Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Clinical Significance of Serum PGC-1 Alpha Levels in Diabetes Mellitus with Myocardial Infarction Patients and Reduced ROS-Oxidative Stress in Diabetes Mellitus with Myocardial Infarction Model
Authors Zhu N, Yan X, Li H, Wang H
Received 17 August 2020
Accepted for publication 19 September 2020
Published 28 October 2020 Volume 2020:13 Pages 4041—4049
DOI https://doi.org/10.2147/DMSO.S276163
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Ning Zhu, Xue Yan, Hongli Li, Huiqin Wang
Department 1 of Cardiology, Baoding First Center Hospital, Baoding 071002, Hebei, People’s Republic of China
Correspondence: Ning Zhu
Department 1 of Cardiology, Baoding First Center Hospital, 320 Changchengbeidajie, Baoding 071002, Hebei, People’s Republic of China
Tel +86-312-5096409
Email [email protected]
Background: In this study, we explored the clinical significance of serum peroxisome proliferator-activated receptor gamma co-activator 1 (PGC-1) alpha levels in diabetes mellitus with myocardial infarction (DMMI) patients and investigated the possible mechanism.
Materials and Methods: Serum samples were obtained from patients with DMMI or normal volunteer in Baoding First Center Hospital. C57BL/6 mice were induced by a single intraperitoneal (i.p.) injection of 100 mg/kg STZ (streptozocin) for in vivo model. Human myocardial cell lines H9C2 cells were induced with high glucose medium (33 mmol/L glucose) for in vitro model. Western blot was used to analyze the protein expressions in this study.
Results: Serum PGC-1 alpha levels were down-regulated in patients with DMMI. There was negative correlation between serum PGC-1 alpha levels and glycated hemoglobin, blood glucose or glucagon in DMMI patients. Recombination of PGC-1 alpha protein decreased the levels of glycated hemoglobin, blood glucose and glucagon, and inhibited oxidative stress and myocardial damage in mice of DMMI. Over-expression of PGC-1 alpha reduced reactive oxygen species (ROS)-oxidative stress, while down-regulation of PGC-1 alpha promoted ROS-oxidative stress via regulation of hemeoxygenase− 1 (HO-1) expression in in vitro model of DMMI. The inhibition of HO-1 expression attenuated the anti-oxidation effects of PGC-1 alpha in vitro.
Conclusion: PGC-1 alpha attenuated ROS-oxidative stress in diabetic cardiomyopathy model, and PGC-1 alpha served as a potential intervention to alleviate DMMI in clinical applications.
Keywords: PGC-1 alpha, ROS, oxidative stress, diabetes mellitus with myocardial infarction
Introduction
Diabetes mellitus is a common endocrine and metabolic disease characterized by hyperglycemia in clinical practice.1 Hyperglycemia is caused by either defective insulin secretion or impaired biological function, or both of them.2 The persistent high blood sugar in patients with diabetes mellitus would cause chronic damage and dysfunction of various tissues, especially eyes, kidneys, heart, blood vessels and nerves.3 Among them, acute myocardial infarction is one of its complications.4 Acute myocardial infarction is characterized by myocardial necrosis caused by acute and persistent coronary ischemia and hypoxia, exerting a certain impact on the quality of life and life safety of patients, especially in elderly patients. Elderly patients have weak body, poor immunity and are prone to combine multiple diseases, thus, great attention should be paid to.5
Recent studies have confirmed that enhanced oxidative stress plays an important role in the pathogenesis and development of diabetes mellitus.6,7 Myocardial ischemia/reperfusion can give rise to a large amount of ROS, and ROS can cause peroxidation reaction in the lipid bilayer of the biofilm, decreasing or even depriving its activity, subsequently causing myocardial damage.8 ROS can also damage the mitochondrial membrane and further initiate the activation of Caspase-3, which leads to the damage of myocardial cells.9 Persistent hyperglycemia can cause excessive production of mitochondrial ROS, thereby affecting transcription and leading to systolic dysfunction. ROS can excessively reduce NO levels, causing myocardial inflammation and endothelial dysfunction.10
PGC-1α is a transcriptional co-activator with close association with the body’s energy metabolism, which plays an important role in the body’s heat production, mitochondrial synthesis, glucose and lipid metabolism, and skeletal muscle fiber type conversion.11 PGC-1α is mainly expressed in tissues rich in mitochondria, such as brown fat, liver and skeletal muscle.12 In recent years, PGC-1α has become a novel research target for the treatment of metabolic diseases, including diabetes mellitus and obesity.13,14 In this study, we explored that clinical significance of serum PGC-1 alpha levels in diabetes mellitus with myocardial infarction (DMMI) patients and its possible mechanism.
Materials and Methods
Clinical Patients
Serum samples were obtained from patients with DMMI (n = 30) or normal volunteer (n = 14) in Baoding First Center Hospital (Baoding, China). Written informed consent was obtained from all participants and the research protocols were approved by the Ethics Committee of Baoding First Center Hospital.
Quantitative RT-PCR
Total RNA was isolated from human serum samples or cells samples using Trizol reagent (Thermo Fisher Scientific) and the RNA purity was detected using spectrophotometer. The synthesis of cDNA was performed by the First Strand cDNA Synthesis Kit (Fermentas, Thermo Fisher Scientific). The real-time PCR was carried out on ABI 7500 Fast Real-Time PCR system (Applied Biosystems, USA) by using SYBRGreen PCR Kit (Takara, Dalian, China). These reactions were incubated at 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 60°C for 40 s, 72°C for 30 s. Relative quantitation of the gene expression was normalized by β-actin mRNA level following the 2-ΔΔCt method and relative to the control group.
Myocardial Function and Creatine Kinase-MB Activity
Myocardial function was intermittently monitored by invasive hemodynamic measurements. Left ventricular systolic pressure (LVSP) and the maximal rates of the increase and decrease in LVSP (±dp/dtmax) were monitored by an electrophysiolograph (BioPAC). Arterial blood was collected at the end of reperfusion to collect the serum (2000 g × 10 min). Creatine kinase (CK) and Creatine kinase-MB (CK-MB) were measured using CK kit and CK-MB kits (Beyotime Biotechnology, China)
Experimental Animals
C57BL/6 mice (6–8 weeks) were purchased from Cavens Lab Animal Co., Ltd. (Changzhou, China) and kept in 26 ± 2°C a humidity of 6070% and a programmed 12 h light/12 h dark cycle. C57BL/6 mice were induced by a single intraperitoneal (i.p.) injection of 100 mg/kg STZ (Sigma-Aldrich). All animal procedures were in accordance with the Principles of Laboratory Animal Care of Baoding First Center Hospital and were approved by the Committee of Baoding First Center Hospital for the Use of Live Animals in Teaching and Research. Mice was anesthetized using injection of pentobarbital sodium (50 mg/kg). Heart was exposed and MI/R was achieved by occluding the left anterior descending (LAD) coronary artery for 45 min followed by reperfusion for 2 h.
Histopathology Examination
The left ventricle of heart samples was put in 10% formaldehyde solution for 24 h at room temperature and dehydrated in ethanol gradient. Samples were embedded in paraffin and cut down into slices (5 μm). Slices were stained with haematoxylin and eosin and observed under a light microscope (Leica Microsystems, Wetzlar, Germany).
Cell Culture and Small Interfering RNA Transfection
Human myocardial cell lines H9C2 cells were purchased from Shanghai Cell bank (Shanghai, China) and maintained in RPMI-1640 medium (Hyclone, Pittsburgh, PA, USA), containing 10% fetal bovine serum (FBS, Hyclone, Pittsburgh, PA, USA) at 37°C with 5% CO2. PGC-1 alpha, si- PGC-1 alpha, PGC-1 alpha +siHO-1, si- PGC-1 alpha +HO-1 and negative plasmids were synthesized by Genepharm (Shanghai, China) and was transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. After 48 h of transfection, cell was induced with HG medium (33 mmol/L glucose) for 24 h.
Western Blot
Cell samples and tissue samples were lysed with RIPA buffer and the protein concentration was determined by BCA assay reagent. Equal amounts of protein samples (50 μg) were separated by SDS polyacrylamide gel electrophoresis, and then transferred to PVDF membrane. The membrane was incubated with the primary antibody: PGC-1α, HO-1 and GAPDH overnight at 4°C and then the secondary antibodies conjugated to horseradish peroxidase were added. Protein blank was visualized by enhanced chemiluminescence (ECL) reagent and calculated with Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
ELISA KIT Analysis
Serum samples were collected at 1000g for 10 min and used to measure MDA, SOD, CAT and GSH activity levels. Cell samples were also collected at 1000g for 10 min and used to measure ROS production levels, MDA, SOD, CAT and GSH activity levels. ROS production levels, MDA, SOD, CAT and GSH activity ELISA kits were purchased from Nanjing Jiancheng Biological Engineering Research Institute Co. LTD (Nanjing, China).
Immunofluorescence Assay
Cell was fixed with 4% paraformaldehyde for 15 min at room temperature and incubated with 0.2% TrisX100 in TBST for 15 min at room temperature. Cell was incubated with 5% BSA in TBST for 1 h at room temperature and then incubated with HO-1 at 4°C overnight. Then, cell was washed with PBS for 20 min and stained with 555- anti-rabbit secondary antibody for 1 h at 37°C. Cell stained with DAPI assay for 15 min at darkness and imaged by fluorescence microscopy (Nikon Eclipse TE3000-U, Japan).
Statistical Methods
Values are expressed as mean ± SD using GraphPad Prism (GraphPad Software, San Diego, CA, USA). A value of p < 0.05 was considered to be significant. Two-way repeated measures ANOVA with Tukey’s post hoc test or Student’s t-tests were performed.
Results
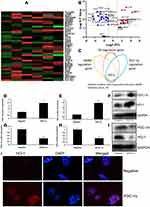
Serum PGC-1α Levels in Patients with DMMI
As shown in Figure 1AC, serum PGC-1α levels were significantly decreased in DMMI patients. Meanwhile, serum PGC-1α levels were inversely proportional to serum CK-MB or CK levels in patients with DMMI (Figure 1D and E).
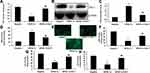
PGC-1α Prevented DMMI in Mice
The application of PGC-1α recombinant protein decreased the serum levels of CK-MB and CK, inhibited cardiomyocyte fibrosis, reduced the levels of LVEDD, LVESD and LVFS and promoted LVEF levels in mice with DMMI (Figure 2).
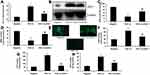
PGC-1α Inhibited ROS-Induced Oxidative Stress in vitro
To examine the mechanism of PGC-1α in DMMI, the expression of PGC-1α was up-regulated in vitro (Figure 3A). Over-expression of PGC-1α attenuated ROS production levels and MDA activity levels, and promoted the activity levels of SOD, CAT and GSH in vitro (Figure 3BG). SiPGC-1α treatment decrease the expression of PGC-1α, increased ROS production and MDA activity levels, and reduced the activity levels of SOD, CAT and GSH in vitro (Figure 3HN).
HO-1 Was an Important Target for the Function of PGC-1α in DMMI
Heat map and volcano figures were used to analyze targets for the function of PGC-1α in DMMI (Figure 4A and B). Consequently, HO-1 was an important target for the function of PGC-1α in DMMI (Figure 4C). Over-expression of PGC-1α induced the protein expression of PGC-1α and HO-1 in vitro (Figure 4DF). While down-regulation of PGC-1α suppressed the protein expression of PGC-1α and HO-1 in vitro (Figure 4GI). Immunofluorescence showed that over-expression of PGC-1α induced the protein expression of HO-1 in vitro (Figure 4J).
Regulation of HO-1 Was Involved in the Function of PGC-1α in DMMI
We further confirmed the role of HO-1 in the function of PGC-1α in DMMI. As a result, over-expression of HO-1 induced the protein expression of HO-1, reduced ROS production levels and MDA activity levels, and increased the activity level of SOD, CAT and GSH in in vitro model following si-PGC-1α (Figure 5). SiHO-1 suppressed the protein expression of HO-1, increased ROS production levels and MDA activity levels, and decreased the activity level of SOD, CAT and GSH in in vitro model following up-regulated expression of PGC-1α (Figure 6).
Discussion
Clinical studies have revealed that the incidence and mortality of myocardial infarction in patients combined with diabetes mellitus are much higher than those in patients with simple myocardial infarction.15,16 Therefore, how to protect ischemia/reperfusion injury in patients with diabetes mellitus has become a research hotspot.17 Relevant experiments have confirmed that significant alterations in cardiomyocytes, stroma, and microvessels can occur under high blood glucose, leading to myocardial remodeling.18 In the case of further progression of myocardial ischemia and hypoxic infarction, there would be not only myocardial cell apoptosis, necrosis and hypertrophy and ventricular hypertrophy, but also the destructed dynamic balance between collagen synthesis and degradation of interstitial fibers, resulting in the interstitial composition and amount changes, thereby further aggravating ventricular remodeling, and ultimately causing severe heart failure and even death.19,20 We initially found that serum PGC-1α levels in diabetes mellitus with myocardial infarction patients demonstrated a significant decrease. Oropeza et al showed that PGC-1 coactivators in β-cells regulate lipid metabolism for insulin secretion coupled to fatty acids.21 These results suggested that PGC-1α may be participated in the pathogenesis of diabetes mellitus with myocardial infarction.
Patients with diabetes mellitus have oxidative stress, and the excessive ROS generated after reperfusion further aggravates the oxidative stress, thereby causing enhanced myocardial damage.7,10,22 In recent years, studies have shown that MIRI combined with diabetes mellitus, reperfusion can lead to the release of a large amount of oxygen-free radicals, which decreases the antioxidant substances such as SOD and NO, and increases the oxidizing substances such as MDA in the blood and myocardial tissue, thereby further aggravating MIRI damage.23,24 SOD activity can be utilized to evaluate the body’s ability to clear oxygen-free radicals, and MDA can indirectly reflect the damage degree of tissues and cells.24 The detection of ROS production, MDA content and SOD, NO activity in the myocardial tissue to reflect the body’s oxidative stress level have shown that APN can decrease the range of MIRI myocardial infarction and reduce myocardial cell apoptosis in diabetes mellitus rats, but also can enhance SOD and NO activity as well as decrease MDA content and ROS production, suggesting that APN has a protective effect on MIRI of diabetes mellitus rats.24 The cause is correlated with improving the defense function of the antioxidant system, inhibiting oxidative damage, and maintaining the body’s oxidant and antioxidant balance.25 In the present study, PGC-1α prevented diabetes mellitus with myocardial infarction in mice and over-expression of PGC-1α inhibited ROS-induced oxidative stress in vitro model. Liang et al indicated that PGC-1 triggers autophagy and attenuates oxidative damage in intestinal epithelial cells.26 These demonstrated that PGC-1α had good anti-oxidation activity in diabetes mellitus with myocardial infarction.
HO-1 is an important antioxidant enzyme that plays a critical role in protecting endogenously and exogenously originated cells from harmful stimuli.27 On the one hand, the antioxidant function of HO-1 is correlated with preventing free heme from involving in the oxidation reaction.28,29 On the other hand, HO-1 and its enzymatic hydrolysate bilirubin and CO, jointly exert antioxidant, anti-inflammatory, inhibit apoptosis and vascular dilation, improve tissue microcirculation, etc.30 The Nrf 2/HO-1 pathway is widely involved in the anti-oxidative stress injury of tissues and organs, including heart, brain, liver, kidney and nervous system, which is one of the most important endogenous protection systems of the body.31 The study results suggested that HO-1 was important targets for the function of PGC-1α in Diabetes mellitus with myocardial infarction, indicating that the role of PGC-1 alpha in the intervention of HIF-lα/VEGF pathway may be related to the regulation ROS-induced oxidative stress in diabetes mellitus with myocardial infarction. Singh et al showed that PGC-1 alpha increased mitochondrial viability and increased VO2 consumption in high fat-induced obesity in mice by HO-1 levels.32 These results showed that PGC-1 alpha is related to inhibiting is related to inhibiting ROS-induced oxidative stress in diabetes mellitus with myocardial infarction by HO-1 expression.
Conclusion
The study indicates that PGC-1α prevented diabetes mellitus with myocardial infarction and reduced relevant oxidative stress. PGC-1α plays a key role in the maintenance of diabetes mellitus with myocardial infarction and PGC-1α may alleviate ROS-induced oxidative stress by activation of HO-1 signaling pathway.
Ethics Approval and Consent to Participate
Serum samples were obtained from patients with DMMI or normal volunteer in Baoding First Center Hospital (Baoding, China). Written informed consent was obtained from all participants and the research protocols were approved by the Ethics Committee of Baoding First Center Hospital.
Consent for Publication
Not applicable.
Funding
Science and Technology Project of Baoding (17ZF183).
Disclosure
The authors declare that they have no competing interests.
References
1. Arnold SV, Spertus JA, Jones PG, et al. Predicting adverse outcomes after myocardial infarction among patients with diabetes mellitus. Circ Cardiovasc Qual Outcomes. 2016;9(4):372–379. doi:10.1161/CIRCOUTCOMES.115.002365
2. Choi SY, Choi BG, Rha SW, et al. Angiotensin-converting enzyme inhibitors versus angiotensin II receptor blockers in acute ST-segment elevation myocardial infarction patients with diabetes mellitus undergoing percutaneous coronary intervention. Int J Cardiol. 2017;249:48–54. doi:10.1016/j.ijcard.2017.08.030
3. Tan X, Benedict C. IncreaSED RISK OF MYOCARDIAL INFARCTION AMONG PATIENTS WITH TYPE 2 DIABETES WHO CARRY THE COMMON RS10830963 VARIANT in the MTNR1B gene. Diabetes Care. 2020;43:2289–2292. doi:10.2337/dc20-0507
4. Triposkiadis F, Xanthopoulos A, Parissis J, Butler J, Farmakis D. Pathogenesis of chronic heart failure: cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail Rev. 2020;10.
5. Lam HCY, Chan JCN, Luk AOY, Chan EYY, Goggins WB. Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: A time series study. PLoS Med. 2018;15(7):e1002612. doi:10.1371/journal.pmed.1002612
6. Bashar T, Akhter N. Study on oxidative stress and antioxidant level in patients of acute myocardial infarction before and after regular treatment. Bangladesh Med Res Counc Bull. 2014;40(2):79–84. doi:10.3329/bmrcb.v40i2.25226
7. Di Filippo C, Cuzzocrea S, Rossi F, Marfella R, D’Amico M. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24(2):77–87. (). doi:10.1111/j.1527-3466.2006.00077.x
8. Shen M, Bai D, Liu B, et al. Dysregulated Txnip-ROS-Wnt axis contributes to the impaired ischemic heart repair in diabetic mice. Biochimica Et Biophysica Acta Molecular Basis Disease. 2018;1864(12):3735–3745. doi:10.1016/j.bbadis.2018.09.029
9. Surekha RH, Srikanth BB, Jharna P, Ramachandra RV, Dayasagar RV, Jyothy A. Oxidative stress and total antioxidant status in myocardial infarction. Singapore Med J. 2007;48(2):137–142.
10. Tao A, Xu X, Kvietys P, Kao R, Martin C, Rui T. Experimental diabetes mellitus exacerbates ischemia/reperfusion-induced myocardial injury by promoting mitochondrial fission: role of down-regulation of myocardial Sirt1 and subsequent Akt/Drp1 interaction. Int J Biochem Cell Biol. 2018;105:94–103. doi:10.1016/j.biocel.2018.10.011
11. Andrzejewski S, Klimcakova E, Johnson RM, et al. PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metab. 2017;26(5):778–87e5. doi:10.1016/j.cmet.2017.09.006
12. Bost F, Kaminski L. The metabolic modulator PGC-1α in cancer. Am J Cancer Res. 2019;9(2):198–211.
13. Cheng CF, Ku HC, Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018;19(11). doi:10.3390/ijms19113447
14. Kaarniranta K, Kajdanek J, Morawiec J, Pawlowska E, Blasiak J. PGC-1α protects RPE cells of the aging retina against oxidative stress-induced degeneration through the regulation of senescence and mitochondrial quality control. The significance for AMD pathogenesis. Int J Mol Sci. 2018;19(8). doi:10.3390/ijms19082317
15. Li W, Li M, Gao C, et al. Impact of type 2 diabetes mellitus on recurrent myocardial infarction in China. Diabetes Vascular Disease Research. 2016;13(6):395–404. doi:10.1177/1479164116653606
16. Liang H, Guo YC, Chen LM, et al. Relationship between fasting glucose levels and in-hospital mortality in Chinese patients with acute myocardial infarction and diabetes mellitus: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16(1):156. doi:10.1186/s12872-016-0331-2
17. Nauck MA, Tornøe K, Rasmussen S, Treppendahl MB, Marso SP. Cardiovascular outcomes in patients who experienced a myocardial infarction while treated with liraglutide versus placebo in the LEADER trial. Diabetes Vascular Disease Research. 2018;15(5):465–468. doi:10.1177/1479164118783935
18. Padovani C, Arruda R, Sampaio LMM. Does type 2 diabetes mellitus increase postoperative complications in patients submitted to cardiovascular surgeries? Brazilian j Cardiovascular Surgery. 2020;35(3):249–253. doi:10.21470/1678-9741-2019-0027
19. Škrlec I, Milić J, Cilenšek I, Petrovič D, Wagner J, Peterlin B. Circadian clock genes and myocardial infarction in patients with type 2 diabetes mellitus. Gene. 2019;701:98–103. doi:10.1016/j.gene.2019.03.038
20. Umar H, Mattiullah K, Nasir Hussain SK, et al. Frequency of newly diagnosed diabetes mellitus in patients with acute myocardial infarction. J Ayub Med Coll Abbottabad. 2014;26(3):368–370.
21. Oropeza D, Jouvet N, Bouyakdan K, et al. PGC-1 coactivators in β-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Molecular Metabolism. 2015;4(11):811–822.
22. Pu Z, Han C, Zhang W, et al. Systematic understanding of the mechanism and effects of Arctigenin attenuates inflammation in dextran sulfate sodium-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1. Am J Transl Res. 2019;11(7):3992–4009.
23. Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi:10.1016/j.freeradbiomed.2018.01.024
24. Liu ZH, Dai DP, Ding FH, et al. Association of serum HMGB2 level with MACE at 1 mo of myocardial infarction: aggravation of myocardial ischemic injury in rats by HMGB2 via ROS. Am J Physiol Heart Circ Physiol. 2017;312(3):H422H36. doi:10.1152/ajpheart.00249.2016
25. Zou J, Fei Q, Xiao H, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. 2019;234(10):17690–17703. doi:10.1002/jcp.28395
26. Liang D, Zhuo Y, Guo Z, et al. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. 2020;170:10–20. doi:10.1016/j.biochi.2019.12.001
27. Chen G, Liu G, Cao D, Jin M, Guo D, Yuan X. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J Nat Med. 2019;73(1):85–92. doi:10.1007/s11418-018-1241-7
28. Der Sarkissian S, Cailhier JF, Borie M, et al. Celastrol protects ischaemic myocardium through a heat shock response with up-regulation of haeme oxygenase-1. Br J Pharmacol. 2014;171(23):5265–5279. doi:10.1111/bph.12838
29. Lee TM, Lin SZ, Chang NC. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO-1 signaling. Free Radic Biol Med. 2014;77:71–81. doi:10.1016/j.freeradbiomed.2014.08.022
30. Koneru S, Penumathsa SV, Thirunavukkarasu M, Zhan L, Maulik N. Thioredoxin-1 gene delivery induces heme oxygenase-1 mediated myocardial preservation after chronic infarction in hypertensive rats. Am J Hypertens. 2009;22(2):183–190. doi:10.1038/ajh.2008.318
31. Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39(1):1–25. doi:10.1016/j.freeradbiomed.2005.03.010
32. Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016;125:8–18. doi:10.1016/j.prostaglandins.2016.07.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.