Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Study of Human Coronavirus NL63, OC43, 229E, HKU1 Infentions in Hospitalized Children from 2015 to 2020
Authors Wen C, Sun L, Zhao MC, Duan SX, Wang L, Cui XW
Received 5 January 2022
Accepted for publication 22 February 2022
Published 16 March 2022 Volume 2022:15 Pages 1093—1101
DOI https://doi.org/10.2147/IDR.S357193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Chan Wen,1,* Ling Sun,1,* Meng-Chuan Zhao,2 Su-Xia Duan,2 Le Wang,2 Xiao-Wei Cui3
1Department of Medical, Children’s Hospital of Hebei Province, Shijiazhuang, 050031, People’s Republic of China; 2Department of Laboratory Medicine, Children’s Hospital of Hebei Province, Shijiazhuang, 050031, People’s Republic of China; 3Children’s Hospital of Hebei Province, Shijiazhuang, 050031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Wei Cui, Children’s Hospital of Hebei Province, No. 133 of Jianhua Street, Yuhua District, Shijiazhuang, 050031, People’s Republic of China, Tel +86 18503292016, Fax +86 031185911109, Email [email protected]
Objective: This study aims to analyze the clinical characteristics of hospitalized children infected with HCoV-NL63, OC43, 229E, HKU1 and provide the basis for disease diagnosis and treatment.
Methods: A retrospective analysis was conducted on clinical manifestations, imaging data, and treatment measures of hospitalized children with positive HCoV-NL63, OC43, 229E, HKU1 from 2015 to 2020.
Results: A total of 1062 children aged 33 days to 12 years were analyzed, including 879 (82.77%) between 33 days to three years. Lower respiratory tract infections were the most common in 698 children positive for HCoVs (65.72%). The incidences of runny nose, cough, pharyngeal hyperemia, and fine crackles in the mild case group (n = 894, 84.18%) were significantly higher than in the severe case group, and the differences were statistically significant (P < 0.01). The incidences of gasp, stridor, and convulsions, the proportion of underlying diseases, such as congenital heart disease, laryngomalacia, and general developmental disorders, anemia, and abnormal liver function, and mixed infections in the severe group (n = 168, 15.82%) were significantly higher than in the mild group, and the differences were statistically significant (P < 0.01 or P < 0.05). Imaging manifestations differed. Pleural effusion and atelectasis occurred in the severe cases. After treatment, patients fully recovered or improved and were discharged from the hospital. There were no deaths.
Conclusion: HCoV-NL63, OC43, 229E, HKU1 infection is most common in children under three years old, and the infection site is mainly the lower respiratory tract. The main clinical manifestations include fever, cough, and runny nose. Inspiratory three concave signs, respiratory failure, and heart failure occurred in the severe cases, with pleural effusion and atelectasis possibly occurring at the same time. Severe cases should be identified early so that they may be given comprehensive treatment in time to improve the prognosis.
Keywords: coronavirus, children, clinical characteristics, infection, respiratory tract
Introduction
Respiratory viral infections in children represent a significant public health problem. Human coronaviruses (HCoVs) is a common respiratory virus, which can cause worldwide spread, particularly in children. Coronaviruses (CoVs) is a zoonotic single-stranded RNA virus. There are four common coronaviruses: HCoV-NL63, HCoV-OC43, HCoV-229E, and HCoV -HKU1. A Chinese study showed that the positive rate of these four HCoVs infections was 4.3% in hospitalized children with acute respiratory tract infection.1 Another prospective study showed that the positive rate of HCoVs accounted for 4% of hospitalized children with respiratory tract infections.2 HCoV -NL63, OC43, 229E, HKU1 infections mainly cause respiratory tract infection symptoms which may be accompanied by digestive and nervous system impairments. Clinical manifestations are diverse, ranging from the common cold to lower respiratory tract infections (such as bronchitis and pneumonia) and even more serious diseases, such as severe acute respiratory distress syndrome and multiple organ failure. Although these four HCoVs infection is widespread worldwide, clinical characteristics vary in different regions and populations. So far, There is a paucity of evidence on the clinical manifestations of HCoVs.
HCoVs has a high rate of genetic variation, and variant viruses can spread from animals to human beings, e.g. the severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003, the Middle East respiratory syndrome (MERS) in 2012, and the 2019 novel coronavirus (2019-nCoV) which is causing epidemics in multiple countries around the world. Compared with adults, SARS-CoV, MERS-CoV, and 2019-nCoV are not common in children, and most children infected were exposed through family gatherings. The symptoms and severity of infected children are mild, and the fatality rate is low. Therefore, our understanding of coronavirus infection in children is still limited, and further research is required on the epidemic and clinical characteristics of coronavirus infection in children.
To expand the existing evidence base and provide new insights into the clinical manifestations of HCoVs, We performed this study in which children infected with four common human coronaviruses (HCoV-NL63, HCoV-OC43, HCoV-229E and HCoV-HKU1) were chosen as the subjects. The infection sites of the children infected with common HCoVs who were hospitalized at the Children’s Hospital of Hebei Province from 2015 to 2020, their department distribution features, clinical features, imaging findings, and clinical treatment measures are summarized to provide the basis for treatment and prevention of children infected with HCoVs.
Subjects and Methods
Subjects
A total of 1062 children under 18 years old who were infected with HCoVs and hospitalized at the Children’s Hospital of Hebei Province from January 2015 to December 2020 were retrospectively study. Inclusion criteria: during hospitalization, secretions were collected from the respiratory tract and tested for 13 respiratory pathogens (including NL63, OC43, 229E, and HKU1). The children with a positive HCoV test result were included in this study. The Ethics Committee of the Children’s Hospital of Hebei Province approved this study (YYLS No. 204).
Methods
Etiological Test
Thirteen respiratory pathogens were tested by multiplex polymerase chain reaction (PCR). The nucleic acid extraction reagent provided by Ningbo Health Gene Technologies Co., Ltd. was used for automatic nucleic acid extraction from the specimens through the automatic nucleic acid extractor Smart LabAssist-32 (Taiwan Advanced Nanotech Inc.). The operation strictly followed the instrument operation manual and kit instructions. Then, the multiplex detection kit for 13 respiratory pathogens (Ningbo Health Gene Technologies Co., Ltd.) was combined with the GeXP multiplex gene expression analysis system (Beckman Coulter, Inc.) for the test.
The 13 respiratory pathogens kit monitors the quality of the samples by simultaneously detecting human RNA and human DNA in samples. The internal control was added into the samples for nucleic acid extraction, so the RT-PCR and electrophoresis process could be monitored. Each experiment requires the use of the negative and positive controls at the same time with clinical samples. If 1) the fluorescence peaks of human DNA and internal controls appear in the peak profile of the negative control result, 2) the peak profiles of 13 respiratory pathogens, human RNA, human DNA and internal controls appear in the peak profile of the positive control result, the experiment is proved to be under control.
Clinical Data Collection
The “Case Data Collection Form” was developed, and the children’s information was registered in the unified standard case data collection form to establish the case database. Clinical information were collected and analyzed by a pediatrician with the following variables: age, sex, clinical diagnosis, underlying disease, Clinical manifestations, vital signs, complication, chest computed tomography (CT), etiological tests results, results of laboratory tests (liver function and myocardial enzyme detection), length of hospital stay, treatment measures, and disease outcomes. According to the clinical manifestations and Guidelines for the Management of Community-Acquired Pneumonia in Children (2013 Revision), children with HCoVs infections were divided into two groups: a mild case group and a severe case group. The cases with one of the following clinical manifestations are considered severe cases: inspiratory depression of the chest wall, nasal ala flap, moaning, central cyanosis, respiratory distress, anorexia or dehydration, disturbance of consciousness (lethargy, coma).3 The cases without the above clinical manifestations are mild cases. The clinical manifestations, signs, underlying diseases, and complications of the two groups were collected and analyzed, respectively.
According to Practice of Pediatrics (8th Edition), upper respiratory tract infection refers to inflammation of the nasal cavity, pharynx, or larynx. Lower respiratory tract infection refers to bronchitis, bronchiolitis, pneumonia, and so on.4 Patients presenting with cough, rhinobyon, rhinorrhea, sneeze, pharyngeal discomfort, or trachyphonia were classified as having upper respiratory tract infection. Patients with bronchopneumonia, pneumonia, asthma, shortness of breath, chest tightness, chest pain, or abnormal pulmonary rales were classified as having lower respiratory tract infection. Bronchopneumonia and pneumonia were diagnosed with chest radiography. Other clinical symptoms were identified by common medical examinations and clinical descriptions. Complication refers to the occurrence of another disease or symptom caused by one disease in the course of its development, the latter being the complication of the former. The elevated myocardial enzymes and liver enzymes are considered as myocardial damage and abnormal liver function.
Statistical Analysis
Statistical software SPSS 25.0 was used for analysis. Enumeration data were described with the number of cases and percentage (%), and the χ2 test was used for the intergroup comparison. P < 0.05 was considered statistically significant.
Results
General Data
Among the 1062 children with HCoVs infections, there were 662 boys (62.34%) and 400 girls (37.66%), with a boy-to-girl ratio of about 1.6:1. In the study group, the youngest infected child was 33 days old, and the oldest infected child was 12 years old. There were 879 cases aged 33 days to three years (82.77%), 117 cases aged three to five years (11.02%), and 66 cases aged above five years (6.21%).
Analysis of HCoVs Infection Sites in Children
Lower respiratory tract infections were the most common infection sites in children, totaling 698 cases (65.72%), including 164 cases (15.44%) of lower respiratory tract and digestive tract infections simultaneously, followed by upper respiratory infections, totaling 350 cases (32.96%), including 45 cases (4.24%) having upper respiratory and digestive tract infections at the same time. The proportion of digestive tract or nervous system infections alone was relatively small, 0.75% and 0.57%, respectively. See Table 1.
 |
Table 1 Proportion of Infection Sites in Children with HCoVs Infection |
Distribution of Inpatient Departments for Children with HCoVs Infections
The highest detection rate of children with HCoVs infections was found in the Pneumology Department (n = 872, 82.11%), followed by the Critical Care Medicine Department (n = 168, 15.82%), the Neurology Department (n = 9, 0.85%), the Gastroenterology Department (n = 8, 0.75%), the Pediatric Surgery Department (n = 3, 0.28%), and the Hematology Department (n = 2, 0.19%). See Table 2.
 |
Table 2 Distribution of Inpatient Departments for Children with HCoVs Infection |
Clinical Manifestations
The clinical manifestations of HCoVs infection were diversified. The hospitalized children were primarily clinically diagnosed with bronchitis, bronchopneumonia, bronchiolitis, and severe pneumonia. The main clinical symptoms were fever, cough, and runny nose, with severe cases having shortness of breath and respiratory distress, combined with respiratory and heart failure. Among the 1062 cases, there were 894 mild cases (84.18%) and 168 severe cases (15.82%). The clinical manifestations of the mild cases primarily included fever, cough, and runny nose, and the clinical signs were pharyngeal hyperemia, antiadoncus, coarse breath sounds, and cough with fine crackles. The clinical manifestations of the severe cases primarily included fever, cough, gasp, shortness of breath, and respiratory distress, frequently combined with respiratory and heart failure. The clinical signs were antiadoncus, stridor, and inspiratory three concave signs. The incidence of runny nose, cough, pharyngeal hyperemia, and fine crackles in the mild case group was significantly higher than in the severe case group (P < 0.01), and the difference was statistically significant. The incidence of gasp and stridor in the severe case group was significantly higher than in the mild case group (P < 0.01), and the incidence of convulsions in the severe case group was higher than in the mild case group (P < 0.05), with statistically significant differences. See Table 3.
 |
Table 3 Comparison of Clinical Features Between Mild and Severe Case Groups of HCoVs Infection in Children |
Underlying Diseases
Among the 1062 children, 124 cases (11.68%) had underlying conditions, including congenital heart disease, general developmental disorders, and laryngomalacia. Of the underlying diseases, the proportion of congenital heart disease in the severe case group was significantly higher than in the mild case group (P < 0.01), and the proportion of general developmental disorders and laryngomalacia was also higher than in the mild case group (P < 0.05), with statistically significant differences. See Table 3.
Complications
Among the 1062 children, 437 cases (41.15%) had complications. The mild cases mainly had complications such as diarrhea, anemia, laryngitis, and abnormal liver function. In addition to the above complications, the severe cases also had myocardial damage, respiratory failure, and heart failure. Also, the proportion of abnormal liver function in the severe case group was significantly higher than in the mild case group (P < 0.01), and the difference was statistically significant. See Table 3.
Chest Imaging Findings
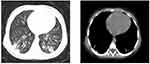
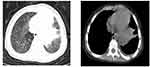
All hospitalized children underwent chest imaging examinations. Due to mild systemic symptoms, the mild cases underwent X-ray examinations, and some received lung Computed Tomography (CT) examinations. All severe patients underwent lung CT examinations. The X-ray findings of the 894 mild cases were mainly an increase of lung markings, and patchy opacities appeared in 233 cases (26.06%). The CT imaging findings of the mild cases showed lesions occurred in the inner zone of the lungs, presenting as dots or fuzzy spot opacities, with blurred margins and thickened lung markings. Generally, there were no lung abscesses or pleural changes, as shown in Figure 1. The CT imaging findings of the 168 severe cases showed pleural effusion on one or both sides in 120 cases (71.43%), atelectasis in 23 (13.69%), ground-glass opacity in the lung field in 15 (8.93%), and pulmonary consolidation in 10 cases (5.95%). See Figure 2.
Clinical Treatment
The children were evaluated and treated comprehensively according to clinical symptoms and signs. The mild cases were given general symptomatic treatment for 3–7 d, including expelling phlegm, relieving cough, and antivirals by interferon atomization. The severe cases were given electrocardiogram (ECG) monitoring and blood saturation monitoring in time, active prevention of complications, treatment of underlying diseases, prevention of secondary infections, and timely organ function support. The active respiratory support strategy is essential for the prognosis of severe cases. It is required to closely observe the patient’s respiration and oxygen saturation. In cases of oxyhemoglobin saturation (SpO2) ≤92% without respiratory distress, oxygen therapy was given. If there was no response and SpO2 continued to drop, noninvasive continuous positive pressure ventilation was used to assist breathing. If necessary, invasive mechanical ventilation was adopted. In addition, various auxiliary examinations were performed for severe patients in time, including routine blood examinations, CRP, arterial blood gas analyses, electrolytes, liver and kidney functions, myocardial enzymes, and blood cultures, with vital signs monitored. Meanwhile, adequate fluid and energy supplies were maintained. According to the examination results and the severity of the disease, personalized treatment plans were developed. Children with bacterial infections were treated with antibiotics. Nutritional myocardial therapy was given to patients with myocardial damage. Those with heart failure were combined with cardioside positive inotropic drugs, diuresis, a vasodilation treatment.
Outcomes
The average hospital stay of the mild cases was 7 days, and all were cured or improved and discharged from the hospital. The average hospital stay of the severe cases was 15 days. After comprehensive treatment, all were cured or improved and discharged from the hospital. There were no deaths.
Discussion
With the development of medical science, HCoVs detection technology has been widely used in clinics. Epidemiological and clinical studies on infection of the four common strains (OC43, NL63, HKU1, and 229E) are increasing. However, there are few clinical studies on HCoVs infections in children with a large sample size. Especially since the outbreak of COVID-19 in 2019, more attention has been paid worldwide to novel coronavirus infections. But the incidence rate of infection in children is lower than in adults, and there are fewer studies on infections in children. Therefore, in the context of COVID-19, studying and analyzing children infected with HCoVs is very important for understanding the characteristics of infection in children and studying the variations of coronavirus and the prevention, control, diagnosis, and treatment of infection. This retrospective study analyzed data from 1062 hospitalized children (including 168 severe cases) with HCoV-NL63, OC43, 229E, HKU1 infection over a six-year period in Hebei, China. Given the study’s duration and large sample size, our results represent an important and additional evidence base on the clinical characteristics of HCoVs infection, which will provide new insights for clinical hospitalization, identification and treatment of severe cases.
HCoVs infection occurs in adults, children, and neonates. Children of all ages, especially infants under one year old, are susceptible.5 This study showed that children infected with human coronavirus were primarily under three years old, accounting for 82.76%, and 53.48% of them were under one year old. This result is consistent with previous studies. Infants and young children should be the key population for prevention and control of HCoVs infection. Infants are more likely to be hospitalized after infection, which should be focused on. The common human coronaviruses (HCoV-OC43, 229E, NL63, HKU1) primarily manifest as respiratory tract infections, which may be related to the imperfect development of non-specific and specific immune functions in the respiratory systems of infants and young children. HCoVs infections may be accompanied by gastrointestinal symptoms. It has been found that among patients with acute upper respiratory tract infections accompanied by gastrointestinal symptoms, those with HCoVs infections account for 11.6%.6 Four types of HCoVs can be detected in the feces of children with HCoVs infections accompanied by diarrhea.7 HCoVs can also cause damage to the nervous system, leading to acute disseminated encephalomyelitis, encephalitis, epilepsy, etc.8 This study showed that most hospitalized children with HCoVs infections had lower respiratory tract infections, and 19.68% had symptoms of digestive tract infections, such as diarrhea, besides respiratory tract infections, and the incidence of pure digestive and nervous system infections is low. These results suggest that other systemic symptoms should be paid attention to during the diagnosis and treatment of HCoVs infectious children, and comprehensive treatment should be given to promote the recovery of patients. HCoVs infection is usually manifested as a mild respiratory system infection. Children with immune suppression can also lead to severe infections.9,10 In this study, 82.11% of children were admitted to the Pneumology Department, while those with severe respiratory infections (15.82%) were admitted to the Critical Care Medicine Department. The Neurology (n = 9), Gastroenterology (n = 8), Hematology (n = 2) and Pediatric Surgery Departments (n= 3) also had sporadic HCoVs infection cases, indicating that HCoVs infection is mainly a respiratory system infection and easily occurs in young children, postoperative patients, and those with low immune functions. The possibility of HCoVs infection should be considered and timely diagnosis should be made when patients presenting with respiratory symptoms, digestive symptoms or neurological symptoms simultaneously, which will promote correct clinical triage and timely treatment.
Respiratory tract infection symptoms caused by HCoVs are mainly cough, fever, and shortness of breath, and the clinical symptoms are diversified, ranging from rhinitis, laryngitis, and upper respiratory tract infections to bronchitis, pneumonia, and severe pneumonia.1 In this study, the clinical symptoms of children with HCoVs were cough, fever, runny nose, shortness of breath, and gasp. This result is consistent with previous research. Severe cases had inspiratory three concave signs, respiratory failure, and heart failure.11,12 It is worth noting that in this study, 44% of patients had no fever, so missed diagnosis should be prevented. Some studies have also shown that HCoVs infection is prone to be complicated with digestive tract symptoms. In this study, it was found that the incidence of diarrhea in children was 12.43%. Patients with positive HCoVs may develop severe respiratory tract infections, and some severe cases require intensive care treatment in the PICU.13 In this study, the proportion of children with HCoVs infections with severe pneumonia was 15.82%. The main manifestations of the mild cases were cough, runny nose, pharyngeal hyperemia, and fine crackles, and the main manifestations of the severe cases included gasp, inspiratory three concave signs, and stridor, indicating that with the aggravation of the disease, children have anoxia manifestations such as rapid breathing, gasp, and inspiratory three concave signs, which should be taken seriously. It has been reported that immune deficiency status, young age, potential lung disease, and respiratory syncytial virus infections in children with HCoVs infections were associated with severe lower respiratory tract infections.10 In this study, among the children with HCoVs infections, 124 cases had underlying conditions, including laryngomalacia, general developmental disorders, and congenital heart disease. The proportion of congenital heart disease in the severe cases was significantly higher than in the mild cases. Among the children with positive HCoVs, 280 cases had mixed infections with other respiratory pathogens, and the proportion of mixed infections in the severe cases was significantly higher than in the mild cases. This reveals that children with congenital heart disease have poor immunity and are prone to develop into severe cases after HCoVs infections. Besides, those with HCoVs infections and other pathogens can easily develop into severe cases. Severe cases are more likely to suffer complications. In this study, the incidence of anemia, liver dysfunction, myocardial damage, respiratory failure and heart failure in severe cases was significantly higher than that in mild cases, which should be taken seriously. Pleural effusion and atelectasis may occur in severe cases, even causing respiratory and heart failure, The chest imaging manifestations of children with HCoVs with lower respiratory tract infections are difficult to distinguish from other respiratory virus infections.14 In mild cases, lung X-ray manifestations were mild, and chest CT imaging showed segmental or large lamellar dense opacities; the lesions occurred in the inner zone of the lung, presenting as dots or fuzzy spot opacities, with blurred margins and thickened lung markings. Generally, there were no lung abscesses, pleural changes, or pleural effusions. The results are consistent with previous research.15 In severe cases, pleural effusion was more common in CT imaging findings, followed by atelectasis, partial lung consolidation, and ground-glass opacity in the lung field. Once severe lung imaging manifestations occur, they should be treated in time.
In terms of clinical treatment, the clinical symptoms of HCoVs infection are similar. There is no specific medicine at present, and the primary treatment method is symptomatic and supportive treatment. The main drug recommended for antiviral therapy for children is α-interferon.16 The interferon can suppress the spread of the virus, play an immunomodulatory role, promote phagocytosis of macrophages for antigens, and directly influence whether the virus can survive in the host body. Mild cases have a good prognosis, and most severe cases have severe pneumonia. No timely treatment or improper treatment measures may cause damage to other organ functions, respiratory failure, heart failure, etc, directly affecting the prognosis. Thus, a comprehensive evaluation is required for severe cases. In this study, the incidence rate of severe pneumonia in hospitalized children with HCoVs infections was 15.82%. All severe cases were complicated with other organ dysfunctions and even had respiratory and heart failure, resulting in treatment difficulties and an increase in mortality. Therefore, it is vital to identify severe cases early and give active treatment. It is necessary to closely monitor the changes in children’s conditions and vital signs and focus on monitoring oxygen saturation, early detection of hypoxia, and early identification of severe and critical cases. Respiratory support should be given actively according to the examination situation. If the condition is aggravated and blood oxygen saturation continues to decline, a noninvasive ventilator should be provided to assist ventilation. For critical cases and those with refractory hypoxemia, timely tracheal intubation and mechanical ventilation should be used for auxiliary treatment. Furthermore, corresponding antibacterial drugs should be applied according to the etiological detection results in time. For patients with myocardial damage, cardiac nutrition therapy should be applied; for patients with heart failure, cardiac strengthening, diuresis and vasodilation should be combined, and traditional Chinese medicine should be combined. After comprehensive treatment in this study, patients fully recovered or improved and were discharged from the hospital. There were no deaths.
In conclusion, through summarizing and analyzing the clinical characteristics of 1062 children with HCoVs infections, the clinical features, imaging characteristics, and treatment measures of children with acute respiratory tract infections caused by HCoVs in Hebei were preliminarily grasped in this study. The four strains of HCoVs infections are more common in children, with various clinical symptoms ranging from common cold-like symptoms to flu-like symptoms, bronchitis, pneumonia and even severe pneumonia. Most cases are mild and have a good prognosis. When severe symptoms occur, they should be transferred to ICU for comprehensive treatment in time. This study emphasizes that HCoVs infection is an important pathogen of respiratory tract infection in children in Hebei, China, and summarizes relevant experience in clinical practice, which will provide a basis for clinical diagnosis, treatment, monitoring, prevention and control of HCoVs infection for pediatricians and public health departments. Moreover, human coronaviruses are variable, and the differences in clinical manifestations of children with different types of HCoVs infections are not covered in this study. Hence, further investigation and exploration are required.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Children’s Hospital of Hebei Province (YYLS No. 204). Participants under the age of 16 have obtained written informed consent from their parents.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
This study was funded by the Hebei medical science research project (No.20210211).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Zeng ZQ, Chen DH, Tan WP, et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37(2):363–369. doi:10.1007/s10096-017-3144-z
2. Calvo C, Alcolea S, Casas I, et al. A 14-year prospective study of human coronavirus infections in hospitalized children: comparison with other respiratory viruses. Pediatr Infect Dis J. 2020;39(8):653–657. doi:10.1097/INF.0000000000002760
3. Li CC, Shang YX, Shen XZ, Chen ZM, Zhao SY. Guidelines for the management of community-acquired pneumonia in children (revised in 2013) (Part I). Chin J Pediatr. 2013;51(10):745–752. Chinese.
4. Jiang ZF, Shen KL. Pediatrics [M].
5. Ottogalli ME, Rodríguez PE, Frutos MC, et al. Circulation of human coronaviruses OC43 and 229E in Córdoba, Argentina. Arch Virol. 2021;166(3):929–933. doi:10.1007/s00705-020-04914-x
6. Minodier L, Masse S, Capai L, et al. Clinical and virological factors associated with gastrointestinal symptoms in patients with acute respiratory infection: a two-year prospective study in general practice medicine. BMC Infect Dis. 2017;17(1):729. doi:10.1186/s12879-017-2823-9
7. Varghese L, Zachariah P, Vargas C, et al. Epidemiology and clinical features of human coronaviruses in the pediatric population. J Pediatric Infect Dis Soc. 2018;7(2):151–158. doi:10.1093/jpids/pix027
8. Singer TG, Evankovich KD, Fisher K, Demmler-Harrison GJ, Risen SR. Coronavirus infections in the nervous system of children: a scoping review making the case for long-term neurodevelopmental surveillance. Pediatr Neurol. 2021;117:47–63. doi:10.1016/j.pediatrneurol.2021.01.007
9. Chang TY, Du CJ, Chang CC, et al. Human coronavirus OC43 infection associated pneumonia in a girl with acute lymphoblastic leukemia: a case report. Medicine. 2020;99(33):e21520.
10. Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of coronavirus infection in children: the role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019;8(1):21–28. doi:10.1093/jpids/pix093
11. Haddadin Z, Chappell J, McHenry R, et al. Coronavirus surveillance in a pediatric population in Jordan from 2010 to 2013: a Prospective Viral Surveillance Study. Pediatr Infect Dis J. 2021;40(1):e12–e17.
12. Zhang Y, Su L, Chen Y, et al. Etiology and clinical characteristics of SARS-CoV-2 and other human coronaviruses among children in Zhejiang Province, China 2017–2019. Virol J. 2021;18(1):89. doi:10.1186/s12985-021-01562-8
13. Trombetta H, Faggion HZ, Leotte J, Nogueira MB, Vidal LR, Raboni SM. Human coronavirus and severe acute respiratory infection in Southern Brazil. Pathog Glob Health. 2016;110(3):113–118. doi:10.1080/20477724.2016.1181294
14. Shan SQ, Xia KJ, Gan DM, Hua LL, Huang J. Prevalence of human coronavirus infection in children with acute lower respiratory tract infection. Chin J Nosocomiol. 2017;27(12):2813–2816. Chinese.
15. Wang JJ, Xie ZD. Research progress in β human coronavirus infection. Chin J Exp Clin Virol. 2021;35(01):22–27. Chinese.
16. Jiang Y, Lu XX, Jin RM, et al. Diagnosis, treatment and prevention of 2019 novel coronavirus in children: experts′ consensus statement (Second Edition). Chin J Appl Clin Pediatr. 2020;35(2):143–150. Chinese.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.