(3):e0151433. doi:10.1371/journal.pone.0151433
106. Te Winkel ML, Appel IM, Pieters R, van den Heuvel-eibrink MM. Impaired dexamethasone-related increase of anticoagulants is associated with the development of osteonecrosis in childhood acute lymphoblastic leukemia. Haematologica. 2008;93(10):1570–1574. doi:10.3324/haematol.12956
107. Liu C, Kawedia J, Cheng C, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26(11):2303–2309. doi:10.1038/leu.2012.102
108. Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent versus continuous PEG-Asparaginase to reduce Asparaginase-associated toxicities: a NOPHO ALL2008 Randomized Study. J Clin Oncol. 2019;37(19):1638–1646. doi:10.1200/jco.18.01877
109. Mogensen SS, Schmiegelow K, Grell K, et al. Hyperlipidemia is a risk factor for osteonecrosis in children and young adults with acute lymphoblastic leukemia. Haematologica. 2017;102(5):e175–e178. doi:10.3324/haematol.2016.160507
110. Mattano LA, Devidas M, Friedmann AM, et al. Effect of dexamethasone (DEX) dose modification on osteonecrosis (ON) risk associated with intensified therapies for standard risk acute lymphoblastic leukemia (SR-ALL): a report from the Children’s Oncology Group (COG) study AALL0331. J Clin Oncol. 2013;31(15_suppl):10002. doi:10.1200/jco.2013.31.15_suppl.10002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
