Back to Journals » Cancer Management and Research » Volume 11
Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis
Authors Zhang Y, Xiao G, Wang R
Received 8 October 2018
Accepted for publication 1 March 2019
Published 7 May 2019 Volume 2019:11 Pages 4185—4200
DOI https://doi.org/10.2147/CMAR.S190006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Yi Zhang, Guoliang Xiao, Rong Wang
Department of General Surgery, the First People‘s Hospital of Neijiang, Neijiang, Sichuan 641000, People’s Republic of China
Background: Numerous studies have reported that systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) correlate with tumor progression and prognosis in various types of human cancer. The aim of this study is to systematically investigate the clinical significance of SII and CAR in esophageal cancer (EC).
Methods: We searched a number of databases for articles reporting the effect of pretreatment SII and CAR on the survival of EC patients. Review Manager 5.3 and STATA/SE 14.1 were applied in this meta-analysis. The multivariable-adjusted hazard ratio (HR) was used for calculating the relationship between SII and CAR and overall survival (OS), and the odds ratio (OR) was applied for the clinical pathology.
Results: Five original studies for SII and seven original datasets for CAR were included for analysis. Increased SII showed a significant association with shorter OS in EC patients after surgery (HR: 1.34, 95% CI: 1.15–1.53, P<0.001) and high CAR indicated worse long-term OS in EC (HR: 1.60, 95% CI: 1.29–1.90, P<0.001). Different subgroup analyses were also confirmed the prognostic roles in EC patients. Furthermore, the adverse impacts of elevated SII and CAR on tumor progression were revealed in the infiltration depth, lymph node metastasis, and clinical stage.
Conclusions: Both pretreatment SII and CAR might be promising predictors of cancer survival and tumor progression in EC. Further studies are warranted to verify the clinical usefulness in patients with EC.
Keywords: systemic immune-inflammation index, C-reactive protein-to-albumin ratio, esophageal cancer, prognosis, clinical pathology, meta-analysis
Introduction
Esophageal cancer (EC) is the sixth major cause of cancer-related death globally, and esophageal squamous cell carcinoma (ESCC) is the predominant pathological subtype worldwide.1,2 Above half of all diagnosed EC cases occurred in Asian countries and the five-year survival rates for the patients with this disease are still low and unsatisfactory.3,4 Prognosis-related indicators could contribute to prognostic assessment as well as individualized treatment. Thus, searching for prognostic indicators that are non-invasive and easily-available is significant and required for clinical practice.
Increasing evidence has shown systemic inflammatory responses and nutritional status were involved in tumor development and considered as important factors associated with clinical prognosis in various types of cancers.5–8 And therefore a series of biological indicators based on inflammatory and/or nutritional status have been reported as tumor biomarkers.9–11 Among them, systemic immune-inflammation index (SII), defined as neutrophil × platelet/lymphocyte, was initially known as an indicator of the host inflammatory status as well as a prognostic marker in hepatocellular carcinoma.12 And the good prognostic value of SII was subsequently reported in EC and other tumors.13,14 Another inflammation-based score, C-reactive protein-to-albumin ratio (CAR), based on C-reactive protein and albumin, also aroused great concern in the prediction in multiple tumors, including EC.15–17 And different cutoffs of these two parameters have been utilized in previous studies to display the predictive values in cancer patients.13–16
However, the relationships between pretreatment SII and CAR and clinical outcomes of EC are inconsistent, their values as prognostic tumor markers in EC remain elusive. And to the best of our knowledge, no meta-analysis regarding the clinical significance of both SII and CAR in EC patients is available. Therefore, we searched all relevant literature to perform an integrated meta-analysis to fully address the clinical values of SII and CAR in EC sufferers. According to PICOS principles, the participants with primary EC were included, and we compared the related clinical outcomes in EC patients with high SII/CAR and those with low SII/CAR. Any studies designed that in compliance with screening criteria were considered eligible and collected for further analysis.
Materials and methods
In this study, we performed the meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.
Search strategy and study selection
Two authors searched eligible articles independently in PubMed, Web of Science, Embase, Cochrane Library databases and Google Scholar from the inception of the databases to September 1, 2018. The search was restricted to the English language. The following keywords and their combinations were used in searching: “carcinoma” or “cancer” or “tumor”, and “esophageal”, and “systemic immune-inflammation index” or “SII”, or “C-reactive protein-to-albumin ratio” or “CAR” or “C-reactive protein/albumin”. The detailed search strategy was illustrated in the Supplementary materials section. Additionally, the references in the eligible publications were also reviewed for potential studies.
Selection criteria
Published articles were included in this meta-analysis if they met the following criteria: 1) All patients collected were histopathologically confirmed to be primary esophageal cancer; 2) The SII and/or CAR were measured prior to treatment; 3) The hazard ratios (HRs) with their 95% confidence interval (95% CI) were reported in multivariate analysis; 4) Original articles were published in English; 5) Only the most complete data were included if overlapping data were found in more than one study. Any studies that were reviews, abstracts, conferences, or posters are excluded in this meta-analysis.
Data extraction
For each study, the following information was extracted by two authors: the name of first author, year of publication, country, study type, pathological type, included period, number of patients, age distribution, end-point, follow-up time, cut-off selection, treatments, stage of cancer, cutoff values and HRs with the 95% CIs. Furthermore, the number of patients for the clinical pathology (including tumor grade, infiltration depth, lymph node metastasis, and clinical stage) was directly extracted from the eligible studies.
Quality assessment
Two authors independently assessed the quality of each study in the meta-analysis, we used the method that was detailed described in the study by Perisanidis et al18 It contained a total of eight items, and each study included in this meta-analysis could get a final score ranged from 0 to 8 after quality assessment.
Statistical analysis
The risk of bias summary and risk of bias graph were applied using Review Manager 5.3, and the data analyses were performed with STATA/SE 14.1 in this meta-analysis. The cohort-specific HRs and 95% CIs for cancer survival were extracted from multivariate cox proportional hazard models. In addition, subgroup analysis was conducted to explore the prognostic values of SII and CAR in EC patients. And for the associations between SII/CAR and clinicopathologic characteristics in EC cases, the pooled ORs and 95% CIs were evaluated using STATA/SE 14.1. Cochran’s Q test and Higgins I square were used to determine the statistical heterogeneity across studies. I2>50% or P<0.1 was considered as heterogeneity, then a random-effects model was used. Otherwise, a fixed-effects model was utilized to combine the data when there was no significant heterogeneity. Publication bias was assessed by Begg’s funnel plot and Begg’s test. And the sensitivity analysis was utilized by omitting individual study one-by-one to assess the robustness of the results. And P-value less than 0.05 was considered statistically significant.
Results
Literature characteristics
After primary retrieval, a total of 210 relevant articles were incorporated into our initial assessment after exclusion of the duplications, and then 189 articles were further excluded by screening the titles and abstracts. The remaining 21 full-text articles were assessed for eligibility. Among them, 10 articles were further removed with the following reasons: such as HRs were not reported in multivariate analysis, no survival data available, and SII/CAR was measured after treatment. Finally,11 published articles were included in this meta-analysis.14,16,19–27 Among them, 4 articles were for SII, 6 articles were for CAR, and 1 article was for both SII and CAR. The process of identifying studies is shown in Figure 1.
 | Figure 1 Flow diagram of included studies for this meta-analysis. |
For the relationship between SII and survival rate in EC patients, five cohort studies were collected with a total of 2292 cases. Among them, four studies were published in 2017 or later and one study was published before 2017. All these studies assessed patients from East Asian countries (China and Japan), and the endpoints of OS and CSS were addressed in 4 studies and 1 study, respectively, and the CSS was integrated into the meta-analysis of OS. In terms of pathological types, 4 articles reported ESCC, 1 article studied the mixture of ESCC, EAC, and others. The cut-off value of SII varied in different studies, ranging from 307 to 650 with a mean of 462.
For the correction of CAR in EC patients, 7 studies selected for analysis comprised 1742 patients, with sample sizes ranging from 116 to 468 patients. Among them, 2 studies were published in 2015, 2 articles were released in 2017, and 3 studies were published in 2018. 1 study assessed patients from Australia and the rest 6 were conducted in East Asian populations (China and Japan). All the studies assessed the correction of CAR with OS. In terms of pathological types, 5 studies worked on ESCC, 1 studies focused on the mixture of ESCC and EAC, and another one study worked on the mixture of ESCC, EAC, and other types. There was a wide range of the cut-off value of CAR, ranging from 0.023 to 0.95 with the mean of 0.277.
And the relevant data of clinicopathologic characteristics for SII and CAR, including tumor grade, infiltration depth, lymph node metastasis, and clinical stage are shown in Table S1. The quality of the 12 included cohort studies was good with an average quality score of 7 (range 5–8, Figure 2, Table S2). In addition, the risk of bias summary and risk of bias graph for SII and CAR were presented in Figures S1 and Figure S2, respectively. The main characteristics of all cohort studies are summarized in Table 1.
 | Table 1 Main characteristics of all included studies |
 | Figure 2 Quality assessment of 12 cohort studies included in the meta-analysis according to predefined eight items. |
 | Figure S1 Risk of bias assessment for SII. Abbreviations: SII, systemic immune-inflammation index. |
 | Figure S2 Risk of bias assessment for CAR. Abbreviations: CAR, C-reactive protein-to-albumin ratio. |
SII in esophageal cancer
Overall survival (OS)
Five studies with a total of 2292 cases explored the effect of SII on OS in esophageal cancer. As no obvious heterogeneity observed among studies, the fixed effects model was used (I2=16.0%, phet=0.312). The pooled results indicated that high SII was significantly related with shorter OS in EC patients following surgery (HR: 1.34, 95% CI: 1.15–1.53, P<0.001; Figure 3).
 | Figure 3 Meta-analysis of the correlation between SII and OS.Abbreviations: OS, overall survival; SII, systemic immune-inflammation index; HR, hazard ratio; 95%CI, 95% confidence interval. |
As shown in Table 2, when stratified by the pathological type, high SII had a significantly worse OS in ESCC (HR: 1.34, 95% CI: 1.14–1.53, P<0.001). In addition, statistically significant pooled multivariable adjusted HR values >1 were consistently calculated in subgroup meta-analyses stratified by cut-off value (≥462 vs <462), follow-ups (≥5 years vs <5 years) and cancer staging (Non-metastatic vs < Mixed).
 | Table 2 Subgroup analysis of the correlation between SII and OS |
Clinical pathology
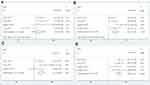
Five articles with 2,292 patients coved the effect of high SII on histological grade. The fixed effect model was used (I2=10.0%, Phet=0.349), the combined results showed that there was no significant difference between SII status and histological grade (OR: 1.07, 95% CI: 0.87–1.30, P=0.528; Figure 4A).
Four studies, consisting of 1,994 patients, explored the relationship between SII and depth of tumor invasion. The pooled analysis revealed the pooled OR of 2.10 with 95% CI: 1.72–2.56 (P<0.001) (Figure 4B) with no significant heterogeneity (I2=42.4%, Phet=0.157). Patients with high SII have deeper infiltration depth when compared with those with low SII.
A total of four studies with 1,994 patients reported the relationship between SII and lymph node metastasis. As shown in Figure 4C, the random effect model was used (I2=60.8%, Phet=0.054), the patients with esophageal cancer were at significantly greater risk of lymph node metastasis (OR: 1.51, 95% CI: 1.09–2.09, P<0.001).
Five studies selected comprised 2,292 patients assessed the association between SII and clinical stage. The estimated proportion of heterogeneity (I2) between five SII studies was 50.4% (P=0.089), the random effect model was applied. As indicated in Figure 4D, high SII was correlated with the advanced clinical stage in patients with esophageal cancer (OR: 2.00, 95% CI: 1.51–2.65, P<0.001).
CAR in esophageal cancer
Overall survival (OS)
A total of 1,742 cases from seven articles reported the effect of CAR on OS in esophageal cancer. The fixed effects model was used for no obvious heterogeneity (I2=35.7%, Phet=0.155). The combined results showed that patients with elevated CAR were expected to suffer worse long-term OS (HR: 1.60, 95% CI: 1.29–1.90, P<0.001; Figure 5).
 | Figure 5 Meta-analysis of the correlation between CAR and OS.Abbreviations: OS, overall survival; CAR, C-reactive protein-to-albumin ratio; HR, hazard ratio; 95%CI, 95% confidence interval. |
As summarized in Table 3, when stratified by the pathological type, CAR could be a significant prognostic biomarker in ESCC (HR: 1.64, 95% CI: 1.30–1.98, P<0.001). In addition, significant associations between high CAR level and poor OS were also found in the subgroup analyses of cut-off value (≥0.277 vs <0.277), treatments methods (With-surgery vs Mixed) and clinical staging (Non-metastatic vs Mixed).
 | Table 3 Subgroup analysis of the correlation between CAR and OS |
Clinical pathology
A total of four articles with 1,193 patients reported the relationship between CAR and histological grade. The pooled results indicated that no significant association was observed between CAR and histological grade (OR: 1.26, 95% CI: 0.69–2.32, P=0.456; random-effects; Figure 6A)
Four articles with 1,088 patients reported the correlation between CAR and infiltration depth. As shown in Figure 6B, significant heterogeneity was observed (I2=56.0%, Phet=0.078; random-effects), the overall results showed that the patients with high CAR more likely to have deeper tumor invasion (OR: 2.42, 95% CI: 1.48–3.98, P<0.001)
Only three articles with 972 cases coved the correlation of high CAR on lymph node metastasis. The fixed effect model was employed (I2=21.7%, Phet=0.279), the combined analysis revealed the pooled OR of 1.83 with 95% CI: 1.34–2.49 (P<0.001) (Figure 6C), suggesting that high CAR was significantly associated with lymph node metastasis.
Four articles, consisting of 1,150 patients, explored the association between CAR and clinical stage. No obvious heterogeneity was found, the fixed effect model was utilized (I2=0.0%, Phet=0.788). As indicated in Figure 6D, the patients with high CAR tended to have advanced tumor stages compared those with low CAR (OR: 2.90, 95% CI: 2.20–3.81, P<0.001).
Publication bias
The Begg’s funnel plots were shown in Figure 7, and the P-values of Begg’s were 0.086 for SII and 1.000 for CAR. These results showed that there was no publication bias in the current study.
 | Figure 7 Publication bias assessment for OS with SII (A) and CAR (B).Abbreviations: OS, overall survival; SII, systemic immune-inflammation index; CAR, C-reactive protein-to-albumin ratio. |
Sensitivity analysis
The sensitivity analysis indicated that the results of the combined analysis were robust (Figure 8).
 | Figure 8 Sensitivity analysis for OS in SII (A) and CAR (B).Abbreviations: OS, overall survival; SII, systemic immune-inflammation index; CAR, C-reactive protein-to-albumin ratio. |
Discussion
Serum biological markers are considered non-invasive and obtained easily in daily clinical practice. Nowadays, many blood-related parameters, including lymphocyte count, plasma fibrinogen and albumin, have been reported as prognostic markers in various malignant tumors.28–31 But a single blood indicator is often not stable and reliable for they are inevitably susceptible to many other factors. And some comprehensive index based on two or three hematological parameters have been identified, such as platelet to lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR) and neutrophil/lymphocyte ratio (NLR), they all showed the potential prognosis prediction in multiple cancers.32–35
SII, as a combination of three blood-related factors, neutrophil, platelet, and lymphocyte, was shown to be related with prognosis in upper gastrointestinal (GI) cancers. For example, some researchers have reported that SII might be a useful indicator in predicting survival and clinicopathologic characteristics in patients with gastric cancer.13,36,37 In addition, SII has shown to be superior to PLR, NLR, and MLR as a predictive biomarker in ESCC.14,16 And CAR, based on serum C-reactive protein and albumin, was also revealed the great usefulness as a predictor of survival in various types of tumors, including upper GI cancers. A number of studies have indicated that preoperative high CAR was related with poor prognosis of patients with gastric cancer,38–41 and also could act as a predictor for short-term complications in gastric cancer patients following gastrectomy.42 And the prognostic values of CAR were also found in the patients with EC.19,20
SII and CAR, they are both nutrition and inflammation-based indexes although they are based on different serum parameters. The data of these serum factors can be available in routine blood tests, and they are cheap, non-invasive, and easily accessible. And the two indexes could better reflect systemic inflammatory response and also showed better predictive performance in tumors. As yet, until now, there are no studies that comprehensively assess the prognostic and clinicopathological roles of both SII and CAR in cancer of the esophagus. In order to provide new insights that helping understanding the clinical values of SII and CAR in EC patients, a total of twelve original datasets were included to demonstrate the relationship between SII/CAR and clinical relevance in oesophageal cancer. The combined results showed that high pretreatment SII/CAR was significantly associated with worse clinical outcomes in EC patients.
Our study is the first meta-analysis to evaluate the clinical roles of both SII and CAR in EC patients. We found that high pretreatment SII and CAR were closely related to worse clinical outcomes in EC. Both high pretreatment SII and CAR were significantly related to shorter OS in EC patients. And the subgroup meta-analyses also further confirmed the clinical roles of these two indexes in this disease. As for the relationships between SII or CAR and clinical pathological factors in EC, we found that elevated SII and CAR were significantly related with deeper infiltration depth, positive lymph node metastasis, and advanced clinical stage.
Nevertheless, there exist some limitations to our study. First, the total number of included studies were still relatively small and only the articles published in English were searched. Second, the included studies conducted in Asian countries are the majority, which might entail the preferences of the population. Third, most of the studies focused on the prognostic roles of these two indexes on ESCC, as the difference in the pathology of EAC, their prognosis prediction on EAC need further validated. Fourth, there were a diversity of cut-off values that were used to divide the patients into high and low SII/CAR groups. Additionally, many other factors, such as postoperative treatment, tumor stage, they could also affect the survival time of the patients.
In conclusion, our study provides clear evidence that both SII and CAR are correlated with clinical outcomes in EC and could be used as a noninvasive prognostic marker for this disease. In this meta-analysis, the cut-off values for SII (462) and CAR (0.277) are recommended that would be useful for predicting outcomes. Given the limitations mentioned, further larger multi-center studies are required to further confirm our findings.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933–7943. doi:10.3748/wjg.v21.i26.7933
2. Alsop BR, Sharma P. Esophageal Cancer. Gastroenterol Clin North Am. 2016;45(3):399–412. doi:10.1016/j.gtc.2016.04.001
3. Codipilly DC, Qin Y, Dawsey SM, et al. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88(3):413–426. doi:10.1016/j.gie.2018.04.2352
4. Ohashi S, Miyamoto S, Kikuchi O, et al. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149(7):1700–1715. doi:10.1053/j.gastro.2015.08.054
5. Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi:10.1016/S1470-2045(14)70263-3
6. Soria-Valles C, López-Soto A, Osorio FG, et al. Immune and inflammatory responses to DNA damage in cancer and aging. Mech Ageing Dev. 2017;165(Pt A):10–16. doi:10.1016/j.mad.2016.10.004
7. Bail J, Meneses K, Demark-Wahnefried W. Nutritional status and diet in cancer prevention. Semin Oncol Nurs. 2016;32(3):206–214. doi:10.1016/j.soncn.2016.05.004
8. Toles M, Demark-Wahnefried W. Nutrition and the cancer survivor: evidence to guide oncology nursing practice. Semin Oncol Nurs. 2008;24(3):171–179. doi:10.1016/j.soncn.2008.05.005
9. Zhang Y, Wang L, Lin S, et al. Preoperative albumin-to-globulin ratio as a significant prognostic indicator in urologic cancers: a meta-analysis. Cancer Manag Res. 2018;10:4695–4708. doi:10.2147/CMAR.S178271
10. Zhang Y, Zhang X. Prognostic value of aspartate aminotransferase to platelet ratio index as a noninvasive biomarker in patients with hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:3023–3032. doi:10.2147/CMAR.S174095
11. Li YJ, Yang X, Zhang WB, et al. Clinical implications of six inflammatory biomarkers as prognostic indicators in Ewing sarcoma. Cancer Manag Res. 2017;9:443–451. doi:10.2147/CMAR.S146827
12. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
13. Chen L, Yan Y, Zhu L, et al. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–867. doi:10.2147/CMAR.S151026
14. Geng Y, Shao Y, Zhu D, et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Sci Rep. 2016;6:39482. doi:10.1038/srep39482
15. Deng TB, Zhang J, Zhou YZ, et al. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Medicine (Baltimore). 2018;97(50):e13505. doi:10.1097/MD.0000000000013505
16. Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore). 2017;96(4):e5886. doi:10.1097/MD.0000000000005886
17. Shimizu T, Ishizuka M, Suzuki T, et al. The value of the C-reactive protein-to-albumin ratio is useful for predicting survival of patients with child-pugh class A undergoing liver resection for hepatocellular carcinoma. World J Surg. 2018;42(7):2218–2226. doi:10.1007/s00268-017-4446-0
18. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. doi:10.1016/j.ctrv.2015.10.002
19. Wang L, Wang C, Wang J, et al. A novel systemic immune-inflammation index predicts survival and quality of life of patients aftercurative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077–2086. doi:10.1007/s00432-017-2451-1
20. Ishibashi Y, Tsujimoto H, Hiraki S, et al. Prognostic value of preoperative systemic immunoinflammatory measures in patients with esophageal cancer. Ann Surg Oncol. 2018;25(11):3288–3299. doi:10.1245/s10434-018-6651-y
21. Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune‐inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2018. doi:10.1002/jcp.27052 [[Epub ahead of print]].
22. Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. doi:10.1186/s12885-015-1584-3
23. Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-Reactive Protein/Albumin Ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9):e0138657. doi:10.1371/journal.pone.0138657
24. Otowa Y, Nakamura T, Yamamoto M, et al. C-reactive protein to albumin ratio is a prognostic factor for patients with cStage II/III esophageal squamous cell cancer. Dis Esophagus. 2017;30(12):1–5. doi:10.1093/dote/dox107
25. Jomrich G, Paireder M, Gleiss A, et al. Comparison of inflammation-based prognostic scores in a cohort of patients with resectable esophageal cancer. Gastroenterol Res Pract. 2017;2017:1678584. doi:10.1155/2017/1678584
26. Kunizaki M, Tominaga T, Wakata K, et al. Clinical significance of the C-reactive protein-to-albumin ratio for the prognosis of patients with esophageal squamous cell carcinoma. Mol Clin Oncol. 2018;8(2):370–374. doi:10.3892/mco.2017.1527
27. Yu X, Wen Y, Lin Y, et al. The value of preoperative glasgow prognostic score and the C-Reactive protein to albumin ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. 2018;9(5):807–815. doi:10.7150/jca.22755
28. Wu Z, Zhang J, Cai Y, et al. Reduction of circulating lymphocyte count is a predictor of good tumor response after neoadjuvant treatment for rectal cancer. Medicine (Baltimore). 2018;97(38):e11435. doi:10.1097/MD.0000000000011435
29. Ji R, Ren Q, Bai S, et al. Prognostic significance of pretreatment plasma fibrinogen in patients with hepatocellular and pancreatic carcinomas: A meta-analysis. Medicine (Baltimore). 2018;97(25):e10824. doi:10.1097/MD.0000000000010824
30. Chen Z, Shao Y, Wang K, et al. Prognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysis. Onco Targets Ther. 2016;9:6701–6710. doi:10.2147/OTT.S108469
31. Bozkaya Y, Erdem GU, Demirci NS, et al. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2018;35(2):1–8.
32. Wang J, Zhou X, He Y, et al. Prognostic role of platelet to lymphocyte ratio in prostate cancer: A meta-analysis. Medicine (Baltimore). 2018;97(40):e12504. doi:10.1097/MD.0000000000012504
33. Wang W, Liu W, Zhang N, et al. Preoperative platelet-lymphocyte ratio is an independent prognostic factor in ampullary carcinoma following pancreaticoduodenectomy. Oncol Lett. 2018;16(4):4879–4888. doi:10.3892/ol.2018.9285
34. Solmaz Medeni S, Acar C, Olgun A, et al. Can neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and platelet-to-lymphocyte ratio at day +100 be used as a prognostic marker in multiple myeloma patients with autologous transplantation?. Clin Transplant. 2018;32(9):e13359. doi:10.1111/ctr.13359
35. Zhao Z, Zhao X, Lu J, et al. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Arch GynecolObstet. 2018;297(4):849–857. doi:10.1007/s00404-018-4678-8
36. Huang L, Liu S, Lei Y, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7(28):44185–44193. doi:10.18632/oncotarget.9923
37. Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune- inflammation index in patients with gastric cancer.Chin J Cancer. 2017;36(1):75. doi:10.1186/s40880-017-0243-2
38. Liu X, Sun X, Liu J, et al. Preoperative C-Reactive Protein/Albumin Ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–345. doi:10.1016/j.tranon.2015.06.006
39. Toyokawa T, Muguruma K, Tamura T, et al. Comparison of the prognostic impact and combination of preoperative inflammation-based and/or nutritional markers in patients with stage II gastric cancer. Oncotarget. 2018;9(50):29351–29364. doi:10.18632/oncotarget.25486
40. Mao M, Wei X, Sheng H, et al. C-reactive protein/albumin and neutrophil/lymphocyte ratios and their combination predict overall survival in patients with gastric cancer. Oncol Lett. 2017;14(6):7417–7424. doi:10.3892/ol.2017.7179
41. Saito H, Kono Y, Murakami Y, et al. Prognostic significance ofthe preoperative ratio of C-Reactive protein to Albumin andneutrophil-lymphocyte ratio in gastric cancer patients. World J Surg. 2018;42(6):1819–1825. doi:10.1007/s00268-017-4400-1
42. Sun F, Ge X, Liu Z, et al. Postoperative C-reactive protein/albumin ratio as a novel predictor for short-term complications following gastrectomy of gastric cancer. World J Surg Oncol. 2017;15(1):191. doi:10.1186/s12957-017-1258-5
Supplementary materials
 | Table S1 Relevant data of clinicopathologic characteristics for SII and CAR in EC patients |
 | Table S2 Study quality of all included cohort studies in the meta-analysis |
Supplementary File 1. Literature search strategy in PUBMED
((((C-reactive[All Fields] AND protein-to-albumin[All Fields] AND (“Ratio (Oxf)“[Journal] OR “ratio“[All Fields])) OR (“automobiles“[MeSH Terms] OR “automobiles“[All Fields] OR “car“[All Fields])) OR ((“c-reactive protein“[MeSH Terms] OR (“c-reactive“[All Fields] AND “protein“[All Fields]) OR “c-reactive protein“[All Fields] OR “c reactive protein“[All Fields]) AND (“albumins“[MeSH Terms] OR “albumins“[All Fields] OR “albumin“[All Fields]))) OR ((systemic[All Fields] AND immune-inflammation[All Fields] AND (“abstracting and indexing as topic“[MeSH Terms] OR (“abstracting“[All Fields] AND “indexing“[All Fields] AND “topic”[All Fields]) OR ”abstracting and indexing as topic”[All Fields] OR ”index”[All Fields])) OR (”Stat Interface”[Journal] OR ”sii”[All Fields]))) AND ((((”carcinoma”[MeSH Terms] OR ”carcinoma”[All Fields]) OR (”neoplasms”[MeSH Terms] OR ”neoplasms”[All Fields] OR ”cancer”[All Fields])) OR (”tumour”[All Fields] OR ”neoplasms”[MeSH Terms] OR ”neoplasms”[All Fields] OR ”tumor”[All Fields])) AND esophageal[All Fields]) AND (”0001/01/01”[PDAT]: ”2018/09/01”[PDAT]) AND (”loattrfull text”[sb] AND English[lang])
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.