Back to Journals » OncoTargets and Therapy » Volume 10
Clinical significance of SLP-2 in hepatocellular carcinoma tissues and its regulation in cancer cell proliferation, migration, and EMT
Authors Huang Y, Chen Y, Lin X, Lin Q, Han M, Guo G
Received 22 June 2017
Accepted for publication 29 August 2017
Published 20 September 2017 Volume 2017:10 Pages 4665—4673
DOI https://doi.org/10.2147/OTT.S144638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samir Farghaly
This paper has been retracted.
Yijie Huang,1,* Yexi Chen,2,* Xiaoqi Lin,2 Qingjun Lin,2 Ming Han,2 Guohu Guo2
1Department of General Surgery, Guangdong General Hospital, Guangzhou, 2Department of General Surgery, The Second Affiliated Hospital of Shantou University Medical College, Shantou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Stomatin-like protein 2 (SLP-2) gene was significantly upregulated in a variety of tumor tissues and found to be involved in proliferation and metastasis. However, its functional role in hepatocellular carcinoma (HCC) remains unknown. Our study was to investigate the function of SLP-2 in cell proliferation, migration, invasion, cell apoptosis, and the process of epithelial–mesenchymal transition (EMT) in HCC. SLP-2 mRNA and protein expression in HCC were assessed by qRT-PCR and immunohistochemical staining. In vitro, we determined cell proliferation, migration, invasion, and cell apoptosis by CCK-8, transwell, and flow cytometry assays, respectively. SLP-2 was found to be upregulated at both mRNA and protein levels in HCC tissues, and its aberrant overexpression was linked with poor prognosis in patients with HCC. SLP-2 downregulation by siRNAs significantly suppressed cell proliferation, migration, invasion, anti-apoptosis abilities, and inhibited EMT process in vitro. In conclusion, the present study demonstrated the overexpression of SLP-2 in HCC tissues for the first time. As an effective regulator involved in cell proliferation, migration, invasion, cell apoptosis, and EMT, SLP-2 could be a novel therapeutic target for patients with HCC who express high levels of SLP-2.
Keywords: hepatocellular carcinoma, stomatin-like protein 2, prognosis, proliferation, migration, epithelial–mesenchymal transition
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer, and its morbidity and mortality have demonstrated an ever-pressing health trend in China, according to a recent annual report of cancer epidemiology.1 Considerable progress has been made in the diagnosis and treatment of HCC, including novel target chemotherapy, hyperemia therapy, and other multidisciplinary managements.2,3 However, the prognosis of patients with HCC remains extremely poor, which is mainly challenged by metastasis and recurrence of neoplasms. Consequently, there is an urgent need to provide effective strategies for the diagnosis and treatment of tumors. Molecular bio-techniques are preferred among the newer strategies, as emerging evidence has showed that genetic alterations were implicated in the HCC cell proliferation and migration processes.4–6 Unfortunately, only a few of these biomarkers are satisfactory. Therefore, continual efforts in screening new biomarkers for HCC remain necessary.
Stomatin-like protein 2 (SLP-2, also known as STOML2), a member of the stomatin superfamily, is a major protein which locates on the mitochondrial inner membrane. This protein does not contain a NH2-terminal hydrophobic domain compared to other family members. Meanwhile, SLP-2 may play a major role in organizing sphingolipid and cholesterol-rich lipid rafts, regulating ion channel conductance, and linking other integral membrane proteins to the peripheral cytoskeleton.7 The SLP-2 protein was found to be overexpressed in many human cancer tissues, including papillary thyroid carcinoma,8 esophageal cancer,9,10 gastric cancer,11 endometrial adenocarcinoma,12 and breast cancer.13 Emerging evidences showed that SLP-2 possessed an ability to promote proliferation and adhesion in the esophageal squamous cell carcinoma cell line TE12.9,10 These results suggested that SLP-2 was a novel cancer-related gene, and a potential drug target to block proliferation and migration in tumor cells. However, expression of SLP-2 and its clinical significance in HCC has not yet been uncovered. Thus, we conducted the present study to analyze the level of SLP-2 expression in HCC tissues, as well as its associated clinical implications. In addition, for better mechanistic understanding of how SLP-2 is involved in carcinogenesis, we conducted further experiments to study the effects of SLP-2 on cell proliferation, apoptosis, migration, invasion, and the EMT process in HCC cell lines.
Materials and methods
Ethics statement
This study was approved by the Second Affiliated Hospital of Shantou University Medical College Ethics Committee according to the Helsinki Declaration. Informed consent was obtained either from the patient or their next of kin for all tissue samples used.
Tissue samples
Between 2012 and 2016, HCC tissue and adjacent normal liver tissues were collected from 60 patients who underwent radical surgeries for HCC in the Second Affiliated Hospital of Shantou University Medical College (Guangdong, People’s Republic of China). Pathological features of these tissue samples were evaluated by the Department of Pathology, The Second Affiliated Hospital, according to the World Health Organization standards. Characteristics and follow-up data of patients were obtained from medical records.
Cell lines
L02, HepG2, HCCLM3, MHCC97H, and BEL-7702 cell lines were purchased from Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, People’s Republic of China). All cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS; Gibco), and then incubated with 5% CO2 at 37°C in a humidified atmosphere.
Quantitative reverse transcription PCR
Total RNA of the 60 paired fresh tissue samples, extracted with RNAiso Plus reagent (Takara, Tokyo, Japan), was reversely transcribed into cDNA by Prime Script RT Master Mix (Takara) according to the manufacturer’s protocol. The relative mRNA level was assessed by quantitative polymerase chain reaction (qPCR) with SYBR Premix Ex Taq kit (Takara) and normalized to GAPDH with the following primers: SLP-2 forward (F), 5′-CCAGCAGAGCAGCCTCATC-3′ and reverse (R), 5′-TCCTTGAGTGACGCCAGGA-3′. GAPDH F, 5′-CAGGAGGCATTGCTGATGAT-3′ and R, 5′-GAAGGCTGGGGCTCATTT-3′. Changes in the expression were calculated by the 2−ΔΔCT method. For statistical analysis, these 60 patients were grouped into high and low categories based on the median of relative SLP2 expression.13
Immuohistochemistry
For immunohistochemistry (IHC), 4 μm-thick sections were cut from 10% formalin-fixed paraffin-embedded tissue samples. These sections were routinely deparaffinized, hydrated, and repaired in a microwave for 10 min, treated in 3% H2O2 to eliminate endogenous peroxidase for 15 min, then subjected to 10% normal goat non-immune block for 30 min, and incubated with rabbit anti-human SLP-2 antibody (1:200; Cat NO 10348-1-AP, Proteintech, Rosemont, IL, USA) at 4°C overnight. After washing with PBS, the sections were incubated with streptavidin-peroxidase conjugated goat anti-rabbit secondary antibody (ZSGB, People’s Republic of China). After extensive washing, 3,3-diaminobenzidine (DAB, ZSGB) was used for visual reaction. Immunostaining was reviewed by two pathologists independently. SLP-2 protein expression in 60 paired tissue samples were classified into positive and negative groups according to Zhao et al.14
siRNA transfection
HepG2 and HCCLM3 cells were cultured in six-well plates. The siRNAs targeting SLP-2 and negative control (NC) were transfected in the cells using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) and Opti-MEM (Thermo Fisher Scientific) following the manufacturer’s instruction. The sequences of siRNAs and NC were as follows: siRNA-1, 5′-GCAGUCAGCCGUAACUCUUTT-3′ and 5′-AAGAGUUACGGCUGACUGCTT-3′; siRNA-2, 5′-GCCUGCGAUACGAGAUCAATT-3′ and 5′-UUGAUCUCGUAUCGCAGGCTT-3′; NC, 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′.
Cell proliferation assay
A total of 2×103 cells per well were seeded in 96-well plates for 24 h, transfected with the indicated siRNAs, and the cell number was then assessed by Cell Counting Kit 8 (CCK-8; KeyGEN, Nanjing, People’s Republic of China) following the kit assay protocol at 0, 24, 48, 72, and 96 h after transfection. Briefly, 10 μL of CCK 8 solution was added to each well and incubated for 2 h at 37°C. The solution was then measured spectrophotometrically at 450 nm.
Cell apoptosis assay
Transfected cells were collected and treated with Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI). Apoptosis Detection Kit (KeyGEN) according to the manufacturer’s instructions. Flow cytometry was undertaken by BD FACSCalibur flow cytometer systems (Becton Dickinson, Franklin Lakes, NJ, USA).
Transwell migration and invasion assay
After transfection with the indicated siRNAs for 48 h, 5×104 cells were harvested in 200 μL serum-free medium, and added to the upper chambers with 8 μm pores in 24-well plates (Corning Incorporated, Corning, NY, USA). The migration was induced by 10% FBS medium in the lower chambers. After 24 h, the cells were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet. The numbers of migrated cells were counted in five different fields under an inverted microscope (Nikon, Tokyo, Japan). For the invasion assay, all conditions were as described above after the inserts were pre-coated with 50 μL Matrigel (1:8; Corning Incorporated).
Western blotting assay
Western blotting (WB) was carried out using rabbit anti-human antibodies including E-Cadherin (1:1,000; Cat No 9782T), N-Cadherin (1:1,000), Vimentin (1:1,000; Cat No 9782T), Snail (1:1,000; Cat No 9782T), and β-actin (1:1,000; Cat No 9782T, Cell Signaling Technology, Boston, MA, USA). Transfected cells were lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitor; 30 μg total protein was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Corp, Billerica, MA, USA). Non-specific antibodies were blocked by 5% BSA in PBST for 1 h. The membranes were then incubated with primary antibodies at 4°C overnight, and subsequently incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:1,000; Cat. No 9782T, Cell Signaling Technology) for 1 h. The immunoblotted proteins were detected by enhanced chemiluminescence (ECL; Millipore).
Statistical analysis
All data were analyzed with the SPSS version 19 for Windows (SPSS, Chicago, IL, USA) and GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Levels of SLP-2 mRNA in HCC tissues and in adjacent normal tissues were compared by the Mann–Whitney test. Correlations between SLP-2 expression and clinicopathological parameters were evaluated by the χ2 test. Tumor-free survival was analyzed using the Kaplan–Meier method, and the differences was evaluated by the log-rank test. Comparisons among multiple groups were determined using one-way ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
SLP-2 exhibited overexpression in HCC
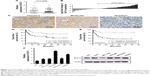
To investigate the role of SLP-2 in HCC, we first detected the levels of SLP-2 mRNA and protein expression in 60 cases of HCC tissues and adjacent normal tissues, respectively. On qRT-PCR, an elevated SLP-2 mRNA level was observed in cancerous tissues, compared with the adjacent normal tissues (Figure 1A). SLP-2 upregulation (tumor/non-tumor ≥1) was found in 39 cases (65%, Figure 1B). With IHC, positive expression of the SLP-2 protein was detected in the cytoplasm of hepatocytes (Figure 1C), with the positive rate of SLP-2 in cancerous tissues being significantly higher than that in the adjacent normal tissues (56.7% vs 13.3%). Furthermore, we found that SLP-2 was overexpressed in HCC cells compared to that in the normal liver cell line L02 (Figure 1F).
Overexpression of SLP-2 predicts poor prognosis in patients with HCC
To evaluate the clinical benefits of SLP-2, the expression data from tumor tissue samples were subjected to statistical correlation with various clinicopathological parameters, and the results were summarized in Tables 1 and 2. Upregulation of SLP-2 mRNA was strongly associated with AFP level, tumor size, metastasis, and recurrence. Similar results were observed in the SLP2 protein analysis. Positive expression of SLP-2 was significantly associated with tumor size, TNM stage, metastasis, and recurrence. The prognostic potential of SLP-2 was evaluated by the Kaplan–Meier method with log-rank test, and high expression of SLP-2 associated with poor tumor-free survival (Figure 1D and E).
Silencing SLP-2 inhibits cell proliferation and induces cell apoptosis
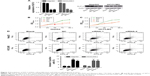
Our statistical data showed that overexpression of SLP-2 was correlated with tumor size and metastasis, which inspired us to examine the functional role of SLP-2 in HCC cell lines. To investigate the effects of SLP-2 on the proliferation of HCC cells, siRNA-mediated knockdown of SLP2 was employed in the HepG2 and HCCLM3 cells. As shown by qRT-PCR and WB results (Figure 2A), two series of siRNA targeting SLP-2 efficiently reduced SLP-2 expression in HepG2 and HCCLM3 cells. Furthermore, a CCK8 assay indicated that cell proliferation was significantly inhibited after 48-h SLP-2-specific siRNA treatment in two HCC cell lines (Figure 2B and C). Evasion of apoptosis results in unlimited growth of cancer cells. After knockdown of SLP-2, apoptosis of HepG2 and HCCLM3 cells was found to remarkably increase (Figure 2D). It seemed that SLP-2 regulates cell proliferation by inducing apoptosis.
Silencing SLP-2 represses cell migration and invasion
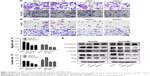
Next, we examined the role of SLP-2 in HCC cell migration and invasion by a transwell migration assay. As shown in Figure 3A–D, both the number of migrated and invaded HepG2 and HCCLM3 cells that got through the chambers decreased significantly in the SLP-2 knockdown group, indicating that SLP-2 was a tumor-promoting gene in HCC, and might strengthen the migration and invasion of HCC cells.
Silencing SLP-2 suppresses the EMT process
EMT has been increasingly recognized to occur during the development of HCC. To identify whether SLP-2 is involved in the process of EMT and consequently promotes HCC metastasis, we next investigated the role of SLP-2 in HCC cell EMT and found EMT results in loss of epithelial markers and gain of mesenchymal markers. Western blot showed that expression of E-cadherin was upregulated, whereas N-cadherin, Vimentin, and Snail were downregulated in SLP-2 knockdown groups (Figure 3E). Therefore, we inferred that SLP-2 may prompt EMT processes in HCC.
Discussion
Hepatectomy and liver transplantation are preferred therapeutic options for HCC that, however, cannot guarantee full recovery. The main factors responsible for this phenomenon are the biological characteristics of metastasis and recurrence in HCC.15–20 Tumorigenesis results from multifactorial, multistep, multigene, and multi-mutation elements. It would be beneficial to, first, discover new biomarkers involved in HCC oncogenesis and progress that predict early tumor recurrence, then clarify their molecular mechanisms, and design better targeted therapy.3
SLP-2 was a peripheral cytoskeleton-related protein and was found to be highly associated with multiple malignancies. Recent studies on gastric cancer, endometrial cancer, breast cancer, laryngeal cancer, glioma, and ovarian cancer have all shown that the SLP-2 was overexpressed and actively involved during cancer development and progression.7–12,14 However, very little is known regarding its oncogenic potential in HCC. In the present study, we initially investigated the SLP-2 expression status in HCC at both the mRNA and protein levels. The results of the immunohistochemistry and qRT-PCR study were cross-supported, verifying that SLP-2 was markedly overexpressed in HCC tissues as compared with normal counterparts. Further statistical analysis indicated that overexpression of SLP-2 was significantly associated with APF levels, tumor size, TNM stage, metastasis, and recurrence of patients with HCC. These findings suggested that SLP-2 was a potential oncogene to predict poor prognosis of patients with HCC.
SLP-2 is a novel but unusual member of the stomatin gene superfamily.21,22 The human stomatin protein family comprises five members, including stomatin, SLP-1, SLP-2, SLP-3, and the kidney-specific protein podocin. SLP-2 and stomatin share a stomatin-homology region and are part of the much larger SPFH (stomatin–prohibitin–flotillin–HflK/C) superfamily, whereas SLP-2 lacks the typical amino-terminal transmembrane domain present in other stomatins.23 Emerging evidence have shown that stomatins were involved in the organization of the peripheral cytoskeleton, the formation of sphingolipid- and cholesterol-rich lipid rafts, and the assembly of ion channels and mechanosensation receptors.24,25 Furthermore, SLP-2 have been shown to enhance invasiveness in glioma and esophageal squamous cell carcinoma (ESCC) cells, potentially through the upregulation of matrix metalloproteinase 9 (MMP-9), mainly through an NF-κB signaling pathway.10,26 Our research also suggested SLP-2 highly correlated with cell motility abilities, as the tumor size, metastasis, and recurrence of HCC were both significantly associated with SLP-2. However, the exact mechanism underlying SLP-2 involvement in cell motility abilities in HCC remains unclear, including cell proliferation, apoptosis, migration, and invasion.
Previous studies have suggested that knockdown of SLP-2 may dramatically reduce the growth rate of tumor cells in vitro and in vivo and inhibit cell attachment.10 In order to explore the effect of SLP-2 on HCC, we used SLP-2-targeting siRNAs to knockdown SLP-2 in the HCC cell lines to study the effect of SLP-2 downregulation. The results showed that cell proliferation after SLP-2 silencing was downregulated compared with the control group, which suggested that SLP-2 might promote cell proliferation in HCC cell lines. Consistently, we also found that depletion of SLP-2 suppressed HCC cell lines proliferation in vivo, as the tumor growth on mice was inhibited by 50% in SLP-2 downregulated group. Thus, SLP-2 siRNA induced significantly more cell apoptosis (twofold higher) in HepG2 cells than did the control group. Moreover, SLP-2 siRNA did inhibit cell migration and invasion abilities. Taken together, SLP-2 depletion might result in changes in the capacity of HCC cells, including cell proliferation, apoptosis, migration, and invasion.
Previous studies have shown that metastasis was a characteristic feature of HCC and a principal factor related to poor prognosis in patients with HCC.2,18–20 Consequently, identification of the molecular mechanisms responsible for control of the metastatic potential of HCC is crucial in order to inhibit these processes. EMT under stimulation is correlated with tumor metastasis. During EMT, epithelial cells lose polarity and gain greater ability for migration.27–29 A complex network of transcriptional regulation orchestrates the process of EMT during development, and distinct aspects of the physiologic changes characterizing EMT are regulated by the coordinated and overlapping activity of a number of transcriptional factors.3,30
In this study, cell-function experiments suggested SLP-2 might be associated with metastasis. In order to discover its further mechanisms, we tested EMT-related proteins and found that knockdown of SLP-2 upregulated protein expression levels of E-cadherin and downregulated N-cadherin, Vimentin, and Snail, which conferred evidence that SLP-2 is involved in the EMT process.
In summary, this study for the first time showed that SLP-2 is overexpressed in HCC tissues and is negatively associated with the prognosis of patients with HCC. We found that SLP-2 knockdown significantly inhibited tumor cell proliferation, apoptosis, migration, invasion, and EMT progression. Our study results shed a new light on HCC pathogenesis, and SLP-2 might be utilized as a potential novel target for effective treatment of HCC. Meanwhile, prospective studies and enlargement of the evidence base with larger samples should be conducted to draw definitive conclusions and validate the diagnostic value of SLP-2 in HCC.
Author contributions
Conception and design of experiments: GG and MH. Performance of the experiments: YH and YC. Data analysis: YH and YC. Contribution of reagents/materials/tools: XQ and QL. Manuscript writing: YH and YC. All authors have read and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest with regard to this work.
References
Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12. | ||
Marrero JA. Multidisciplinary management of hepatocellular carcinoma: where are we today? Semin Liver Dis. 2013;33(Suppl 1):S3–S10. | ||
Finn RS. Emerging targeted strategies in advanced hepatocellular carcinoma. Semin Liver Dis. 2013;33(Suppl 1):S11–S19. | ||
Liu X, Huang Y, Yang D, et al. Overexpression of TRIM24 is associated with the onset and progress of human hepatocellular carcinoma. PLoS One. 2014;9(1):e85462. | ||
Ma L, Ji L, Yu Y, Wang J. Novel molecular targets for diagnosis and treatment of hepatocellular carcinoma. Discov Med. 2015;19(102):7–14. | ||
Li D, Satomura S. Biomarkers for hepatocellular carcinoma (HCC): an update. Adv Exp Med Biol. 2015;867:179–193. | ||
Wang Y, Morrow JS. Identification and characterization of human SLP-2, a novel homologue of stomatin (band 7.2b) present in erythrocytes and other tissues. J Biol Chem. 2000;275(11):8062–8071. | ||
Bartolome A, Boskovic S, Paunovic I, Bozic V, Cvejic D. Stomatin-like protein 2 overexpression in papillary thyroid carcinoma is significantly associated with high-risk clinicopathological parameters and BRAFV600E mutation. APMIS. 2016;124(4):271–277. | ||
Zhang LY, Ding F, Liu ZM, Li WD, Liu ZH, Li YD. [Effect of stomatin-like protein 2 (SLP-2) gene on growth and proliferation of esophageal squamous carcinoma cell line TE12]. Ai Zheng. 2005;24(2):155–159. Chinese. | ||
Zhang L, Ding F, Cao W, et al. Stomatin-like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12(5):1639–1646. | ||
Liu D, Zhang L, Shen Z, et al. Increased levels of SLP-2 correlate with poor prognosis in gastric cancer. Gastric Cancer. 2013;16(4):498–504. | ||
Cui Z, Zhang L, Hua Z, Cao W, Feng W, Liu Z. Stomatin-like protein 2 is overexpressed and related to cell growth in human endometrial adenocarcinoma. Oncol Rep. 2007;17(4):829–833. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. | ||
Zhao CB, Bao JM, Lu YJ, et al. Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am J Cancer Res. 2014;4(4):369–377. | ||
Sun F, Ding W, He JH, Wang XJ, Ma ZB, Li YF. Stomatin-like protein 2 is overexpressed in epithelial ovarian cancer and predicts poor patient survival. BMC Cancer. 2015;15:746. | ||
Lu X, Zhao H, Yang H, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100(6):488–493. | ||
Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–1468. | ||
Fontanella R, Pelagalli A, Nardelli A, et al. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016;370(1):100–107. | ||
Pelagalli A, Nardelli A, Fontanella R, Zannetti A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int J Mol Sci. 2016;17(7). pii: E1102. | ||
Lu Y, Zhu M, Li W, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med. 2016;20(3):549–558. | ||
Tavernarakis N, Driscoll M, Kyrpides NC. The SPFH domain: implicated in regulating targeted protein turnover in stomatins and other membrane-associated proteins. Trends Biochem Sci. 1999;24(11):425–427. | ||
Yamashita H, Kitayama J, Ishikawa M, Nagawa H. Tissue factor expression is a clinical indicator of lymphatic metastasis and poor prognosis in gastric cancer with intestinal phenotype. J Surg Oncol. 2007;95(4):324–331. | ||
Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200(1):39–46. | ||
Wu C, Luo Z, Chen X, et al. Two-dimensional differential in-gel electrophoresis for identification of gastric cancer-specific protein markers. Oncol Rep. 2009;21(6):1429–1437. | ||
Cho WC. Proteomics in translational cancer research: biomarker discovery for clinical applications. Expert Rev Proteomics. 2014;11(2):131–133. | ||
Song L, Liu L, Wu Z, et al. Knockdown of stomatin-like protein 2 (STOML2) reduces the invasive ability of glioma cells through inhibition of the NF-κB/MMP-9 pathway. J Pathol. 2012;226(3):534–543. | ||
Bogachek MV, De Andrade JP, Weigel RJ. Regulation of epithelial-mesenchymal transition through SUMOylation of transcription factors. Cancer Res. 2015;75(1):11–15. | ||
Wan T, Zhang T, Si X, Zhou Y. Overexpression of EMT-inducing transcription factors as a potential poor prognostic factor for hepatocellular carcinoma in Asian populations: a meta-analysis. Oncotarget. Epub 2017 June 2. | ||
Saunders LR, McClay DR. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development. 2014;141(7):1503–1513. | ||
Rogers CD, Jayasena CS, Nie S, Bronner ME. Neural crest specification: tissues, signals, and transcription factors. Wiley Interdiscip Rev Dev Biol. 2012;1(1):52–68. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.