Back to Journals » OncoTargets and Therapy » Volume 9
Clinical utility of 5-aminolevulinic acid HCl to better visualize and more completely remove gliomas
Authors Halani S, Adamson DC
Received 29 May 2016
Accepted for publication 30 July 2016
Published 12 September 2016 Volume 2016:9 Pages 5629—5642
DOI https://doi.org/10.2147/OTT.S97030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Sameer H Halani,1 D Cory Adamson1,2
1Department of Neurosurgery, Emory University School of Medicine, Atlanta, GA, USA; 2Neurosurgery Section, Atlanta VA Medical Center, Decatur, GA, USA
Abstract: Surgical resection is typically the first line of treatment for gliomas. However, the neurosurgeon faces a major challenge in achieving maximal resection in high-grade gliomas as these infiltrative tumors make it difficult to discern tumor margins from normal brain with conventional white-light microscopy alone. To aid in resection of these infiltrative tumors, fluorescence-guided surgery has gained much popularity in intraoperative visualization of malignant gliomas, with 5-aminolevulinic acid (5-ALA) leading the way. First introduced in an article in Neurosurgery, 5-ALA has since become a safe, effective, and inexpensive method to visualize and improve resection of gliomas. This has undoubtedly led to improvements in the clinical course of patients as demonstrated by the increased overall and progression-free survival in patients with such devastating disease. This literature review aims to discuss the major studies and trials demonstrating the clinical utility of 5-ALA and its ability to aid in complete resection of malignant gliomas.
Keywords: aminolevulinic acid, 5-ALA, fluorescence, glioblastoma multiforme, high-grade glioma, resection
Introduction
Gliomas are the most common primary brain tumors and account for over 70% of brain tumors.1,2 The 2007 World Health Organization (WHO) classification of gliomas is based on the aggressive morphological features visualized under the microscope and is graded as follows:3 grade I and II gliomas are benign, well-differentiated, and can often be adequately managed medically and surgically.4 WHO grade III and IV gliomas (or glioblastoma multiforme [GBM]) are highly invasive cancers with 5-year survival rates of 24% and 4%, respectively.5–7 However, recent works by Brat et al8 have led to a paradigm shift in the classification of gliomas by focusing on molecular markers: namely, the mutational statuses of isocitrate dehydrogenase and codeletion of 1p and 19q chromosomal arms were found to better predict the clinical behavior of these tumors compared to histological assessment alone.9 As such, the 2016 WHO guidelines for classification of gliomas incorporates these new molecular signatures in defining new classes of disease. The isocitrate dehydrogenase-wild-type status of tumors, including GBM, comprise the malignant high-grade gliomas (HGGs) and are particularly devastating because of their infiltrative nature, which largely contributes to universal recurrence, despite multiple therapeutic approaches.3
Surgical resection is typically the first line of treatment for gliomas. Multiple studies have demonstrated that the extent of resection is a recognized prognostic factor in HGGs and lower-grade gliomas (LGGs).10–17 However, the neurosurgeon faces a major challenge in achieving maximal resection of gliomas; their infiltrative nature makes it difficult to discern tumor margins from normal brain with conventional white-light microscopy alone. This obstacle is worsened when tumors are in close proximity to eloquent structures, such as the motor cortex. Consequently, the neurosurgeon must balance achieving maximal resection of malignant tumor with preservation of normal brain parenchyma. To aid in resection of these infiltrative tumors, fluorescence-guided surgery has gained much popularity in intraoperative visualization of malignant gliomas, with 5-aminolevulinic acid (5-ALA) leading the way. First introduced by Stummer et al in 1998,18 5-ALA has since become a safe, effective, and inexpensive method to visualize, improve the extent of resection, and therefore improve the clinical course of patients with such devastating disease.10,18–21 This review aims to discuss the history of 5-ALA and identify the major studies in the literature demonstrating the clinical utility of 5-ALA in the resection of malignant gliomas.
Biochemistry of 5-ALA and intraoperative fluorescence
5-ALA is a part of the heme biosynthetic pathway found in all living mammalian cells.22 As part of this pathway, 5-ALA is metabolized and transformed through a series of reactions in the mitochondria into protoporphyrin IX (PpIX), a fluorescent metabolite.21 In physiologically normal cells, PpIX is chelated by ferrochelatase into heme.
Exogenous 5-ALA acts as an orally administered “prodrug” and has demonstrated marked penetration of the blood–brain barrier (BBB), most notably in areas with a high density of malignant cells.10,23 There are many theories describing the mechanisms of and reasoning for accumulation of 5-ALA and PpIX in malignant cells. Decreased ferrochelatase levels, increased uptake of PpIX by ATP-binding cassette B6, increased cellular density, increased proliferative activity by malignant tumor cells, increased angiogenesis, and increased BBB permeability in areas of malignancy have all been implicated in increased fluorescence of gliomas.10,23–27 In normal cells, the synthesis of PpIX is regulated by a feedback control system (therefore avoiding its accumulation), and it has been suggested that this feedback system can be set out of control when high amounts of exogenous 5-ALA are administered.28
The protocol for 5-ALA use includes oral administration at a dose of 20 mg/kg body weight 3 hours before anesthesia.4 5-ALA is rapidly absorbed into the bloodstream within 1 hour, and PpIX levels peak at 4 hours after administration; the neurosurgeon can assume similar kinetics in all patients with regular organ function, regardless of age.10,18,21,29 However, there is a risk of adverse effects in patients with a history of acute or chronic porphyria; it should also not be used in patients who are pregnant due to potential teratogenic effects. Therefore, the timing of 5-ALA administration must account for operating room (OR) preparation time and time for craniotomy to allow for adequate visualization of fluorescence intraoperatively. To detect this fluorescence optimally in the operating room, a blue light source (375–440 nm) is needed as PpIX has an absorption wavelength of 404 nm.22 This allows for a violet–red fluorescence of malignant cells from increased 5-ALA and PpIX accumulation (Figure 1). 5-ALA received approval for use in fluorescence-guided resections of gliomas in 2007 by the European Medicines Agency and is the only agent approved for such use in humans in Europe, Asia, and Australia. Unfortunately, the US Food and Drug Administration (FDA) has not yet approved the use of 5-ALA in the USA. The FDA considers 5-ALA a drug, rather than a surgical aid, which would require a lengthy process in order to gain approval.30
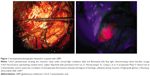  | Figure 1 Intraoperative photographs obtained in a patient with GBM. |
Visualization of gliomas with 5-ALA
Led by Dr Walter Stummer, 5-ALA’s ease of use and ability to demarcate tumor boundaries have led to numerous studies investigating its ability to accurately identify tumors and its efficacy for tumor resection. One of Strummer’s first investigations aimed to identify the efficacy of 5-ALA in visualizing malignant gliomas.18 In this study, fluorescing and nonfluorescing samples were taken from nine patients harboring HGGs and examined histologically. The sensitivity and specificity of 5-ALA-induced fluorescence were 85% and 100%, respectively; fluorescence resulted in greater resection of the tumor in seven of nine patients (Table 1). Of note, the phenomenon of “photobleaching” (ie, diminished fluorescence because of photon-induced structural modification) was also analyzed and it was found that fluorescence decayed to 36% after 25 minutes under blue light and after 87 minutes in white light. This demonstrates that the window of opportunity for the surgeon is limited but crucial.
The 5-ALA Recurrent Glioma Study Group performed a multicentered Phase II trial to determine the positive predictive value (PPV) of 5-ALA fluorescence in 36 patients with recurrent HGGs.31 The PPV of fluorescence in brain tissue with no obvious tumor visible under white light was 93%; the PPV of fluorescence in tissue from obvious tumor bulk was 99.5%. The major conclusions from this study were that 5-ALA can be used to guide resection after radiation and chemotherapy and is effective for the detection of recurrent gliomas.
Díez Valle et al32 analyzed the efficacy of 5-ALA fluorescence in 36 patients with GBM. Fluorescent tissue was sampled from the areas of variable fluorescence from tumor center, tumor margins, and surrounding tissue, and histologically examined. It was seen that strong fluorescence had 100% PPV for identifying tumor cells in the tumor bulk. Tissue with indeterminate fluorescence had a PPV of 97% and a negative predictive value (NPV) of 66%. Panciani et al33 performed a prospective study of 18 patients with presumed GBM to identify the accuracy of 5-ALA combined with neuronavigation for detecting tumor. Fluorescence-guided resection revealed a sensitivity of 91.4% and a specificity of 89.2%. In false-negative samples (ie, no fluorescence but positive tumor presence on histological examination), cellularity was low. This is consistent with previous studies demonstrating that low fluorescence may correlate to low tumor cellular density. When fluorescence was combined with neuronavigation, the sensitivity increased to 97.2% whereas the specificity dropped markedly to 45.9%. This high false-positive rate was likely attributable to a peritumoral inflammatory state with increased reactive mitotic activity. This also illustrates some of the drawbacks of 5-ALA use but does not deter its use particularly in noneloquent areas – the benefits of increased tumor resection (ie, increased overall survival [OS]) outweigh the low risk of long-term neurological deficit. Although several studies report on the sensitivity and specificity of 5-ALA fluorescence in both tumor and peritumoral brain tissue, one must be cognizant of the fact that both of these parameters are dependent on the number of samples taken, as well as the specific location of samples (ie, increased number of samples from nonfluorescent areas can artificially shift results toward “true negatives”). Future studies would benefit from noting areas and the number of tumor samples, as well as providing PPV and NPV in addition to sensitivities and specificities.
Apart from white-light-guided resection, intraoperative magnetic resonance imaging (iMRI) has also emerged as a tool to aid surgeons in achieving greater rates of resection in malignant gliomas. In a prospective study, Coburger et al12 enrolled 45 patients with contrast-enhancing lesions to determine whether 5-ALA provides additional benefit in the detection of invasive tumor compared to iMRI. The study included 45 patients: 34 with HGGs (with 114 histological samples) and eleven with metastatic lesions (with 13 histological samples). In the HGG group, iMRI had a greater number of areas that were “undetectable” but revealed areas with histopathological evidence of solid tumor (n=40, 59%) compared with 5-ALA (n=10, 15%). The specificity of 5-ALA was 0.80 when areas of infiltration were included in the calculation, compared to 0.60 for iMRI. When calculating specificity, 5-ALA was 0.80 when areas of infiltration were included in the calculation, compared to 0.60 for iMRI. This reveals that 5-ALA is a better tool for identifying and resecting tumor margins of HGGs that would otherwise be missed using conventional iMRI. In the metastatic group, three patients were excluded because their tumors did not demonstrate 5-ALA fluorescence. iMRI and 5-ALA showed no significant difference in tumor detection in metastatic lesions. This study further demonstrates the utility of 5-ALA compared to iMRI for HGGs and confirms that its use in metastatic intracranial lesions is limited. Although studies investigating the utility of 5-ALA fluorescence in cerebral metastatic lesions are rare, results demonstrate that metastatic lesions have unpredictable fluorescence behavior.34
Variable intensity of 5-ALA fluorescence and tumor visualization
An emerging question with 5-ALA fluorescence is the association of fluorescent intensity and degree of histologic cellularity. One of the first studies to study variable intensities of fluorescence was performed by Hefti et al.35 They used 5-ALA for the intraoperative delineation of tumor margins in 71 patients, of which 57 cases were HGGs. The remaining cases consisted of LGGs and nonglial pathologies. In this study, 56 of 57 HGGs demonstrated strong fluorescence, whereas no fluorescence was observed in LGGs. In the HGG, the sensitivity and specificity for strong fluorescence were 100% and 98%, respectively, while indeterminate fluorescence showed a sensitivity and specificity of 76% and 85%, respectively. This fluorescent-guided approach was shown to be better than the surgeon’s estimate under white light (specificity: 68%, sensitivity: 66%). The areas of indeterminate fluorescence are noted to be infiltrative zones, which have tumor as well as normal cells.
Nabavi et al31 explored the gradient of macroscopic fluorescence even further in a prospective study. Thirty-three patients with HGGs received 5-ALA, and during resection, samples were taken from “weak” and “strong” areas of fluorescence as determined by the surgeon and confirmed with spectrometry, as well as nonfluorescing areas near and distant from brain for blinded histological assessment. To minimize bias, this study included four neurosurgical centers, and all neuropathologists and neuroradiologists were blinded. They found that “strong” fluorescence corresponded to solidly proliferating tumor and high cell densities, while “weak” fluorescence corresponded to infiltrating tumor and medium cell densities. Among all samples, the PPV of fluorescence was 96.2% in strong fluorescent areas and 92.2% in weak fluorescent areas. However, the NPV (nonfluorescing biopsy without tumor) was 39.5%. Given that these HGGs are highly infiltrative, this is not surprising. Fluorescing tissue was knowingly left unresected in 23 cases due to proximity to eloquent areas; residual MRI enhancement in the postoperative tumor bed was detected in 15 of these 23 patients but in none of the patients with no residual fluorescence, signifying the importance of 5-ALA in achieving gross total resection (GTR) in HGGs.
Yamada et al36 investigated the role of 5-ALA in conjunction with iMRI for the resection of intracranial malignant gliomas. In 99 consecutive cases, tumor bulk was resected using neuronavigation with both iMRI and guidance of 5-ALA. Of these tumors, 64% were histopathologically identified as GBM (WHO grade IV), with the remaining cases being WHO grade III. The mean tumor resection rate was 95%±8% (range: 60%–100%) with 51 cases achieving GTR; the extent of resection was determined by postoperative MRI. In addition to the extent of resection, this study evaluated the degree of fluorescence (ie, “strong” vs “weak”). All tumors demonstrated intraoperative fluorescence, and neoplastic elements were revealed on histological examination in 94% of “strong” fluorescent areas and 83% of weak fluorescent areas. The diagnostic efficacy of 5-ALA-induced fluorescence revealed sensitivities of 99% in tumor bulk, 93% in infiltrative tumor margins, and 95% overall; the PPV of fluorescence in areas of tumor bulk identified by iMRI was 100%.
In a single-center, single-arm, open-label Phase II clinical trial, Lau et al37 investigated the correlation of intensity of 5-ALA fluorescence with the degree of tumor cellularity. Fifty-nine patients with HGGs were enrolled (GBM: 47 patients, grade III glioma: 12 patients), and 211 intraoperative biopsies were examined from areas of fluorescence graded by increasing intensity (0–3) as determined by the surgeon; tumor samples were studied by a neuropathologist for cellular density (Figure 2). The results showed the overall PPV of 5-ALA intensity of three was predictive of tumor presence across all tumor types (all tumors: 97%, GBM: 97.2%, high-grade tumors: 100%, recurrent tumors: 93.8%). However, the NPV (ie, absence of fluorescence as an indicator of no tumor) is fairly low (all tumors: 37.7%, GBM: 43.9%, high-grade tumors: 16.7%, and recurrent tumors: 31.0%). In addition, increased fluorescent intensity is a strong predictor of increased tumor cellular density, but this is not as reliable for lower intensity (ie, lower cellular density). The utility of 5-ALA in improving visualization of tumor bulk is apparent once again; however, this study demonstrates some of the limitations of 5-ALA. For example, tumor-infiltrated tissue may not illuminate, and that nontumor areas may illuminate secondary to inflammation and reactive changes and are important considerations for the surgeon. Inflammatory reactions in bacterial, chemical, or irradiated scar tissue may cause false-positive findings, as strong inflammatory reactions have been shown to induce a high false-positive rate.38,39 A study by Utsuki et al38 found that false-positive rates were rare at the initial resection of HGGs unless there was remarkable inflammatory cell infiltration (one out of 31 cases had a false-positive finding). Notably, the false-positive rate was much higher in recurrent cases (five of eleven cases of recurrent resection had false-positive findings). This was attributed to the damaged BBB from inflammatory cell infiltration associated with prior surgical and radiation interventions.38 Therefore, the neurosurgeon cannot simply resect the entire 5-ALA signal and should remain cautious when attempting to employ intraoperative 5-ALA to discriminate radiation- and chemotherapy-induced tissue changes from true disease progression.40
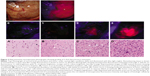  | Figure 2 Representative intraoperative photographs showing grading of 5-ALA fluorescence intensities. |
Clinical impact of 5-ALA: improved extent of resection and survival
The impact of 5-ALA-guided resection on survival is a leading reason for use in glioma resection and has been investigated in several studies (Table 2). Stummer et al19 performed a study to analyze the effect of fluorescence-guided resection on postoperative imaging and survival in 52 patients with GBM. Complete resection of contrast-enhancing tumor occurred in 33 patients (63.4%). In 18 of the remaining 19 patients, fluorescent tissue was not resected due to safety considerations, predicting residual enhancement on MRI. The authors also demonstrated that survival was related to residual tissue fluorescence (no residual fluorescence, mean survival 101 weeks vs residual vague fluorescence, 79 weeks vs residual solid fluorescence, 51 weeks, P=0.0006) and residual contrast enhancement on postoperative MRI (no residual enhancement, 103 weeks vs residual enhancement 54 weeks, P=0.0016).
In one of the largest randomized-control trials performed for this niche, the ALA Glioma Study Group investigated the effect of 5-ALA on the extent of resection, progression-free survival (PFS), OS, and morbidity.41 Three hundred twenty-two patients with suspected malignant glioma were randomly assigned to 5-ALA-guided or conventional white-light resection. Primary end points were identified as the number of patients without contrast-enhancing tumor on postoperative MRI and 6-month PFS. It is important to note that there are two commonly used, but slightly different, definitions for the extent of resection: 1) GTR is defined as residual contrast-enhancing tumor volume ≤1 cm3; and 2) complete resection of enhancing tumor (CRET) is defined as no residual contrast enhancement >0.175 cm3 on postoperative MRI.42 CRET was achieved in 90 of 139 (65%) patients in the 5-ALA group compared with 47 of 131 (36%) in the control group (difference between groups 29%, 95% confidence interval [CI]: 17–40, P<0.0001). In addition, patients allocated 5-ALA had a greater 6-month PFS than the control group (41.0% in the 5-ALA group vs 21.1% in white-light group, difference between groups 19.9%, 95% CI: [9.1–30.7], P=0.0003). The median PFS was 5.1 months in the 5-ALA group vs 3.6 months in the control group. CRET patients had a median survival of 17.9 months compared to 12.9 months in patients who did not achieve CRET (P<0.0001). Despite more complete resection in the 5-ALA group, there was no difference in the frequency of adverse effects between the two groups.
Further analysis of this trial was performed by the ALA Glioma Study Group to determine the effect of improved resections on safety.43 The analysis included 176 patients in the 5-ALA group and 173 patients in the white-light group. CRET was achieved in 63.6% of patients in the 5-ALA group and 37.6% in the white-light group (P<0.0001). Six-month PFS was 35.2% for those in the 5-ALA group compared to 21.8% for those in the white-light group (P=0.004); there was no difference observed in OS (14 months in both groups). The incidence of repeat operations was 30% in the 5-ALA group compared to 39% in the white-light group (P=0.0311), reinforcing earlier findings that 5-ALA facilitates more complete resections. Most notably, this analysis showed improved OS for patients with complete resections than for those without complete resections (median 16.7 months in the 5-ALA group vs 12 months in the control, P<0.0001).
Aldave et al11 performed a retrospective review of 52 patients from a single institution to determine the prognostic value of residual fluorescent tissue in GBM patients who underwent 5-ALA-guided resection. In this study, the aim of surgery in all cases was the resection of all fluorescent tissue in the surgical field with the exception of eloquent areas; 27 patients had residual fluorescence and 25 had no residual fluorescence. Inclusion criteria for this study included 100% resection of MR-contrast-enhancing tumor. As well established in other studies, univariate analysis demonstrated age, and the Karnofsky performance score (KPS) had significant correlation with OS. Multivariate analysis showed that age, MGMT status, tumor volume, eloquent location, residual fluorescence, and additional immunotherapy were significant, ie, age >55 years with hazard ratio (HR): 3.3 (P=0.021), residual fluorescence HR: 2.5 (P=0.041), eloquent location HR: 2.9 (P=0.022), preoperative volume HR: 1.1 (P=0.002), and immunotherapy with HR: 4.2 (P=0.004). The median OS was 27.0 months (95% CI: 22.4–31.6) in patients without residual fluorescence compared to 17.5 months (95% CI: 12.5–22.5) in those with residual fluorescence (P=0.015). Complication rate, including new neurological deficit 30 days after surgery, was 8% for the residual fluorescence group compared to 18.5% in patients without residual fluorescence; however, the difference was not significant (P=0.267). Ultimately, this study further demonstrates the clinical utility of 5-ALA for greater tumor resection of GBMs and in increasing OS after CRET is achieved.
As part of a prospective Phase II 5-ALA trial, Cordova et al44 examined the utility of 5-ALA in achieving a greater extent of resection and its effect on OS in patients with newly diagnosed or recurrent GBMs. Thirty patients were prospectively enrolled; the median extent of resection was 94.8% (range: 70%–100%) with a median residual tumor volume of 0.76 cm3 (range: 0–15.3 cm3). CRET (defined as <0.75 cm3 on postoperative contrast-enhanced MRI) was achieved in nine patients. Although this study did not have a control group, it did demonstrate that 5-ALA is helpful at decreasing residual tumor volume and providing more complete tumor resections in GBMs and improved OS (HR: 0.94, 95% CI: 0.89–0.99).
Several systematic reviews have been performed to identify the clinical impact of 5-ALA. In a 2014 Cochrane Review, image-guided surgery of various modalities for the resection of HGGs (and one study with LGGs included) was assessed, with 5-ALA included.17 The median survival was 15.2 months (95% CI: 12.9–17.5) in the intervention arm vs 13.5 months (95% CI: 12.0–14.7) in the control arm (HR: 0.82, 95% CI: 0.62–1.07). The median PFS was 5.1 months (95% CI: 3.4–6.0) in the intervention arm vs 3.6 months (95% CI: 3.2–4.4) in the control arm. The studies included in this review have already been discussed earlier.41,45 In a 2013 systematic review and meta-analysis, Zhao et al20 aimed to identify the added value of 5-ALA-induced fluorescence-guided resection of HGGs compared with conventional neuronavigation-guided resection. This study included ten studies for analysis and used only prospective studies designed for level 1 or level 2 evidence.19,32,33,35,41,43,46–49 Across these studies, 5-ALA-guided resection demonstrated an overall sensitivity of 0.87, specificity of 0.89, a positive likelihood ratio (LR) of 7.62 (95% CI: 3.87–15.01), a negative LR of 0.14 (95% CI: 0.09–0.23), and a diagnostic OR of 53.06 (95% CI: 18.70–150.51) (Figure 3).20 Two studies from the systematic review found that 5-ALA-guided resection was able to detect more tumor lesions compared to conventional neuronavigation-guided surgery.20,33,35 Survival data were reported in three studies that found that patients in the 5-ALA group had a better 6-month PFS (46% and 41% in the 5-ALA group vs 28.3% and 21.2% in the white-light group) and OS (14.3%, 12.3%, and 16% in 5-ALA groups vs 13.7%, 5.6%, and 15% in the white-light group).41,43,48
  | Figure 3 Forest plot for sensitivity and specificity of 5-ALA-guided resection of GBM in studies included in a meta-analysis from Zhao et al. |
Utility of 5-ALA visualization for tumors in eloquent areas
The ability of 5-ALA to delineate tumor margins has opened many doors in exploring gliomas that include eloquent areas. In general, “eloquent areas” include primary motor and sensory cortex, the basal ganglia, thalamus, hypothalamus, cerebral peduncles, the brainstem, the dentate nucleus, language areas (identified by fMRI), the primary visual cortex, and essential white matter tracts linked to these eloquent regions.50 Schucht et al51 also explored the benefits of 5-ALA-guided resection in motor eloquent areas. In a prospective study, 67 patients underwent 5-ALA-guided surgery for GBM adjacent to the corticospinal tract. Patients were monitored with continuous dynamic monopolar motor mapping coupled to an acoustic motor-evoked potential alarm. In all 67 cases, 5-ALA was useful in identifying tumor tissue. Thirty-eight patients had complete resection of 5-ALA fluorescent areas. In the remaining 16 patients, surgery was stopped secondary to mapping findings; each of these patients had 5-ALA fluorescence remaining. However, on postoperative MRI, nine of these 16 patients had CRET despite residual fluorescence. At 1-day postop, 20 patients had worsening of motor status; 13 of these patients had complete motor recovery at the time of discharge. At 3-month evaluation, three patients (4%) presented with persistent motor deficit; the median KPS for all patients at 3 months was 80 (range: 40–100). This study further demonstrates that 5-ALA fluorescence allows for better visualization of tumor margins outside of those visualized with contrast-enhanced MR. In addition, combining 5-ALA and mapping facilitates high rates of CRET in motor eloquent areas without compromising neurological outcomes.
Della Puppa et al50 prospectively investigated the utility of 5-ALA in HGG resection in eloquent areas with the assistance of functional mapping. Tumors had to be <10 mm from eloquent areas. Thirty-one patients (median age: 57 years, range: 27–79 years) with MR scans consistent with HGGs were enrolled. Twenty-two patients were newly diagnosed and nine had recurrent tumor. CRET was achieved in 23 patients; the other eight patients had residual fluorescent tumor intentionally because a functional area or cortical tract was identified or motor-evoked potential amplitudes were reduced in fluorescent areas. Preoperative impairment was detected in six cases, including hemiparesis (n=5) and minimal aphasia (n=1). On the seventh postoperative day, 20 patients had neurological impairment; five of the 19 had worsening of a previous deficit, while 14 had a new deficit. At 1-month evaluation, 14 of 20 patients had fully recovered, while six patients had persistent deficits; four of these six were unchanged in comparison to preoperative state. At the 3-month evaluation, a median KPS of 100 was detected in 25 patients (compared to 27 patients preoperatively). Ultimately, only 3% of patients had a new severe, postoperative deficit, and all patients with permanent postoperative impairment were those affected by recurrent tumor. Patients who underwent awake surgery had 0% late morbidity (n=6). The first surgery was significantly associated with a lower morbidity (P<0.05) and a higher KPS (P-value <0.01). This study demonstrates that 5-ALA combined with functional mapping can achieve maximal resection of HGGs in eloquent areas with relatively low morbidity.
Combining imaging with 5-ALA use for complete resection of gliomas
As technology has advanced, other imaging modalities, including iMRI and intraoperative brain mapping/monitoring, have been used more frequently in conjunction with 5-ALA to improve resections in patients with malignant gliomas (Table 2). In a 2011 retrospective study by Tsugu et al,52 patients who underwent craniotomy with neuronavigation and received 5-ALA were analyzed. The average tumor resection rate in those with positive 5-ALA fluorescence was 92.6%. Those who did not undergo resection with iMRI had an average resection rate of 91.8%. Twelve of the 33 patients did not exhibit 5-ALA fluorescence. In these patients, surgery with iMRI resulted in a resection rate of 89.2%, while those without iMRI guidance had a resection rate of 68.7%. This study demonstrated that the combination of iMRI and 5-ALA has a synergistic effect on resection rates in glioma surgery.
Eyüpoglu et al53 prospectively enrolled 37 patients with HGGs based on MR findings for resection with 5-ALA and iMRI. 5-ALA-alone resections achieved 62% CRET with an increase to 100% with the addition of iMRI. Of note, the authors stated that when complete resection of fluorescence was achieved, often times continuing in areas identified from iMRI would later demonstrate new areas of fluorescence. Stummer54 astutely commented on this finding:
This assessment […] best describes how fluorescence, which gives 2-D […] information and may sometimes be hidden behind overhanging edges or obscured by blood, can be used to wisely augment the capabilities of more complex and non-real-time iMRI, which gives 3-D information, to safely optimize resection.
Furthermore, Stummer’s54 comments introduce an important fact about 5-ALA – it is a tool that is used by a surgeon. Therefore, in addition to the “tool” failing, the user may lack appropriate training to effectively use the tool. With this in mind, the European Medicines Agency has coupled reimbursement and 5-ALA usage to a mandatory training course for surgeons using it.29 This is an important medical precedent that the FDA and health insurance companies will keep in mind when deciding on the usage of 5-ALA in the US.
Hauser et al55 also utilized 5-ALA and iMRI in GBM resection but did so in a reverse fashion. In this prospective study of 12 patients with histologically confirmed GBM, 5-ALA fluorescence was first used to achieve complete resection of all fluorescent tissue; accuracy was assessed with histologic samples as references. Next, after complete fluorescent resection was achieved, iMRI was performed to assess accuracy in detecting remnant tumor. A total of 117 samples were taken from 12 patients. Ninety-three samples were from fluorescent areas (25 strong, 37 moderate, and 31 weak fluorescence), and tumor infiltration was identified in 95.7% of these samples. Moderate and strong fluorescent area samples showed tumor in 100% of samples; weak fluorescence corresponded to tumor in 87.1% of samples. Postoperatively, eleven of 12 patients had residual contrast enhancement after complete resection of fluorescent tissue was achieved. From these enhancing areas, 18 of 28 biopsy samples showed solid tumor or zones of tumor infiltration. Nine of the remaining samples showed reactive brain tissue, and one showed no pathology. Sensitivity of 5-ALA for detecting tumor tissue was 81%, with a specificity of 43%; PPV was 96% and NPV was 12.5%. This study demonstrated that although 5-ALA is useful in identifying GBM with high certainty, some malignant tissue might not fluoresce.
In a large retrospective study, Schatlo et al7 studied outcome measures in 200 patients with a histologic diagnosis of HGG who either underwent resection with both 5-ALA and iMRI (n=55) or no iMRI (n=145). The non-iMRI group included 58 patients who received 5-ALA, and was confounded by less favorable KPS compared to the iMRI group (good in 95% of iMRI patients vs 73% of non-iMRI patients). The strongest predictor of GTR was noneloquent localization of the tumor (OR: 0.082, P<0.01). Most notably, 5-ALA use was the second strongest predictor of GTR (OR: 3.188, P<0.05). The median PFS in the non-MRI group was 7.0 vs 10.6 months in the 5-ALA/iMRI group, but this did not reach statistical significance (P=0.19); the extent of resection was a strong predictor of PFS (HR: 1.45, 95% CI: 1.061–1.971), but neither iMRI nor 5-ALA was a strong predictor of PFS. The median OS was 13.8 months in the non-iMRI group and 17.9 months in the 5-ALA/iMRI group (P=0.043). Although patients who received 5-ALA did have higher rates of GTR, there was no effect observed on PFS and OS in this study. This study shows that combined 5-ALA and iMRI enhance rates of GTR and that GTR improves OS.
Coburger et al13 further evaluated the advantages of 5-ALA use in conjunction with iMRI prospectively in 33 patients with GBMs. These patients were matched with a retrospective control group who underwent resection with only iMRI to assess for differences in extent of resection, PFS, and OS. Postoperative tumor volume was significantly smaller in the 5-ALA/iMRI group compared to the iMRI group alone (0.08 vs 0.7 cc, respectively, P<0.017). The mean extent of resection was 99.7% in the 5-ALA/iMRI group and 97.4% in the iMRI group (P<0.004); the minimum extent of resection in the combined group was 97% compared to 87% in the control group. This difference in mean extent of resection was more markedly pronounced in patients with tumors in eloquent locations (ie, tumors involving the motor or language system) with 5-ALA/iMRI at 99.6% vs iMRI at 96% (P<0.006). A significantly higher rate of GTR was achieved in the 5-ALA/iMRI group compared to the iMRI alone group (100% vs 82%, P<0.010). Complication rates were similar in both groups. Interestingly, median survival analysis showed no difference in PFS (6 months in both groups) or OS between the two groups (18 months in the combined group vs 17 months in the control group). Although this study did demonstrate an improvement in the extent of resection in resection guided by concurrent 5-ALA and iMRI use, this study did not demonstrate a relationship between increased extent of resection and survival.
Schucht et al56 retrospectively studied the impact of multimodal 5-ALA guidance with intraoperative brain mapping and monitoring on the rate of CRET, GTR, and incidence of new neurological deficits. Fifty-three patients were deemed eligible for CRET based on preoperative MRI (ie, exclusion criteria: tumor infiltration of eloquent areas, not unilateral, multifocal). GTR (ie, residual contrast-enhancing tumor volume ≤1 cm3) was achieved in 96% of patients (n=51). CRET (ie, no residual contrast enhancement >0.175 cm3 on postoperative MRI) was achieved in 47 of 53 patients (89%); 33 of 34 noneloquent tumors achieved CRET, while only 14 of 19 eloquent tumors (shifting or compressing vs infiltration) achieved CRET. This study demonstrates that rates of surgical success can exceed those of the 5-ALA Study Group (96% vs 65%) when using combined imaging modalities, such as intraoperative mapping.41 However, we must also remember that the 5-ALA Study Group included surgeons who were beginners of intraoperative fluorescent use and that expertise has readily developed in the years since that study.54
5-ALA use for visualization of LGGs
Recent technological advances have led to the use of intraoperative confocal microscopy for the visualization of 5-ALA fluorescence of LGGs. Intraoperative confocal microscopy adapts conventional confocal microscope technology to provide high-resolution subsurface imaging of living tissue in vivo, allowing visualization of cellular elements and cytoarchitecture.57 The technology is encased within a handheld, 190×6.6 mm, sterilizable, 135°-angled, rigid probe that displays images in real time on an attached external monitor at up to 1,000× magnification.57 A foot switch enables operator control of the focal plane position over a range of 250 μm.57 In humans, intraoperative confocal microscopy performed using intravenous fluorescein can distinguish the histological features of gliomas, meningiomas, hemangioblastomas, and central neurocytomas in vivo and ex vivo, thereby enhancing the optical resolution beyond that achieved with light microscopy.57,58 However, confocal microscopy is not familiar to most neurosurgeons and is not yet in common use.
In a study by Sanai et al,57 ten patients with WHO grade I and II gliomas underwent resection with 5-ALA. Although macroscopic tumor fluorescence was not appreciated intraoperatively, confocal microscopy identified fluorescence at the cellular level, correlating with tumor infiltration on histological analysis. Further studies were performed by Valdes et al16 to assess the applicability of 5-ALA to LGGs. In a prospective study, 12 patients with presumed LGGs received 5-ALA prior to surgery, and fluorescence was assessed qualitatively and quantitatively. Qualitative assessment (ie, the surgeon) had a diagnostic accuracy of 38.0%, while quantitative assessment showed a diagnostic accuracy of 67%. Of note, tumor specimens did demonstrate diagnostically significant levels of PpIX in areas of histologically confirmed tumor. Although this study confirmed the poor diagnostic utility of 5-ALA in LGGs, the increased levels of PpIX in LGGs may open the door to new avenues of exploration for visualization of fluorescence.
Given the paradigm shift toward molecular classifications of gliomas,8 Jaber et al59 investigated the value of 5-ALA in LGGs and HGGs lacking characteristic imaging features of GBM. In 166 tumors (82 grade II, 76 grade III, 8 grade I), fluorescence correlated with higher WHO grade (P<0.001) and Ki-67/MIB-1 index (P<0.001). Fluorescence was best predicted by contrast enhancement (P<0.01), a tumor volume of >10.6 cm3 (P=0.011), and an increased 18F-FET-PET uptake ratio (P=0.019). These findings can help determine the malignant morphology of patients preoperatively, identify which patients should get 5-ALA, and thereby ultimately facilitate both a greater extent of tumor resection and clinical outcome.
Adverse effects of 5-ALA
5-ALA is a well-tolerated and safe drug.60 To minimize the risk of adverse effects, patients administered this drug must not have a history of acute or chronic porphyria. It should also not be used in patients who are pregnant due to potential teratogenic effects. After oral administration, patients may experience gastrointestinal discomfort including nausea and diarrhea, hypotension, and photodermatosis, although these are uncommon. Two patients experienced adverse effects in one clinical trial with 71 patients receiving 5-ALA: one patient had reddening of the face and arms 11 hours after oral administration, which persisted for 2 days; the second patient developed generalized edema and required monitoring in the intensive care unit for 4 days.35 However, the most common side effect of 5-ALA administration is sensitization of the skin and was seen in several studies, albeit minor.44,60 This effect lasts for 24 hours and requires patients to avoid direct sunlight or strong room light exposure. This effect must also be addressed in the operating room, and patients should be draped for protection from overhead lights. Exposure to light can result in rubor of the skin and sunburn. As a result, other phototoxic substances such as tetracyclines, sulfonamides, fluoroquinolones, and hypericin extracts should be avoided for 24 hours.
Summary
It is apparent that 5-ALA provides benefits in aiding in resection of HGGs. Multiple studies have demonstrated that 5-ALA aids in visualization of HGGs in areas of tumor bulk, with both sensitivity and specificity as high as 100%.18,35 In addition, 5-ALA is useful in identifying areas where tumor infiltrates into normal brain parenchyma – areas that are not easily visualized using white-light microscopy alone – with sensitivity and specificity of 93% and 85%, respectively.35,36 In addition, tumors with variable fluorescence have been found to have an inverse relationship with tumor cell density, thereby allowing strong fluorescence to serve as a surrogate for aggressive tumor behavior. Visualization has the obvious added benefit of increased extent of resection, which has been found time and time again to provide for improved clinical course, including OS and PFS. Most studies to date show an increase in OS as much as 9.5 months (range: 4–9.5 months) when CRET is achieved with 5-ALA compared to incomplete resection of enhancing tissue,11,19,41,43 and a 1.5-month increase in PFS with 5-ALA use compared to white light.41 This provides significant benefit in the context of a universally fatal disease. Moreover, the ability to identify areas of infiltration with 5-ALA that would otherwise be unnoticed helps overcome a major obstacle the neurosurgeon faces when resecting HGGs, especially those without iMRI to assist. It is apparent that 5-ALA provides safer resection margins in eloquent areas, allowing one to maximally decrease tumor burden with preservation of brain function. Other complementary imaging modalities, including iMRI and brain mapping, have allowed for even greater extent of resections and have also improved clinical course in these patients. However, these additional imaging techniques are much more costly compared to 5-ALA alone, and their cost–benefit ratio requires further investigation. There is still much to learn about the use of 5-ALA in LGGs; however, the advent of confocal microscopy may provide a new avenue for improving survival in this subset of patients as well.
Future directions
5-ALA provides added benefit in visualizing HGGs and aiding with maximal resections of these infiltrative tumors. The benefits have been demonstrated many times and have been shown to improve OS in a terminal disease. Many studies are starting to explore a combined approach with other modalities for visualization and improving the resection of these tumors. For example, the use of iMRI and 5-ALA has proven synergistic. Moving forward, combined imaging modalities will certainly pave the way for improved clinical outcomes in patients with HGGs. One weakness of 5-ALA is its applicability to LGGs and metastatic disease, but studies in these areas are lacking. Future directions for LGGs may include visualizing lower concentrations of PpIX with alternate light sources to help identify tumor borders and creating a more promising outcome in this subset of patients as well. There are studies being performed currently to better target tumor cells with fluoroprobes attached to proteins. For example, conjugation of EGF to 5-ALA can target EGFR + gliomas, ultimately improving their visualization and resection (Figure 4). These will not only allow for improved identification of tumor cells, but will also extend the life of intraoperative tumor fluorescence.
Disclosure
The authors report no conflicts of interest in this work.
References
Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. | ||
DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. | ||
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. | ||
Babu R, Adamson DC. Fluorescence-guided malignant glioma resections. Curr Drug Discov Technol. 2012;9(4):256–267. | ||
Smoll NR, Schaller K, Gautschi OP. Long-term survival of patients with glioblastoma multiforme (GBM). J Clin Neurosci. 2013;20(5):670–675. | ||
Smoll NR, Hamilton B. Incidence and relative survival of anaplastic astrocytomas. Neuro Oncol. 2014;16(10):1400–1407. | ||
Schatlo B, Fandino J, Smoll NR, et al. Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro Oncol. 2015;17(12):1560–1567. | ||
Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. | ||
Halani SH, Brat DJ. Defining neoplastic diseases differently: an emerging paradigm from The Cancer Genome Atlas lower-grade gliomas project. Mol Cell Oncol. 2016;3(2):e1074333. | ||
Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77(5):663–673. | ||
Aldave G, Tejada S, Pay E, et al. Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery. 2013;72(6):915–920; discussion 920–911. | ||
Coburger J, Engelke J, Scheuerle A, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus. 2014;36(2):E3. | ||
Coburger J, Hagel V, Wirtz CR, Konig R. Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS One. 2015;10(6):e0131872. | ||
Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. | ||
Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. | ||
Valdes PA, Jacobs V, Harris BT, et al. Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg. 2015;123(3):771–780. | ||
Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev. 2014;1:Cd009685. | ||
Stummer W, Stocker S, Wagner S, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42(3):518–526. | ||
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003–1013. | ||
Zhao S, Wu J, Wang C, et al. Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS One. 2013;8(5):e63682. | ||
Stummer W, Stocker S, Novotny A, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B. 1998;45(2–3):160–169. | ||
Eljamel MS. Fluorescence image-guided surgery of brain tumors: explained step-by-step. Photodiagnosis Photodyn Ther. 2008;5(4):260–263. | ||
Ennis SR, Novotny A, Xiang J, et al. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959(2):226–234. | ||
Zhao SG, Chen XF, Wang LG, et al. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol. 2013;20(13):4379–4388. | ||
Stummer W, Reulen HJ, Novotny A, Stepp H, Tonn JC. Fluorescence-guided resections of malignant gliomas – an overview. Acta Neurochir Suppl. 2003;88:9–12. | ||
Inoue K, Karashima T, Kamada M, et al. Regulation of 5-aminolevulinic acid-mediated protoporphyrin IX accumulation in human urothelial carcinomas. Pathobiology. 2009;76(6):303–314. | ||
Xia Y, Huang Y, Lin L, Liu X, Jiang S, Xiong L. A comparative study on the enhancement efficacy of specific and non-specific iron chelators for protoporphyrin IX production and photosensitization in HaCat cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29(6):765–770. | ||
Otake M, Nishiwaki M, Kobayashi Y, et al. Selective accumulation of ALA-induced PpIX and photodynamic effect in chemically induced hepatocellular carcinoma. Br J Cancer. 2003;89(4):730–736. | ||
EuropeanMedicinesAgency. Gliolan; 5-Aminolevulinic Acid Hydrochloride. Churchill, London: EuropeanMedicinesAgency; 2014. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000744/human_med_000807.jsp&mid=WC0b01ac058001d124. Accessed May 24, 2016. | ||
Awad AJ, Sloan A. The use of 5-ALA in glioblastoma resection: two cases with long-term progression-free survival. Cureus. 2014;6(9):e202. | ||
Nabavi A, Thurm H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas. Neurosurgery. 2009;65(6):1070–1077. | ||
Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, Aristu Mendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2010;102(1):105–113. | ||
Panciani PP, Fontanella M, Garbossa D, Agnoletti A, Ducati A, Lanotte M. 5-aminolevulinic acid and neuronavigation in high-grade glioma surgery: results of a combined approach. Neurocirugia (Astur). 2012;23(1):23–28. | ||
Kamp MA, Grosser P, Felsberg J, et al. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien). 2012;154(2):223–228; discussion 228. | ||
Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly. 2008;138(11–12):180–185. | ||
Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y. Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clinical Neurology Neurosurg. 2015;130:134–139. | ||
Lau D, Hervey-Jumper SL, Chang S, et al. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg. 2016;124(5):1300–1309. | ||
Utsuki S, Oka H, Sato S, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo). 2007;47(5):210–213; discussion 213–214. | ||
Filbeck T, Roessler W, Knuechel R, Straub M, Kiel HJ, Wieland WF. 5-aminolevulinic acid-induced fluorescence endoscopy applied at secondary transurethral resection after conventional resection of primary superficial bladder tumors. Urology. 1999;53(1):77–81. | ||
Kamp MA, Felsberg J, Sadat H, et al. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir. 2015;157(2):207–213; discussion 213–204. | ||
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre Phase III trial. Lancet Oncol. 2006;7(5):392–401. | ||
Vogelbaum MA, Jost S, Aghi MK, et al. application of novel response/progression measures for surgically delivered therapies for gliomas: response assessment in neuro-oncology (RANO) working group. Neurosurgery. 2012;70(1):234–243; discussion 243–234. | ||
Stummer W, Tonn JC, Mehdorn HM, et al. Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J Neurosurg. 2011;114(3):613–623. | ||
Cordova JS, Gurbani SS, Holder CA, et al. Semi-automated volumetric and morphological assessment of glioblastoma resection with fluorescence-guided surgery. Mol Imaging Biol. 2016;18(3):454–462. | ||
Pichler W, Maier A, Rappl T, Clement HG, Grechenig W. Delayed hypopharyngeal and esophageal perforation after anterior spinal fusion: primary repair reinforced by pedicled pectoralis major flap. Spine (Phila Pa 1976). 2006;31(9):E268–E270. | ||
Stummer W, Nestler U, Stockhammer F, et al. Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J Neurooncol. 2011;103(2):361–370. | ||
Roberts DW, Valdes PA, Harris BT, et al. Glioblastoma multiforme treatment with clinical trials for surgical resection (aminolevulinic acid). Neurosurg Clin N Am. 2012;23(3):371–377. | ||
Eljamel MS, Goodman C, Moseley H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med Sci. 2008;23(4):361–367. | ||
Eljamel MS. Which intracranial lesions would be suitable for 5-aminolevulenic acid-induced fluorescence-guided identification, localization, or resection? A prospective study of 114 consecutive intracranial lesions. Clin Neurosurg. 2009;56:93–97. | ||
Della Puppa A, Pellegrin S, d’Avella E, et al. 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir. 2013;155(6):965–972. | ||
Schucht P, Seidel K, Beck J, et al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014;37(6):E16. | ||
Tsugu A, Ishizaka H, Mizokami Y, et al. Impact of the combination of 5-aminolevulinic acid–induced fluorescence with intraoperative magnetic resonance imaging–guided surgery for glioma. World Neurosurg. 2011;76(1–2):120–127. | ||
Eyupoglu IY, Hore N, Savaskan NE, et al. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One. 2012;7(9):e44885. | ||
Stummer W. Commentary: combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery. 2016;78(4):484–486. | ||
Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays RL. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery. 2016;78(4):475–483. | ||
Schucht P, Beck J, Abu-Isa J, et al. Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery. 2012;71(5):927–935; discussion 935–926. | ||
Sanai N, Snyder LA, Honea NJ, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115(4):740–748. | ||
Sanai N, Eschbacher J, Hattendorf G, et al. Intraoperative confocal microscopy for brain tumors: a feasibility analysis in humans. Neurosurgery. 2011;68:ons282–ons290. | ||
Jaber M, Wolfer J, Ewelt C, et al. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery. 2016;78(3):401–411. | ||
Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008;55:20–26. | ||
Stummer W, Tonn JC, Goetz C, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310–319; discussion 319–320. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.