Back to Journals » OncoTargets and Therapy » Volume 15
Clinical Response to Neoadjuvant Immunotherapy Combined with Targeted Therapy and Chemotherapy in Oral Squamous Cell Carcinoma: Experience in Three Patients
Authors Tian Y , Zhang L, Jin N , Wan Z, Zhang H, Zhang H, Zhang L
Received 5 January 2022
Accepted for publication 29 March 2022
Published 8 April 2022 Volume 2022:15 Pages 353—359
DOI https://doi.org/10.2147/OTT.S355349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Yu Tian,1,2,* Lei Zhang,3,* Nenghao Jin,1,2,* Zhiyi Wan,3 Henghui Zhang,4,5 Haizhong Zhang,2 Lei Zhang2
1Department of Stomatology, Medical School of Chinese PLA, Beijing, People’s Republic of China; 2Department of Stomatology, The First Medical Center, Chinese PLA General Hospital, Beijing, People’s Republic of China; 3Department of Medicine, Genecast Biotechnology Co., Ltd, Wuxi, Jiangsu, People’s Republic of China; 4Biomedical Innovation Center, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Ninth School of Clinical Medicine, Peking University, Beijing, China; School of Oncology, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haizhong Zhang; Lei Zhang, Department of Stomatology, The First Medical Center, Chinese PLA General Hospital, No. 28, Fuxing Road, Beijing, People’s Republic of China, Tel/Fax +86 1066938316, Email [email protected]; [email protected]
Abstract: Immune checkpoint inhibitors (ICIs) pembrolizumab and nivolumab have been approved for the treatment of head and neck squamous cell carcinoma (HNSCC) and used in neoadjuvant immunotherapy in clinical trials. However, combination of ICIs with targeted therapy and chemotherapy was rarely used in pre-surgical HNSCC patients. Herein, we encountered three cases of patients with oral squamous cell carcinoma (OSCC) who all had good responses to neoadjuvant immunotherapy (anti-PD-1 inhibitors) combined with nimotuzumab (anti-EGFR monoclonal antibody) plus paclitaxel. Both Case 1 and Case 2 underwent the same neoadjuvant therapeutic combination (nivolumab, nimotuzumab and paclitaxel) and exhibited a marked tumor shrinkage even complete disappearance by radiological evaluation. Moreover, pathological response was observed in post-surgical tissues of Case 1. Additionally, Case 3 with tongue squamous cell carcinoma also had satisfactory tumor regression (complete healing of his tongue ulcer upon treatment) after receiving similar neoadjuvant therapy with sintilimab (another PD-1 inhibitor), nimotuzumab and paclitaxel. We characterized their potential causes behind favorable treatment outcomes. While there were differences in driver mutations and tumor mutation burden (TMB) identified in pre-treatment tumor tissues among the three patients, numerous CD68+ (macrophages) infiltrates were common for all the cases. Of note, the majority (> 80%) of the total macrophages were molecularly defined as PD-L1-positive macrophages. Given the high expression of PD-L1 in macrophages is associated with better immunotherapy outcomes, we propose that the high proportion of CD68+PD-L1+ cells in total macrophages alone could serve as a promising biomarker for neoadjuvant immunotherapy in combination with other therapies in HNSCC.
Keywords: head and neck squamous cell carcinoma, oral squamous cell carcinoma, neoadjuvant immunotherapy, PD-L1, CD68, macrophage
Introduction
Head and neck cancers usually arise from the squamous cells in oral cavity, oropharynx, hypopharynx, or larynx, being referred to as head and neck squamous cell carcinoma (HNSCC). While patients with locoregionally advanced HNSCC accounting for approximately 60% of all HNSCC cases tend to receive multimodal therapies including surgery and chemoradiotherapy, over half of them experience recurrence. The current first-line standard of care treatment for recurrent or metastatic HNSCC is the combination of cetuximab, platinum and 5-fluorouracil (5-FU), which gives rise to the median overall survival (OS) of less than 10 months.1
Immune checkpoint inhibitors (ICIs) targeting PD-1 (programmed cell death protein 1) or PD-L1 (programmed death-ligand 1) have revolutionized the treatment paradigm for various cancers. In 2016, two immunotherapeutic agents, PD-1 monoclonal antibodies, nivolumab and pembrolizumab were approved by the US Food and Drug Administration (FDA) for the treatment of patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (R/M HNSCC) refractory to platinum-based therapy. In 2019, the FDA approved pembrolizumab combined with platinum and fluorouracil for the first-line treatment of patients with metastatic or unresectable, recurrent HNSCC, while pembrolizumab monotherapy is indicated for the first-line treatment of PD-L1-positive (CPS > 1) patients with metastatic or unresectable, recurrent HNSCC based on the results of KEYNOTE-048.2 In addition to immunotherapy, nimotuzumab targeted therapy, an EGFR (epidermal growth factor receptor)-targeted monoclonal IgG1 antibody, has been approved for the treatment of HNSCC in several countries including Cuba, Argentina, Colombia, Peru, India, and Ukraine. In China, nimotuzumab is administered in combination with radiation for nasopharyngeal carcinomas.3 Besides, paclitaxel is a potent chemotherapeutic drug routinely used for HNSCC. It is reported that partial response to weekly paclitaxel monotherapy was observed in 43.3% (26/60) platin-resistant advanced patients with head and neck cancer in the setting of first- and second-line treatments.4 A recently published case report showed considerable clinical benefit of neoadjuvant use of single-agent pembrolizumab prior to surgical resection in an OSCC patient.5 To date, the benefits of immunotherapy and targeted therapy plus chemotherapy in HNSCC, especially in giant or unresectable HNSCC remain to be determined.
Herein, we reported three cases of oral squamous cell carcinoma (OSCC) who exhibited good responses to neoadjuvant immunotherapy combined with nimotuzumab plus paclitaxel and further characterized related molecular features of the patients.
Methods
The clinical data of the three OSCC patients were collected from Department of Oral Surgery of PLA General Hospital (Beijing, China). The pre-treatment tumor tissues were subjected to next generation sequencing (NGS) using a panel encompassing 769 cancer-related genes. Bioinformatic analyses were carried out at a CAP (College of American Pathologists)-certified laboratory (GeneCast Biotechnology Co., Ltd). Isolated DNA was fragmented (150–200 bp) and captured DNA libraries were sequenced by using the instrument of NovaSeq 6000 system (Illumina) with the average depth over 4500×. PD-L1 test (IHC staining) used for Case 1 and Case 3 was 22C3 staining and PD-L1 test (IHC staining) used for Case 2 was SP142 staining. The tumor immune microenvironment of the three patients were characterized by multiplex immunohistochemistry (mIHC) as previously described.6 The panel used for the mIHC comprised the following antibodies: anti-CD8 (clone SP16, 1:100), anti-PD-1 (clone UMAB199, 1:50), anti-PD-L1 (clone SP142, 1:25), and anti-CD68 (clone KP1, 1:500). Tyramine signal amplification (TSA) visualization and slide scanning were performed with the opal seven-color mIHC kit (#811001KT, PerkinElmer) and PerkinElmer Vectra (Vectra 3.0.5), respectively. Quantitative analyses were conducted using InForm Advanced Image Analysis software (inForm 2.3.0; PerkinElmer).
Results
Case Presentation
Case 1
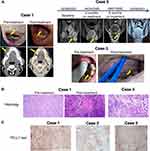
In March 2020, a 69-year-old patient with loosening of tooth in mandibular right teeth for two months and painless white hyperplasia in the right lower alveolar gingiva was taken to our hospital. He had a history of diabetes for 4 years and his blood glucose was controlled with insulin. Contrast-enhanced computed tomography (CT) showed a diffusely infiltrating mass in the right mandibular gingiva with gross invasion of the mandible (Figure 1A). Histopathological examination of the pre-treatment biopsy revealed poorly differentiated squamous cell carcinoma (SCC, pT2N0M0) (Figure 1B). Immunohistochemical staining indicated positive expression of CK5, p63, p40, EGFR, and Ki-67 (+70%), as well as negative expression of p16. Moreover, PD-L1 positivity (TPS 40%, CPS 45) was detected in tissue specimens based on PD-L1 test (22C3 staining) (Figure 1C). Thereafter, the patient received neoadjuvant immunotherapy (nivolumab) in combination with nimotuzumab and paclitaxel. Nivolumab 3 mg/kg and 100 mg nimotuzumab were given every other week, and paclitaxel was administered every other month. An obvious tumor shrinkage was observed in CT after 5 courses of nivolumab with nimotuzumab and 2 courses of paclitaxel (Figure 1A). In early July 2020, he underwent a composite resection for the tumor as well as neck dissection. Postoperative pathologic examination revealed scattered well differentiated SCC in mucosa and the absence of carcinoma in mandible and lymph nodes (Figure 1B). Furthermore, the patient has not relapsed (disease-free survival >16 months) and remains stable.
Case 2
A 64-year-old man diagnosed as having base of tongue SCC with cervical node metastasis (pT3N3M0) was admitted to our hospital in February 2020. Neck contrast-enhanced magnetic resonance imaging (MRI) revealed a 5.0 cm x 5.3 cm infiltrating mass arising from the base of the tongue, multiple cervical lymphadenopathies and narrow pharyngeal cavity (Figure 1A). PD-L1 test (SP142 staining) of the biopsied specimens showed a very high expression of PD-L1 (98%) in tumor cells and a relatively low expression of PD-L1 (10%) in immune cells (Figure 1C). Like Case 1, the patient was treated with the neoadjuvant therapeutic regimen (nivolumab in combination with nimotuzumab and paclitaxel). In this case, paclitaxel was given every 4 weeks, while nivolumab 3 mg/kg and 200 mg nimotuzumab were administered every 2 weeks and every week, respectively. A markedly reduced mass (1.8 cm x 3.6 cm) and alleviated stenosis of pharyngeal cavity were observed in contrast-enhanced MRI after 2 months of treatment (Figure 1A). Given the good response, both paclitaxel and nimotuzumab were given every 4 weeks starting from the second 3-month treatment. After 6 months of treatment, the follow-up MRI showed a complete disappearance of the mass and pharyngeal cavity with eumorphism (Figure 1A). He was very satisfied with the treatment outcome and thus chose not to receive surgery. At this time, the patient remained in good condition (disease-free survival >14 months).
Case 3
A 70-year-old man suffering from pain of left-side tongue body and ulcerative lesion (2 cm x 2.5 cm x 0.5 cm) with basal muscle-invasion of lingual margin (Figure 1A) for one month was admitted to hospital in September 2020. An enlarged lymph node with mobility and smooth was palpable in his left-side neck. Histopathological examination of biopsied tongue specimen revealed moderately differentiated SCC (T2N1M0) infiltrating striated muscle (Figure 1B). PD-L1 test (22C3 staining) indicated an extremely high expression of PD-L1 (TPS 99%, CPS 100) (Figure 1C). Like Case 1 and Case 2, this patient underwent neoadjuvant treatments with nimotuzumab (200 mg), paclitaxel (200 mg), and sintilimab (200 mg), another anti-PD-1 monoclonal antibody that has been approved for marketing in China. After treatment, his tongue ulcer healed completely, but no distinct change in lymph node enlargement was observed. At the end of April 2021, the patient received left-side lymph node dissection, and histological examination of resected lymph nodes revealed metastatic moderately differentiated SCC with a large area of necrosis. Immunohistochemical staining showed positive expression of EGFR and Ki-67 (+80%) as well as negative expression of p16. He remained in good recovery without recurrence until the latest follow-up in November 2021.
Genomic Features and Immune Microenvironment
To determine genomic features that are potentially associated with response to the treatments, we performed sequencing on tumor tissues collected from the three patients. As presented in Table 1, while we identified two major driver mutations HRAS p.Q61K and TERT p.C250T in Case 1 as well as two loss-of-function mutations in tumor suppressor genes (PTEN p.R233*TP53 p.C176*) in Case 2, no oncogenic driver mutation was found in Case 3. Differential mutational profiles among the three patients appeared to be inconsistent with their favorable outcomes with similar therapies. Of note, tumor mutation burden (TMB) value in Case 1 was much higher than that in Case 2 or Case 3 (14.46/Mb, 1.93/Mb, and < 1/Mb, respectively) (Table 1). Besides, all three patients had microsatellite stability (MSS). In general, high TMB observed in Case 1 may avail to good response to immunotherapy, but it is not applicable for other two patients with low TMB. Such differential mutational profiles among the three individuals appeared to be insufficient for fully explaining their favorable outcomes under similar therapies.
 |
Table 1 List of Driver Mutations Identified in Three Cases of Patients |
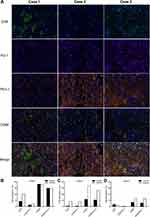
Furthermore, we characterized immunophenotypes of pre-treatment tumor tissues of the patients. The most abundant infiltrating CD8+ lymphocytes were observed in Case 1 (Figure 2A). Quantitative analysis showed that the percentages of CD8+ and CD8+PD-1+ lymphocytes in the tumour area of Case 1 were 8.6% and 2.7%, respectively. The percentage of CD8+ and CD8+PD-1+ lymphocytes in the stromal area was 21% and 3.84%, respectively. (Figure 2B). Compared with Case 1, the level of CD8+ lymphocytes was markedly decreased in both Case 2 and Case 3 (Figure 2A, C, D). Notably, numerous CD68+ (macrophages) infiltrates were detected in all three cases, particularly in Case 1 and Case 2 (Figure 2A–D). PD-L1 test showed that while PD-L1 was highly expressed in the three patients (Figure 1C), PD-L1-positive macrophages accounted for the majority (> 80%) of total macrophages in all the cases (Figure 2B–D).
Discussion
In the present study, all three patients responded effectively to anti-PD-1 inhibitors combined with anti-EGFR targeted therapy and chemotherapy. This observation could be explained by the high proportion of CD68+PD-L1+ cells in total macrophages, rather than genomic features or the status of CD8+ lymphocytes. The finding in this study is concordant with the previous reports showing that the high proportion of CD68+PD-L1+ cells in total macrophages is associated with immunotherapy outcome in melanoma,7 non-small cell lung cancer,8 and breast cancer.9 It has been demonstrated that OSCC inhibits antitumor immunity by inducing PD-L1 expression in tumor-associated macrophages, causing apoptosis in T cells.10 Moreover, infiltration of CD68+ tumor-associated macrophage has been found to be significantly associated with the high expression of PD-L1 in OSCC tumor cells.11
Neoadjuvant immunotherapy has demonstrated good safety and tolerance with promising response data in HNSCC patients.12,13 In the recent years, there have been over 15 clinical trials regarding neoadjuvant immunotherapy with ICIs in HNSCC, implicating promising prospect of this approach in head and neck malignancies.13 Accordingly, the predictive biomarkers for this treatment paradigm in HNSCC remain to be further studied. As a matter of fact, in spite of the correlation of PD-L1 positivity, high TMB, and CD8+ lymphocytes with pathologic responses to neoadjuvant immunotherapy in HNSCC, about half of patients did not respond to pre-surgical treatment with ICIs alone yet. Neoadjuvant immunotherapy combined with other treatments might benefit more HNSCC patients. Besides, neoadjuvant therapy using paclitaxel combined with other drugs has been studied in HNSCC for many years. Given that cytotoxic chemotherapy may facilitate the release of antigen peptides from cancer cells and subsequently trigger immunogenic cell death as well as synergism with immunotherapies,14 we reason that paclitaxel improved relative lack of CD8 T cells in Case 2 and Case 3 to a certain extent and strengthened desired immunotherapy outcomes. Hence, paclitaxel could be essential for the neoadjuvant immunotherapy of the two patients.
Despite the correlation of PD-L1 positivity, high TMB and CD8+ lymphocytes with pathologic responses to neoadjuvant immunotherapy in HNSCC, approximately half of the patients failed to respond to pre-surgical treatment with ICIs alone.12 The observations in this study suggest that combination of PD-1 inhibitors with chemotherapy and anti-EGFR targeted therapy may improve the response rate and survival outcome in PD-L1-positive OSCC patients, regardless of their TMB values, driver mutations or the status of CD8 lymphocytes.
Conclusion
Overall, our work provided new evidence for the application of neoadjuvant immunotherapy combined targeted therapy and chemotherapy in OSCC while the high proportion of CD68+PD-L1+ cells in total macrophages may serve as a promising biomarker for neoadjuvant immunotherapy, though it remains to be verified in more cases.
Ethics Statement
This study was approved by the Ethics Committee of Chinese PLA General Hospital (No. S2021-308-02). Written informed consents were obtained from all patients.
Acknowledgments
This study was granted by the National Key Sci-Tech Special Project of China (No. 2018ZX10302207) and Major military logistics project (No. AWS17J004-ZHZ).
Disclosure
Zhiyi Wan is an employee of Genecast Biotechnology Co., Ltd. The authors declare that they have no other potential conflicts of interest in this work.
References
1. Bamford J, Webster RM. The SCCHN drug market. Nat Rev Drug Discov. 2017;16(4):235–236. doi:10.1038/nrd.2016.261
2. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, Phase 3 study. Lancet. 2019;394(10212):1915–1928. doi:10.1016/S0140-6736(19)32591-7
3. Xu MJ, Johnson DE, Grandis JR. EGFR-targeted therapies in the post-genomic era. Cancer Metastasis Rev. 2017;36(3):463–473. doi:10.1007/s10555-017-9687-8
4. Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol. 2009;129(11):1294–1299. doi:10.3109/00016480802590451
5. Olmos M, Glajzer J, Büntemeyer TO, et al. Neoadjuvant immunotherapy of oral squamous cell carcinoma: case report and assessment of histological response. Front Oncol. 2021;11:720951. doi:10.3389/fonc.2021.720951
6. Pang X, Qian J, Jin H, et al. Durable benefit from immunotherapy and accompanied lupus erythematosus in pancreatic adenocarcinoma with DNA repair deficiency. J Immunother Cancer. 2020;8(2):e000463. doi:10.1136/jitc-2019-000463
7. Toki MI, Merritt CR, Wong PF, et al. High-plex predictive marker discovery for melanoma immunotherapy-treated patients using digital spatial profiling. Clin Cancer Res. 2019;25(18):5503–5512. doi:10.1158/1078-0432.CCR-19-0104
8. Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26(4):970–977. doi:10.1158/1078-0432.CCR-19-1040
9. Ahmed FS, Gaule P, McGuire J, et al. PD-L1 protein expression on both tumor cells and macrophages are associated with response to neoadjuvant durvalumab with chemotherapy in triple-negative breast cancer. Clin Cancer Res. 2020;26(20):5456–5461. doi:10.1158/1078-0432.CCR-20-1303
10. Jiang C, Yuan F, Wang J, Wu L. Oral squamous cell carcinoma suppressed antitumor immunity through induction of PD-L1 expression on tumor-associated macrophages. Immunobiology. 2017;222(4):651–657. doi:10.1016/j.imbio.2016.12.002
11. Suárez-Sánchez FJ, Lequerica-Fernández P, Suárez-Canto J, et al. Macrophages in oral carcinomas: relationship with cancer stem cell markers and PD-L1 expression. Cancers. 2020;12(7):1764. doi:10.3390/cancers12071764
12. Stafford M, Kaczmar J. The neoadjuvant paradigm reinvigorated: a review of pre-surgical immunotherapy in HNSCC. Cancers Head Neck. 2020;5(1):4. doi:10.1186/s41199-020-00052-8
13. Cabezas-Camarero S, Pérez-Segura P. Neoadjuvant immunotherapy in head and neck cancer: rationale, current evidence and future perspective. Crit Rev Oncol Hematol. 2022;169:103569. doi:10.1016/j.critrevonc.2021.103569
14. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi:10.1158/2326-6066.CIR-15-0064
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.