Back to Journals » Journal of Inflammation Research » Volume 16
Clinical Significance and Diagnostic Utility of NLR, LMR, PLR and SII in the Course of COVID-19: A Literature Review
Authors Kosidło JW, Wolszczak-Biedrzycka B , Matowicka-Karna J , Dymicka-Piekarska V , Dorf J
Received 28 October 2022
Accepted for publication 18 January 2023
Published 11 February 2023 Volume 2023:16 Pages 539—562
DOI https://doi.org/10.2147/JIR.S395331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Jakub Wiktor Kosidło,1 Blanka Wolszczak-Biedrzycka,2,3 Joanna Matowicka-Karna,4 Violetta Dymicka-Piekarska,4 Justyna Dorf4
1Students’ Scientific Club at the Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Bialystok, Poland; 2Department of Psychology and Sociology of Health and Public Health, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland; 3Warmia and Mazury Oncology Center of the Hospital of the Ministry of the Interior and Administration, Olsztyn, Poland; 4Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Bialystok, Poland
Correspondence: Justyna Dorf, Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Waszyngtona 15a St., 15-269, Bialystok, Poland, Tel +48 85 8 31 87 16, Email [email protected]
Abstract: Nowadays, society is increasingly struggling with infectious diseases that are characterized by severe course and even death. Recently, the whole world has faced the greatest epidemiological threat, which is COVID-19 caused by SARS CoV-2 virus. SARS CoV-2 infection is often accompanied by severe inflammation, which can lead to the development of different complications. Consequently, clinicians need easily interpreted and effective markers of inflammation that can predict the efficacy of the treatment and patient prognosis. Inflammation is associated with changes in many biochemical and hematological parameters, including leukocyte counts and their populations. In COVID-19, changes in leukocytes count populations such as neutrophils, lymphocytes or monocytes are observed. The numerous research confirm that indicators like neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelets-to-lymphocyte ratio (PLR) and systemic inflammatory index (SII) may prove effective in assessment patient prognosis and choosing optimal therapy. Therefore, in this review, we would like to summarize the latest knowledge about the diagnostic utility of systemic inflammatory ratios – NLR, LMR, PLR and SII in patients with COVID-19. We focused on the papers evaluating the diagnostic utility of inflammatory ratios using ROC curve published in the recent 3 years. Identification of biomarkers associated with inflammation would help the selection of patients with severe course of COVID-19 and high risk of death.
Keywords: COVID-19, inflammatory ratios, NLR, LMR, PLR, SII
Graphical Abstract:
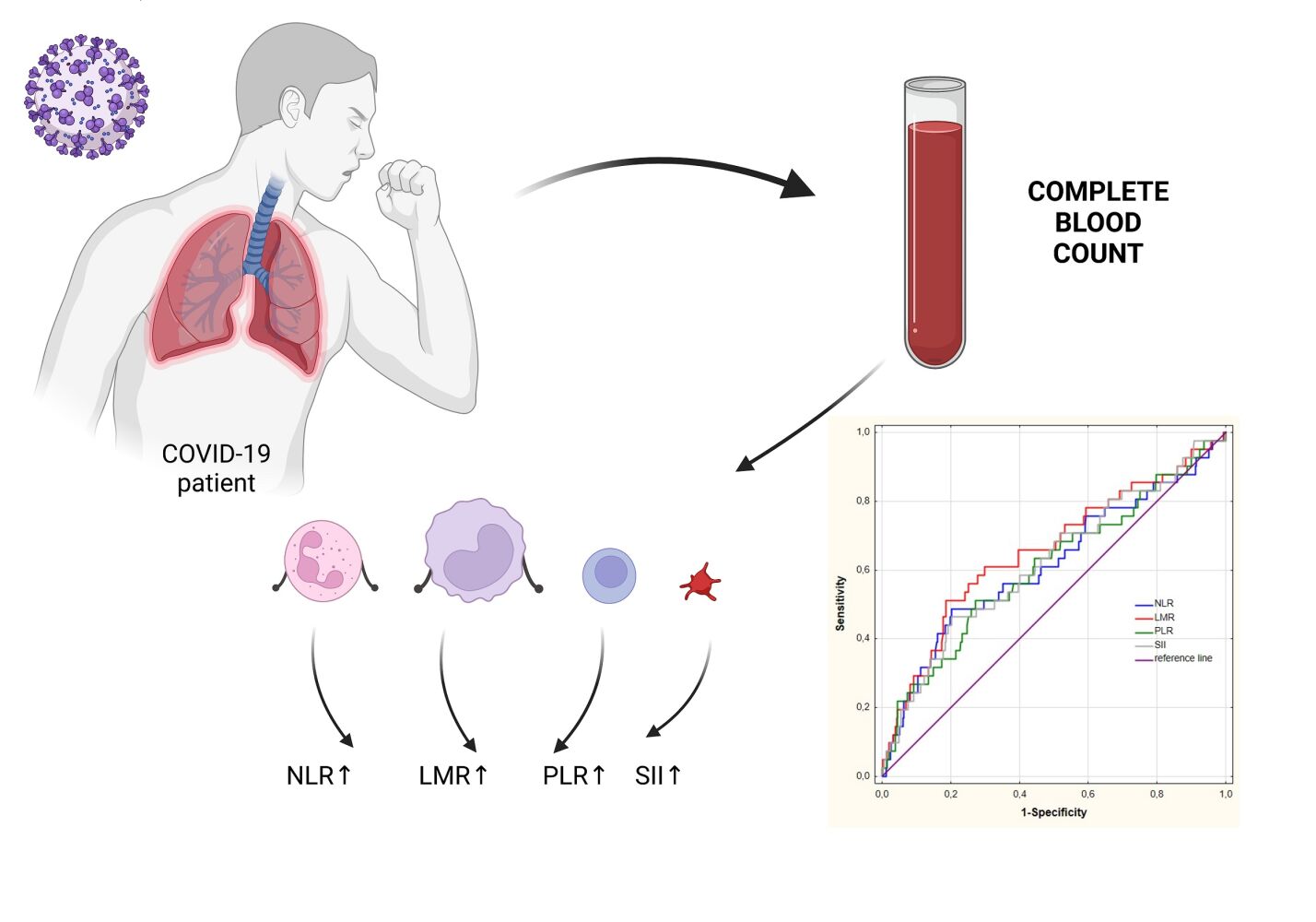
Introduction
Every day, the human body comes into contact with numerous pathogens that may trigger the development of various diseases. In recent years, SARS-CoV-2 virus which causes COVID-19 (coronavirus disease) has become particularly important and is now a serious health problem. The first cases of COVID-19 were reported in Wuhan, Hubei Province, China in November 2019, while in Poland the first case of the disease was confirmed on March 4, 2020.1–3 The total number of COVID-19 cases dated to the beginning of July 2022 worldwide was over 562 million, of which about 6.37 million resulted in death of a patient. In Poland, there were about 6.03 million confirmed cases of COVID-19 and almost 116,000 deaths. The rapidly growing statistics on the number of infections and deaths prompted researchers to accurately classify the etiological agent, delve into the mechanism of the disease development and its complications, and search for markers, effective treatment plans and ways to prevent infection.
It has been discovered that SARS-CoV-2 virus is a member of the Coronaviridae family which also includes over 40 different species of viruses that can infect animals and humans (Figure 1).1,24 The first human coronaviruses were detected in the early 1960s. Some of them, including CoV-229E, CoV-OC43, HCoV-NL63 and HCoV-HKU1, are etiological agents of common upper respiratory tract infections.4–6 Other coronaviruses include SARS-CoV-1 which caused the epidemic of severe acute respiratory syndrome (SARS) in the years 2002–2004,7,8 and MERS-CoV which was responsible for the development of a small epidemic of Middle East respiratory syndrome (MERS) characterized by high mortality rate.4,9–11 The most infectious virus belonging to the Coronaviridae family is SARS-CoV-2 which is responsible for the global pandemic in 2020 that caused the most severe socio-economic crisis to date.1,12–14
 |
Figure 1 Taxonomic classification of coronavirus. |
 |
Figure 2 Host cell infection and cytokine storm in COVID-19. |
The course of SARS-COV-2 virus infection is characterized by high variability, depending on the patient’s condition as well as individual predisposition.15 According to the reports prepared by WHO-China, patients with COVID-19 most frequently develop a dry cough, fever, fatigue, and loss of taste or smell. Less common symptoms include sore throat, headache, muscle aches, and diarrhoea. Dyspnea, difficulty breathing, absent-mindedness, chest pain, or loss of speech should prompt the patient to immediately report for diagnosis and treatment, as these symptoms may be harbingers of a severe course of infection, which may ultimately lead to respiratory failure and death of the patient. Patients from risk groups, such as the elderly, the obese, people with chronic diseases, those with immunodeficiency, and those who have not been vaccinated, are particularly vulnerable.15,16 It is well known that majority of symptoms are caused by inflammation which is why many scientists are conducting research and trying to discover a new specific and sensitive marker that would reflect the severity of inflammation and thus the condition of the patient. This review is the first summary of the latest knowledge on the diagnostic utility of systemic inflammatory ratios – neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelets-to-lymphocyte ratio (PLR) and Systemic Inflammatory Index (SII) and their application in monitoring of patients with COVID-19. Identification of biomarkers associated with inflammation would help the selection of patients with severe course of COVID-19 and high risk of death.
Search Strategy
We searched PubMed database was searched for sources using the following keywords: NLR, neutrophil-to-lymphocyte ratio, PLR, lymphocyte-to-monocyte ratio, LMR, platelets-to-lymphocyte ratio, PLR, systemic inflammatory index, SII, inflammation, inflammatory markers, inflammatory ratios, COVID-19, SARS-CoV-2, diagnostic utility, ROC curve, AUC. Preference was given to the sources which were published within the past 3 years.
SARS-CoV-2 Structure and Infection Mechanisms
SARS-CoV-2 is an enveloped betacoronavirus. The membrane (M) protein, the most abundant structural protein, is mainly responsible for the shape of the envelope. The genetic material of the virus consists of a single strand of ribonucleic acid with positive polarity located inside the protein capsid. The viral RNA chain consists of approximately 30,000 nucleotides and encodes four structural proteins: spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid (N).17–20
Once the virus has entered the body, the S1 subunit of the spike protein (S) attaches to the angiotensin-converting enzyme (ACE2) receptor that is present in the airway epithelium. The ACE2 receptor is physiologically involved in regulating the water and electrolyte balance of the cell. Another important protein is transmembrane serine protease 2 (TMPRSS2), which belongs to the group of scavenger receptors whose main function is to transfer a wide range of ligands into the cell by endocytosis (Figure 2). This protein is highly expressed in the nasal epithelium, respiratory tract and pneumocytes, and weaker expression in the intestines, which explains why SARS-CoV-2 initially localizes in these areas. In addition, TMPRSS2 protein with proteolytic functions cleaves and activates viral glycoproteins. The S2 protein is then released from the domain, facilitating the fusion of the virion with the host cell, or passage of the capsid via endocytosis into the cell. The E protein of SARS-CoV-2 virus contributes to acceleration of the process of infecting host cells. It belongs to small proteins classified as viroporins which combine with the cell membrane, forming protein-lipid pores in it, increasing the permeability of the membrane.17,18,20 Further steps of infection include the opening of the endosome and the proteolytic cleavage of the capsid in the cytoplasm, which leads to the release of the viral genetic material. Nucleoprotein N is responsible for proper packing of the ribonucleic chain and the formation of the ribonucleocapsid. Then, in a further step, the processes of replication, transcription and translation take place, enabling the formation of new virions and, through exocytosis, their export outside the host cells. During the replication of the genetic material of the virus and the translation process, the immune system may be activated.17,18,20
Classifications of SARS-CoV-2 Variants
The process of duplication of genetic material in coronaviruses may lead to the formation of new mutations.21 Due to the emergence of many different variants of SARS-CoV-2 virus characterized by varying severity of symptoms or virulence, the WHO introduced nomenclature based on the letters of the Greek alphabet to designate the key strains of the virus.22 The table presents coronavirus variants (Table 1).23
 |
Table 1 Coronavirus Variants. |
Inflammation
Inflammation is most commonly caused by infectious agents such as bacteria, viruses, fungi, as well as non-infectious agents including trauma, toxic substances, radiation, etc.24 The human body defends itself against these agents through a passive and active immune responses. The passive response includes all mechanisms that are functionally active all the time, or initiated after the cells are exposed of harmful agents. These include cilia of epithelial cells that move mucus, tight intercellular junctions, and proteins dissolved in vascular and tissue fluids. Furthermore, small bioreactive molecules and various types of receptors embedded in the cell membrane that recognize molecular patterns on the surface of pathogens play an important role.25 The function of the adaptive immune response is to activate degradation and removal of pathogens and toxic substances. However, this system could not function without the ability to recognise inflammatory triggers. Lymphocytes, which belong to the leukocyte population, are responsible for detecting pathogens and substances produced by them. Lymphocytes are divided into two main classes: T lymphocytes – included in the immune response cells, and B lymphocytes – responsible for the production of antibodies, associated with a humoral immune response. Protein antibodies can travel in the bloodstream as well as tissue or extracellular fluids, triggering a cellular response of granulocytes or macrophages. On the other hand, T lymphocytes, after detecting foreign agents such as viruses, can directly kill the infected cell by activating the process of apoptosis or by secreting signalling proteins for phagocytes.26,27 Lymphocytes belong to the pool of cells that circulate in the bloodstream, so their number and activity may vary depending on the etiological agent or health status of the exposed person.28
Inflammation and Inflammatory Cells in COVID-19
SARS-CoV-2 virus infection has a complex course and is characterized by, among others, hypoxemia. This is caused by inflammation that sometimes takes the form of an enhanced reaction (hyperinflammation). Consequently, patients with COVID-19 often present a considerable increase in the concentration of pro-inflammatory cytokines in the blood as well as the lung parenchyma.29,30 A large group of cells is involved in this response – mainly monocytes, lymphocytes, neutrophils and, according to recent reports, platelets. These cells detect pathogens as well as damage to the respiratory epithelium through pathogen recognition receptors (PRRs).31 They are located in the cell membrane and in the cytoplasm, which enables them to detect specific ligands. Substances bound (and recognised) by PRRs include viral proteins belonging to the large group of pathogen-associated molecular pattern (PAMP) compounds. They can appear in many tissues, such as the lungs and blood, where pathogens appear as a result of infection. In turn, proteins released from necrotic cells, for instance in the alveoli or airway epithelium, are classified as damage-associated molecular pattern (DAMP).31,32 The binding of PRRs to their ligands, such as DAMP and PAMP, located in the interstitial lung tissue, leads to the induction of cytokines, thus promoting the state of “cytokine storm”.31 This term refers to a self-activating cascade of cytokine production.32 The consequence of this immune system hyperreaction in the course of COVID-19 is the sepsis-like condition observed in patients with severe COVID-19 infection. The cytokine storm involves, among others, such substances as IL-6, IL-8, TNF-α, IFN-γ, IL-17.29–32 On the other hand, the production and secretion of interferons: IFN-α, IFN-β, IFN-γ, which are a major component of the body’s antiviral response, is weakened. Increased activity of cytokines and their decreased effect in the case of interferons result in a change in their ratio to each other, which is observed in a small group of COVID-19 patients who developed a critical illness (Figure 2).32
Macrophages
There are several different classes of alveolar macrophages, including alveolar macrophages, influx monocytic macrophages, transitional macrophages, and monocytes. These different classes of macrophages vary in terms of their function in inflammation. It has been observed that the number of different types of macrophages varies depending on the severity of the SARS-CoV-2 infection. This may lead to a disruption of the precise mechanisms that control inflammation in COVID-19 patients.31,33 Macrophages in critically ill patients induce the expression of many proteins including IL-6, IL-8, TNF-α, and alarmins. In a mild course of infection, monocytes flow into the alveoli, while in a severe course of the disease, inflammatory monocytes are depleted and degraded, and in their place there is an influx of large numbers of hyperinflammatory monocytes. The last of the mentioned isotypes is responsible for increased secretion of cytokines and chemokines.31,33
Neutrophils
Increased production of cytokines in COVID-19 patients has a stimulating effect on the movement of neutrophils from the vascular space to the alveoli. At the same time, it has been observed that elevated concentration of IL-17, which is responsible for neutrophil activation, may contribute to the development of severe form of the disease.31,34 Activation of neutrophils leads to increased expression of a number of proteins such as DAMP, chemokines and cytokines. In addition, neutrophils may undergo degranulation during which defensins, myeloperoxidase, lysozyme, etc., are secreted from the inner granules of the cell into the surrounding environment.31,34 The consequence of this process is intensified production of reactive oxygen species such as H2O2. Simultaneously, the production of antioxidant enzymes acting as anti-inflammatory proteins, such as superoxide dismutase (SOD), decreases, leading to the development of oxidative stress. Consequently, neutrophil infiltration in the lung tissue may lead to additional damage not only to infected but also to normal cells. Increased cell breakdown induced by neutrophil activity results in an increase in extracellular space (DAMP), triggering a cytokine storm.31,34,35
Neutrophils may also be involved in other mechanisms that may trigger pathogen inactivation, including the formation of a neutrophil extracellular trap (NET). This mechanism involves the release of nuclear chromatin fibres derived from neutrophils. At an early stage of activation, the fibres bind to enzymes such as cathepsin G or myeloperoxidase. Such complexes immobilize pathogens and locally concentrate antimicrobial substances, leading to pathogen degradation and thus inhibition of infection.34,36 Complexes of various enzymes with chromatin fibres may have different functions, including anti-inflammatory – via enzymatic neutralization of cytokines and chemokines.27 In turn, interactions of NETs with platelets may prove to be potentially dangerous and lead to damage to the vascular endothelial and an increased risk of vascular complications in the course of COVID-19.36–38
Lymphocytes
Lymphocytes are responsible for the adaptive immune response directed to specific components of the pathogen. The complex process of activation begins at the site of primary infection.31,39 The presence of specific proteins of the viral envelope (E, N, S) is detected by the PRRs of macrophages. Antigen presentation may occur in the bloodstream or in lymphoid organs after antigen-presenting cells (APCs) enter them. These cells, after neutralizing the pathogen in cooperation with dendritic cells, present the virus antigen to naive T lymphocytes. The next step is further maturation and differentiation of naive T cells in the lymphoid organs into CD4+ and CD8+ subpopulations. Proliferating Th lymphocytes (CD4+) stimulate naive B lymphocytes to transform into plasmocytes that produce antibodies directed against viral antigens. Th lymphocytes secrete antiviral IFN-γ in response to viral antigens, leading to its neutralization. They also secrete cytokines, including IL-2, IL-4, and IL-5.40,41 In contrast, the proliferation and maturation of Tc lymphocytes (CD8+) lead to the passage of these cells into the bloodstream. Tc lymphocytes undergo sequestration into the alveoli and airways, where they secrete cytotoxic proteins in response to detection of antigens on the surface of infected cells via MHC class I receptors. Cytotoxic proteins include perforins, granzymes, TNF, TRAIL, etc.39–41 In patients with a severe course of infection, we observe an impaired lymphocyte response with an accompanying decrease in the lymphocyte count.39,42,43
Several mechanisms are responsible for the lymphopenia that occurs in COVID-19 patients. One of them is the migration of Tc lymphocytes to the site of infection as a result of local production of chemokines. As a result of the increased hyperinflammatory response, more cells enter the alveoli, reducing the number of circulating lymphocytes.39,42,44 Lymphopenia may also develop as a result of infection of lymphocytes with SARS-CoV-2 virus, which may lead to the reaction of T cells (CD8+) and specific antibodies with infected immune cells. Moreover, lymphopenia can be caused by increased lymphocyte apoptosis.31,39,45 In patients with severe SARS-CoV-2 infection, we observe impaired lymphopoiesis and stimulation of myelopoiesis caused by elevated levels of cytokines such as IL-1, IL-6, IL-27.39,45,46 Autopsies of patients who died from COVID-19 revealed that the virus was detected in the lung tissue, heart muscle, spleen, kidneys, and intestines. In these patients, tissue damage was quite significant, but this may have been related to the presence of comorbidities. It might be significant that viral RNA was detected in the spleen in all patients. Severe structural abnormalities in this organ were also observed, along with damage to the white pulp in which lymphocytes proliferate.47–49
Platelets
SARS-CoV-2 infection is accompanied by thrombocytopenia. It is assumed that the decrease in platelet count (PLT) may be associated with antibiotic therapy and administration of anticoagulants and anti-inflammatory drugs. However, it may also be related to the role of platelets in fighting infection. It should be noted that thrombocytopenia may be associated with a significant risk due to impaired haemostasis. This process may be accompanied by symptoms such as the appearance of bruises, thrombocytopenic purpura, or impaired blood clotting after injury. PLTs also play a supporting role in fighting infection as they contain pattern recognition receptors (PRRs) that detect pathogens. Binding of the ligand to the receptor leads to platelet activation and secretion of alpha and beta interferons, and interleukins including IL-6, which result in inhibition of platelet production by megakaryocytes. In addition, hyperactive platelets are recognized by the immune system and degraded.50–53
Therefore, interferons can inhibit cell proliferation in the bone marrow, with the exception of granulocytes. This is because macrophages, granulocytes and many other cells secrete growth factors such as GM-CSF and G-CSF. At the same time, a “cytokine storm” is observed in patients with a severe condition, in the course of which there is an abundant secretion of, among others, IL-6 and IL-8. Accordingly, a decrease in PLT level is observed due to sensitivity of megakaryocytes to signals that inhibit platelet formation.50–53
Inflammatory Markers in COVID-19
NLR
Based on the blood count results, we can calculate a number of different ratios that indicate ongoing inflammatory processes in the patient’s body. One of them is NLR (neutrophil-to-lymphocyte ratio). It is the ratio of the absolute number of neutrophils to the number of lymphocytes in 1μL of peripheral blood. One of the first reports describing the use of this parameter dates back to 200154 when NLR was used to assess the severity of inflammation in patients who had undergone surgery to remove colorectal cancer. The study showed that both invasive procedures and active disease processes, including sepsis, contribute to an increase in NLR value.54 As already mentioned above, the number of neutrophils secreting pro-inflammatory cytokines increases in patients with COVID-19, particularly in severe and critical course of the disease. This condition is caused by a strong activation of the non-specific cellular response, and promotes the development of lymphopenia. The combination of increased neutrophil count and decreased lymphocyte count contributes to an elevated value of NLR.31,39,54,55 The advanced course of COVID-19 disease is associated with an imbalance between the levels of neutrophils and lymphocytes. This may be related to the occurrence of a “cytokine storm” in which neutrophils play a significant role as effector cells. It has been observed that the number of neutrophils increases in patients with severe symptoms, while the number of lymphocytes decreases. The neutrophil-to-lymphocyte ratio illustrates the correlation between these two populations, which may be an early indicator of the severe course of the disease.34,38,56,57
The study by Maddani et al was one of the few to demonstrate an excellent (AUC = 0.91) predictive value of the NLR parameter as a predictor of COVID-19 severity.58 Yan et al also obtained in their study a promising value of AUC = 0.945 in their study, with a sensitivity of 97.5% and specificity of 78.1% of NLR for assessing the risk of death of COVID-19 patients. Moreover, they found NLR to be an independent risk factor in predicting death from any cause during hospitalization.59 Wang et al demonstrated a high value (AUC = 0.963, sensitivity 100%; specificity 84%) of NLR as a predictor of patient death in the course of COVID-19 infection.60 Ertekin et al also showed that the NLR factor may be useful in predicting patient’s risk of death (AUC = 0.930, sensitivity 90.9%, specificity 84.4%).61 Reports by Bastug et al, Zhao et al, Gujar et al, and Acar et al indicate a good predictive value of the NLR ratio in predicting a patient’s condition or its deterioration. The AUC values in the studies of these authors were 0.861, 0.76, 0.86, and 0.816, respectively.62–65 According to the results obtained by Waris et al and Ramos-Peñafiel et al, there were no statistically significant differences in NLR values between the study and control groups.66,67 In both studies, the groups were not large enough, so the patients showed a considerable degree of variability within them. The exact data can be found in Table 2.
 |
Table 2 NLR Ratio in Various Studies.58–86 |
PLR
The platelet-to-lymphocyte ratio (PLR) is another parameter calculated from the complete blood count and is referred to as a non-specific marker of inflammation. One of the earliest studies describing PLR involved the evaluation of inflammation in hospital-acquired infections, and changes in PLR values in cases of death in intensive care unit. The researchers demonstrated no statistically significant differences in PLR values compared to fatal and cured cases. However, the observed statistically significant difference in the PLR value indicates that it is more valuable in the group of patients with nosocomial infections compared to those without infections.84 As already mentioned, platelets – apart from their main haemostatic function – play an indirect role in inflammation. In COVID-19, thrombocytopenia occurs as a result of multiple processes caused by a hyperinflammatory state. This is due to the high levels of pro-inflammatory cytokines and the direct activation of platelets present in the course of infection by fragments of broken cells and viral proteins. Activated PLTs show an increased tendency to aggregate, clot, or degrade. As a result, a decrease in PLR is observed in patients in good or intermediate general condition. In contrast, patients in severe condition experience a significant increase in PLR, which is also associated with a decrease in the number of lymphocytes.85
The report by Gujar et al indicates a relatively high diagnostic value (AUC = 0.826) of PLR in predicting the patient’s condition due to current inflammation in the course of COVID-19.64 Acar et al showed a statistically significant difference in PLR values in the groups of survivors vs non-survivors, and a good value of the area under the ROC curve: AUC = 0.742.65 The researchers indicate the possibility of using this coefficient as an auxiliary measure in prognostic evaluation of a patient’s condition and mortality due to COVID-19. Numerous reports confirmed statistically significant differences in PLR values which reached higher levels in patients in severe or critical condition compared to those with a milder course of COVID-19 infection. There are also reports by Bilge et al, Ramos-Peñafiel et al, Citu et al, and Fois et al, which showed no differences in PLR values between the control and study groups. The researchers emphasize the need for a further analysis of this parameter in the evaluation of patients’ condition (Table 3).66,67,73,86
 |
Table 3 PLR Ratio in Various Studies.63–86 |
LMR
Another factor calculated from the complete blood count and used as an indicator for assessing patients’ inflammation and condition is the lymphocyte-to-monocyte ratio (LMR). This parameter represents the ratio of lymphocytes to monocytes in 1 μL of peripheral blood. The use of LMR in COVID-19 patients may prove effective in assessing the course of the disease. The first mention of LMR appeared in the paper by Merekoulias et al, published in 2010, in which the authors described the application of LMR in the screening diagnosis of viral diseases, ie, influenza.87 Zhu et al considered LMR as a marker of systemic inflammation in different types of cancer88 while Ren et al conducted research on the use of LMR as a prognostic factor in patients with cardiovascular disease.89 COVID-19 patients show increased secretion of inflammatory mediators that enhance immune system functions such as granulopoiesis and monocytopoiesis. These cells become activated during infection and intensely secrete pro-inflammatory cytokines including IL-6, IL-8, TNF, IFN, etc., thus inducing a state of “cytokine storm” that inhibits lymphocytopoiesis. In patients with COVID-19, lymphopenia is observed in laboratory tests, and, in more severe or critical cases, monocytosis, which results in a decrease in LMR.
Zhao et al demonstrated a good diagnostic value of the LMR (AUC = 0.73) in differentiating the course of the disease with accompanying inflammation severity. The researchers observed higher LMR value in patients infected with SARS-CoV-2 compared to those with Influenza A virus.64 Citu et al obtained satisfactory prognostic value of LMR (AUC = 0.69) in the assessment the risk of death of patients hospitalised in the course of COVID-19.73 Waris et al, Ramos-Peñafiel et al, and Moisa et al observed a decrease in the value of the ratio with the severity of the disease.67,74,90 However, Bilge et al and Fois et al showed no statistically significant differences in LMR values between patients with different clinical status (Table 4).66,86 Such inconclusive results indicate the need for further analysis of LMR in COVID-19.
 |
Table 4 LMR Ratio in Various Studies.63–98 |
SII
One of the new indicators for assessing systemic inflammation is the systemic immune-inflammation index (SII). This parameter is calculated by multiplying the number of platelets and neutrophils divided by the number of lymphocytes. Initially, SII was analysed for its prognostic value in patients with hepatocellular carcinoma (HCC). Researchers showed that higher SII values in the perioperative period may be correlated with a worse prognosis of the disease. The condition was associated with enhanced inflammation in hepatocellular carcinoma lesions. They also demonstrated that 85% of the study group with increased SII values had HBV infection.91 Another report on SII concerned the evaluation of prognosis in patients with small cell lung cancer (SCLC). It showed a significantly higher level of SII in patients with SCLC. Such results, according to the researchers, were related to increased inflammation caused by strong activation of the immune system.92 Chu et al showed elevated SII values in patients with acute myocardial infarction. The course of myocardial infarction is associated with complex processes leading to increased breakdown of endothelial cells of blood vessels in the heart as well as breakdown of myocytes in the ischemic area. This leads to an intensification of the inflammatory response, which leads to an increase in, among others, number of neutrophils, monocytes, and platelets in the circulating pool.93 Similarly, in the course of COVID-19, there are dynamic changes in the patient’s clinical condition as a result of an increased non-specific cellular response, as in the other diseases mentioned above.
The study by Gujar et al showed a very high diagnostic value of the SII parameter (AUC = 0.841) in differentiating the severity of the course of the disease caused by the SARS-CoV-2 virus. The mean value of SII in seriously ill and critically ill patients hospitalized in the ICU was 2484, and in the control group in home or hospital isolation: 653.3 (p = 0.001).64 A high diagnostic value was observed by Hamad et al, in which the area under the ROC curve (AUC) was 0.819. The study group of critically ill patients hospitalized in the ICU had a mean SII value of 2016.29, while patients who did not require ICU hospitalization had a mean SII value about four times lower: 492.29. In addition, the researchers obtained a diagnostic sensitivity of SII of 50.9%, and specificity – 95.6% in assessing the risk of worsening of the condition of COVID-19 patients, and thus also hospitalization in the ICU.94 Zhao et al showed that the SII shows a good diagnostic value in predicting the exacerbation of a patient’s condition (AUC = 0.72). The studied SII coefficient, together with other biochemical markers, including IL-6, as well as haematological markers and calculated ratios, including NLR and PLR, may be useful in assessing the severity of inflammation (intensity may be related to an inflammatory response) and thus the patient’s condition.63 Moreover, a study by Acar et al demonstrated the diagnostic utility of SII in determining the risk of death of COVID-19 patients (AUC = 0.733).65 SII values were significantly higher in patients whose disease led to death compared to those who recovered.65 These results may indicate that a strong non-specific cellular response may be accompanied by a significant increase in inflammation and thus a worse clinical condition of the patient. However, there are reports by Bilge et al and Citu et al, in which no statistically significant differences in SII values were observed in COVID-19 patients with different clinical conditions (Table 5).66,73
 |
Table 5 SII in Various Studies [63–85] |
Conclusions
In COVID-19, we observe a significant dynamics of changes in the clinical condition of patients, caused by hyperactivation of the immune system. This mechanism is associated with numerous changes in laboratory tests, which is particularly visible in biochemical and haematological parameters. Complete blood count which quantitatively and qualitatively evaluates blood cell populations has proven to be a commonly used and effective tool for assessing the clinical condition of a patient. Factors such as NLR, LMR, PLR or SII, calculated on the basis of the number of individual blood cell populations, may prove useful to clinicians in assessing the patient’s condition and the severity of inflammation in the course of COVID-19. The advantage of determining ratios is also easy interpretation of the calculated parameters, low cost, and routine blood counts at specific intervals.
Numerous studies indicate the high diagnostic value of NLR in assessing the severity of inflammation in the course of infection caused by the SARS-CoV-2 virus. The NLR was higher in patients with a worse clinical condition than mild and moderate as well as in patients who died from COVID-19. This suggests that this ratio can be used as a parameter to assess the risk of health deterioration or death. Furthermore, PLR was statistically significant higher in patients in severe than moderate clinical condition and should be evaluated together with other inflammatory markers. The SII ratio has been proven to be effective in evaluating a patient’s condition. This marker can be very useful in assessing the severity of the disease course and, for example, in the initial selection of optimal therapy. The fewest studies concerned the evaluation of the diagnostic utility of the LMR parameter. Despite the occurrence of an inflammatory response in the course of the disease, patients do not always demonstrate changes in LMR values. On the other hand, some studies show a decrease in the level of LMR in patients with a more severe course of the disease. However, currently there are still relatively few reports clearly confirming the effectiveness of analysed ratios, which should be taken into account in further research.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was granted by the Medical University of Bialystok, Poland (grant number: SUB/1/DN/22/001/2209).
Disclosure
The authors declare that they have no competing interests.
References
1. Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics. 2020;52(11):549–557. doi:10.1152/physiolgenomics.00089.2020
2. Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int J Biol Sci. 2020;16(10):1678–1685. doi:10.7150/ijbs.45053
3. Wang H, Li X, Li T, et al. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020;39(9):1629–1635. doi:10.1007/s10096-020-03899-4
4. National Center for Biotechnology. PubChem taxonomy summary for taxonomy 11118, coronaviridae. PubChem taxonomy summary for taxonomy 11118, coronaviridae; 2022. Available from: https://pubchem.ncbi.nlm.nih.gov/taxonomy/Coronaviridae.
5. Kahn JS, McIntosh K. History and recent advances in coronavirus discovery. Pediatr Infect Dis J. 2005;24(11):S223–S227. doi:10.1097/01.inf.0000188166.17324.60
6. Lau SKP, Woo PCY, Yip CCY, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44(6):2063–2071. doi:10.1128/JCM.02614-05
7. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi:10.1056/NEJMoa030685
8. Rota PA, Oberste MS, Monroe SS, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi:10.1126/science.1085952
9. Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi:10.1056/NEJMoa1211721
10. Omrani AS, Al-Tawfiq JA, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog Glob Health. 2015;109(8):354–362. doi:10.1080/20477724.2015.1122852
11. Pavli A, Tsiodras S, Maltezou HC. Middle East respiratory syndrome coronavirus (MERS-CoV): prevention in travelers. Travel Med Infect Dis. 2014;12(6):602–608. doi:10.1016/j.tmaid.2014.10.006
12. Ren S-Y, Wang W-B, Hao Y-G, et al. Stability and infectivity of coronaviruses in inanimate environments. World J Clin Cases. 2020;8(8):1391–1399. doi:10.12998/wjcc.v8.i8.1391
13. Marquès M, Domingo JL. Contamination of inert surfaces by SARS-CoV-2: persistence, stability and infectivity. A review. Environ Res. 2021;193:110559. doi:10.1016/j.envres.2020.110559
14. Kim D-Y, Shinde SK, Lone S, et al. COVID-19 pandemic: public health risk assessment and risk mitigation strategies. J Pers Med. 2021;11(12):1243. doi:10.3390/jpm11121243
15. Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–1839. doi:10.1016/j.jiph.2020.07.014
16. World Health Organization. WHO COVID-19 dashboard. Geneva: World Health Organization; 2020. Available from: https://covid19.who.int/.
17. Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81(3):357–371. doi:10.1016/j.jinf.2020.06.067
18. Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta. 2020;1866(10):165878. doi:10.1016/j.bbadis.2020.165878
19. Soldevila B, Puig-Domingo M, Marazuela M. Basic mechanisms of SARS-CoV-2 infection. What endocrine systems could be implicated? Rev Endocr Metab Disord. 2022;23(2):137–150. doi:10.1007/s11154-021-09678-6
20. Seyed Hosseini E, Riahi Kashani N, Nikzad H, et al. The novel coronavirus Disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi:10.1016/j.virol.2020.08.011
21. Lauring AS, Frydman J, Andino R. The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol. 2013;11(5):327–336. doi:10.1038/nrmicro3003
22. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–569. doi:10.1038/s41564-020-0688-y
23. CDC. SARS-CoV-2 variant classifications and definitions; 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html.
24. Cologne GI for Q and E in HC. What is an Inflammation? InformedHealth.org; 2010.
25. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2):S3–S23. doi:10.1016/j.jaci.2009.12.980
26. Moro-García MA, Mayo JC, Sainz RM, et al. Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.00339
27. Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–517. doi:10.1038/nm.3547
28. Blumenreich MS. Chapter 153 the white blood cell and differential count. In: Clinical Methods: The History, Physical, and Laboratory Examinations. Butterworth Publishers; 1990. Available from. https://www.ncbi.nlm.nih.gov/books/NBK261/.
29. Soy M, Keser G, Atagündüz P, et al. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–2094. doi:10.1007/s10067-020-05190-5
30. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi:10.1016/S0140-6736(20)30628-0
31. Paludan SR, Mogensen TH. Innate immunological pathways in COVID-19 pathogenesis. Sci Immunol. 2022;7(67):eabm5505. doi:10.1126/sciimmunol.abm5505
32. Tang Y, Liu J, Zhang D, et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01708
33. Kosyreva A, Dzhalilova D, Lokhonina A, et al. The role of macrophages in the pathogenesis of SARS-CoV-2-associated acute respiratory distress syndrome. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.682871
34. Cavalcante-Silva LHA, Carvalho DCM, Lima É de A, et al. Neutrophils and COVID-19: the road so far. Int Immunopharmacol. 2021;90:107233. doi:10.1016/j.intimp.2020.107233
35. Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi:10.1016/j.mehy.2020.110102
36. Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020. doi:10.1172/jci.insight.138999
37. Middleton EA, He X-Y, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi:10.1182/blood.2020007008
38. Borges L, Pithon-Curi TC, Curi R, et al. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:1–7. doi:10.1155/2020/8829674
39. Eijk LE, Binkhorst M, Bourgonje AR, et al. COVID‐19: immunopathology, pathophysiological mechanisms, and treatment options. J Pathol. 2021;254(4):307–331. doi:10.1002/path.5642
40. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437–440. doi:10.1038/s41586-020-2355-0
41. Muntjewerff EM, Meesters LD, van den Bogaart G. Antigen cross-presentation by macrophages. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01276
42. Bobcakova A, Petriskova J, Vysehradsky R, et al. Immune profile in patients with COVID-19: lymphocytes exhaustion markers in relationship to clinical outcome. Front Cell Infect Microbiol. 2021;11. doi:10.3389/fcimb.2021.646688
43. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8(1):36. doi:10.1186/s40560-020-00453-4
44. Anurag A, Jha PK, Kumar A. Differential white blood cell count in the COVID-19: a cross-sectional study of 148 patients. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):2099–2102. doi:10.1016/j.dsx.2020.10.029
45. Fathi N, Rezaei N. Lymphopenia in COVID‐19: therapeutic opportunities. Cell Biol Int. 2020;44(9):1792–1797. doi:10.1002/cbin.11403
46. Chiba Y, Mizoguchi I, Hasegawa H, et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell Mol Life Sci. 2018;75(8):1363–1376. doi:10.1007/s00018-017-2724-5
47. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi:10.1016/S0140-6736(20)31305-2
48. Remmelink M, De Mendonça R, D’Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24(1):495. doi:10.1186/s13054-020-03218-5
49. Edler C, Schröder AS, Aepfelbacher M, et al. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134(4):1275–1284. doi:10.1007/s00414-020-02317-w
50. Hottz ED, Bozza FA, Bozza PT. Platelets in immune response to virus and immunopathology of viral infections. Front Med. 2018;5. doi:10.3389/fmed.2018.00121
51. Delshad M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, et al. Platelets in the perspective of COVID-19; pathophysiology of thrombocytopenia and its implication as prognostic and therapeutic opportunity. Int Immunopharmacol. 2021;99:107995. doi:10.1016/j.intimp.2021.107995
52. Micota B, Sadowska B, Różalska B. The role of blood platelets in infections. Postepy Hig Med Dosw. 2015;69:624–632. doi:10.5604/17322693.1153073
53. Rohlfing A-K, Rath D, Geisler T, et al. Platelets and COVID-19. Hamostaseologie. 2021;41(05):379–385. doi:10.1055/a-1581-4355
54. Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratislava Med J. 2021;122(07):474–488. doi:10.4149/BLL_2021_078
55. Li J, Wang L, Liu C, et al. Exploration of prognostic factors for critical COVID-19 patients using a nomogram model. Sci Rep. 2021;11(1):8192. doi:10.1038/s41598-021-87373-x
56. Kong M, Zhang H, Cao X, et al. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. 2020;148:e139. doi:10.1017/S0950268820001557
57. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:10.1001/jama.2020.1585
58. Maddani SS, Gupta N, Umakanth S, et al. Neutrophil-lymphocyte ratio as a simple tool to predict requirement of admission to a critical care unit in patients with COVID-19. Indian J Crit Care Med. 2021;25(5):535–539. doi:10.5005/jp-journals-10071-23801
59. Yan X, Li F, Wang X, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J Med Virol. 2020;92(11):2573–2581. doi:10.1002/jmv.26061
60. Wang X, Li X, Shang Y, et al. Ratios of neutrophil-to-lymphocyte and platelet-to-lymphocyte predict all-cause mortality in inpatients with coronavirus disease 2019 (COVID-19): a retrospective cohort study in a single medical center. Epidemiol Infect. 2020. doi:10.1017/S0950268820002071
61. Ertekin B, Yortanlı M, Özelbaykal O, et al. The relationship between routine blood parameters and the prognosis of COVID-19 patients in the emergency department. Emerg Med Int. 2021;2021:1–7. doi:10.1155/2021/7489675
62. Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID-19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950. doi:10.1016/j.intimp.2020.106950
63. Zhao Y, Yu C, Ni W, et al. Peripheral blood inflammatory markers in predicting prognosis in patients with COVID-19. Some differences with influenza A. J Clin Lab Anal. 2021;35(1). doi:10.1002/jcla.23657
64. Meena A. Hematological profiles of COVID-19 patients at the Ratlam district, Madhya Pradesh State, India. Bioinformation. 2021;17(7):686–690. doi:10.6026/97320630017686
65. Acar E, Demir A, Yıldırım B, et al. The role of hemogram parameters and C-reactive protein in predicting mortality in COVID-19 infection. Int J Clin Pract. 2021;75:7. doi:10.1111/ijcp.14256
66. Bilge M, Akilli IK, Karaayvaz EB, et al. Comparison of systemic immune-inflammation index (SII), early warning score (ANDC) and prognostic nutritional index (PNI) in hospitalized patients with malignancy, and their influence on mortality from COVID-19. Infect Agent Cancer. 2021;16:1. doi:10.1186/s13027-021-00400-4
67. Ramos-Peñafiel CO, Santos-González B, Flores-López EN, et al. Utilidad de los índices neutrófilo/linfocito, monocito/linfocito y linfocito/plaqueta para el pronóstico de complicaciones asociadas a COVID-19. Gac Med Mex. 2020;156(5):405–411. doi:10.24875/GMM.M20000428
68. Sayed AA, Allam AA, Sayed AI, et al. The use of neutrophil-to-lymphocyte ratio (NLR) as a marker for COVID-19 infection in Saudi Arabia: a case-control retrospective multicenter study. Saudi Med J. 2021;42(4):370–376. doi:10.15537/SMJ.2021.42.4.20200818
69. Chen FF, Zhong M, Liu Y, et al. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J Crit Care. 2020;60:32–37. doi:10.1016/j.jcrc.2020.07.003
70. López-Escobar A, Madurga R, Castellano JM, et al. Hemogram as marker of in-hospital mortality in COVID-19. J Investig Med. 2021;69(5):962–969. doi:10.1136/jim-2021-001810
71. Velazquez S, Madurga R, Castellano JM, et al. Hemogram-derived ratios as prognostic markers of ICU admission in COVID-19. BMC Emerg Med. 2021;21:1. doi:10.1186/s12873-021-00480-w
72. Kilercik M, Demirelce Ö, Serdar MA, et al. A new haematocytometric index: predicting severity and mortality risk value in COVID-19 patients. PLoS One. 2021;16:e0254073. doi:10.1371/journal.pone.0254073
73. Citu C, Gorun F, Motoc A, et al. The Predictive Role of NLR, d-NLR, MLR, and SIRI in COVID-19 Mortality. Diagnostics. 2022;12:1. doi:10.3390/diagnostics12010122
74. Moisa E, Corneci D, Negoita S, et al. Dynamic changes of the neutrophil-to-lymphocyte ratio, systemic inflammation index, and derived neutrophil-to-lymphocyte ratio independently predict invasive mechanical ventilation need and death in critically ill covid-19 patients. Biomedicines. 2021;9(11):1656. doi:10.3390/biomedicines9111656
75. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi:10.1093/cid/ciaa248/5803306
76. Pirsalehi A, Salari S, Baghestani A, et al. Neutrophil-to-Lymphocyte Ratio (NLR) greater than 6.5 may reflect the progression of COVID-19 towards an unfavorable clinical outcome; 2020. Available from: http://ijm.tums.ac.ir.
77. Jain R, Gopal A, Pathak BK, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and their role as predictors of disease severity of coronavirus disease 2019 (COVID-19). J Lab Physicians. 2021;13(01):58–63. doi:10.1055/s-0041-1723057
78. Toori KU, Qureshi MA, Chaudhry A, et al. Neutrophil to lymphocyte ratio (Nlr) in covid-19: a cheap prognostic marker in a resource constraint setting. Pakistan J Med Sci. 2021;37(5):1435–1439. doi:10.12669/pjms.37.5.4194
79. Vafadar Moradi E, Teimouri A, Rezaee R, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. 2021;40:11–14. doi:10.1016/j.ajem.2020.12.003
80. Erdogan A, Can FE, Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J Med Virol. 2021;93(9):5555–5559. doi:10.1002/jmv.27097
81. Seyit M, Avci E, Nar R, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med. 2020. doi:10.1016/j.ajem.2020.11.058
82. Eslamijouybari M, Heydari K, Maleki I, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19 patients and control group and relationship with disease prognosis. Casp J Intern Med. 2020;11:S531–S535. doi:10.22088/cjim.11.0.531
83. Singh Y, Singh A, Rudravaram S, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as markers for predicting the severity in covid-19 patients: a prospective observational study. Indian J Crit Care Med. 2021;25(8):847–853. doi:10.5005/jp-journals-10071-23906
84. Kutlucan L, Kutlucan A, Basaran B, et al. The predictive effect of initial complete blood count of intensive care unit patients on mortality, length of hospitalization, and nosocomial infections. Eur Rev Med Pharmacol Sci. 2016;20(8):1467–1473.
85. Sarkar S, Kannan S, Khanna P, et al. Role of platelet‐to‐lymphocyte count ratio (PLR), as a prognostic indicator in COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2022;94(1):211–221. doi:10.1002/jmv.27297
86. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in-hospital mortality in COVID-19 patients. Molecules. 2020;25(23):5725. doi:10.3390/molecules25235725
87. Merekoulias G, Alexopoulos EC, Belezos T, et al. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr. 2010;2:RRN1154. doi:10.1371/currents.RRN1154
88. Zhu J, Liu C, Wang L, et al. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer. 2017;8(5):737–743. doi:10.7150/jca.17668
89. Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(11):2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
90. Waris A, Din M, Khalid A, et al. Evaluation of hematological parameters as an indicator of disease severity in Covid-19 patients: pakistan’s experience. J Clin Lab Anal. 2021;35(6). doi:10.1002/jcla.23809
91. Hu B, Yang X-R, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
92. Hong X, Cui B, Wang M, et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236(4):297–304. doi:10.1620/tjem.236.297
93. Chu Y-W, Chen P-Y, Lin S-K. Correlation between immune-inflammatory markers and clinical features in patients with acute ischemic stroke. Acta Neurol Taiwan. 2020;29(4):103–113.
94. Hamad DA, Aly MM, Abdelhameid MA, et al. Combined blood indexes of systemic inflammation as a mirror to admission to intensive care unit in COVID-19 patients: a multicentric study. J Epidemiol Glob Health. 2022;12(1):64–73. doi:10.1007/s44197-021-00021-5
95. Caliskan Z, Bozdag E, Sonmez S, et al. Assessment of 7 inflammatory indexes as an early predictor of COVID-19 severity. Cerrahpasa Med J. 2022. doi:10.5152/CJM.2022.22004
96. View of neutrophil-to-lymphocyte ratio, derived neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio as risk factors in critically ill COVID-19 patients, a single centered study. Available from: https://jamc.ayubmed.edu.pk/jamc/index.php/jamc/article/view/8823/3018.
97. Lissoni P. Evidence of abnormally low lymphocyte-to-monocyte ratio in COVID-19-induced severe acute respiratory syndrome. J Immunol Allergy. 2020. doi:10.37191/MAPSCI-2582-6549-1(2)-011
98. Eissa M, Shaarawy S, Abdellateif MS. The role of different inflammatory indices in the diagnosis of COVID-19. Int J Gen Med. 2021;14:7843–7853. doi:10.2147/IJGM.S337488
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
