Back to Journals » ClinicoEconomics and Outcomes Research » Volume 6
Clinical response and hospital costs associated with the empirical use of vancomycin and linezolid for hospital-acquired pneumonia in a Chinese tertiary care hospital: a retrospective cohort study
Authors Song Y, Yang Y, Chen W , Liu W, Wang K, Li X, Wang K, Papadimitropoulos M, Montgomery W
Received 10 April 2014
Accepted for publication 16 June 2014
Published 17 October 2014 Volume 2014:6 Pages 451—461
DOI https://doi.org/10.2147/CEOR.S65900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Yuanlin Song,1,* Yicheng Yang,2,* Wendong Chen,3,4 Wei Liu,2 Kai Wang,2 Xuehai Li,5 Ke Wang,2 Manny Papadimitropoulos,3,6 William Montgomery7
1Department of Pulmonary Medicine, Zhongshan Hospital, Fudan University, 2Lilly Suzhou Pharmaceutical Co, Ltd, Shanghai Branch, Shanghai, People's Republic of China; 3Division of Social and Administrative Pharmacy, Leslie Dan Faculty of Pharmacy, University of Toronto, 4Normin Health, Toronto, ON, Canada; 5VitalStrategic Research Institute, Shanghai, People's Republic of China; 6Global Health Outcomes Research, Eli Lilly, Indianapolis, IN, USA; 7Eli Lilly Australia Pty Ltd, West Ryde, NSW, Australia
*These authors contributed equally to this work
Aims: To evaluate clinical outcomes and allocation of hospital costs associated with empirical use of vancomycin or linezolid for hospital-acquired pneumonia (HAP) in the People's Republic of China.
Methods: Hospital episodes including HAP treated by vancomycin or linezolid between 2008 and 2012 in a Chinese tertiary care hospital were retrospectively identified from hospital administrative databases. Propensity score methods created best-matched pairs for the antibiotics. The matched pairs were used for adjusted comparisons on clinical response and allocation of hospital costs. Multiple regression analyses adjusting residual imbalance after matching were performed to confirm adjusted comparisons.
Results: Sixty matched pairs were created. Adjusted comparisons between vancomycin and linezolid showed similar clinical response rates (clinical cure: 30.0% versus 31.7%, respectively; P=0.847; treatment failure: 55.0% versus 45.0%, respectively; P=0.289) but a significantly lower in-hospital mortality rate for vancomycin (3.3% versus 18.3%, respectively; P=0.013). After further adjusting for the imbalanced variables between matched treatment groups, the risks of treatment failure associated with the two antibiotics were comparable (odds ratio: 1.139; P=0.308) and there was a nonsignificant trend of lower risk of in-hospital mortality associated with vancomycin (odds ratio: 0.186; P=0.055). The total hospital costs associated with vancomycin had a nonsignificant trend of being lower, likely because of its significantly lower acquisition costs (median: RMB 2,880 versus RMB 8,194; P<0.001; 1 RMB =0.16 USD).
Conclusion: In tertiary care hospitals in the People's Republic of China, empirical treatment of patients with HAP with vancomycin had a comparable treatment failure rate but likely had a lower in-hospital mortality rate when compared with linezolid. Vancomycin also costs significantly less for drug acquisition than linezolid when treating HAP empirically.
Keywords: methicillin-resistant Staphylococcus aureus, treatment failure, mortality, antibiotics
A Letter to Editor has been received and published for this article.
Introduction
Hospital-acquired pneumonia (HAP) is the second most common nosocomial infection, occurring in 0.5%–2% of general hospital admissions1,2 and in 15%–20% of patients in intensive care units,3 likely because of the prevalent use of mechanical ventilation. HAP is a life-threatening condition that is associated with increased in-hospital mortality of 22%–60%.4 With extended hospital length of stay and an increasing number of patients with multidrug-resistant HAP, treating HAP is expensive. One episode of HAP has been shown to increase hospitalization costs by up to USD 40,000 in the USA.5
The incidence of HAP in Chinese tertiary care hospitals ranges from 5.9% in general hospitalized patients6 to 28.2% in critically ill patients.7 Overall mortality in Chinese patients with diagnosed HAP was reported as 25.3%.8 Of the reported deaths, Pseudomonas aeruginosa and Staphylococcus aureus infections accounted for 70.6% and 66.7% of cases, respectively. Differences have been observed in the causative pathogens patterns associated with HAP in the People’s Republic of China compared to Western countries, with more than half of Chinese patients with HAP caused by Gram-negative pathogens.7,8 As a result, it is expected that Chinese patients might respond differently to the empirical use of different antibiotics. Vancomycin and linezolid are the first-line treatments for methicillin-resistant S. aureus (MRSA),1 and with the increasing risk of HAP caused by MRSA,9 the empirical use of vancomycin and linezolid for HAP is becoming commonplace in the People’s Republic of China. However, because the HAP causative pathogen(s) is not routinely identified in Chinese tertiary care hospitals, the empirical use of vancomycin and linezolid for suspected MRSA infection is common. There is a paucity of evidence concerning the use of vancomycin and linezolid in the People’s Republic of China for the treatment of difficult HAP under routine clinical practice. Thus, to address the current evidence gap, this retrospective cohort study was conducted using a hospital administrative database to collect information from routine clinical use of vancomycin and linezolid for patients with HAP and compare their associated impact on clinical outcomes and health resource utilization.
Patients and methods
A representative tertiary care hospital in Shanghai, People’s Republic of China was selected to conduct this retrospective cohort study. The study observation time was set from January 1, 2008 to February 28, 2012, which was the time period that both vancomycin and linezolid had hospital prescription records and were available for use at the hospital.
Identifying eligible hospital episodes
Linked hospital administrative databases, using a unique patient identifier, were used to identify eligible hospital episodes with occurrence of HAP. The hospital prescription database was searched using the key words “vancomycin” and “linezolid” during the study observation time. The hospital radiological examination database was further interrogated for any chest radiograph or computed tomography reports containing the key words for radiological signs for pneumonia including infiltrate, consolidation, and/or pleural effusion. The hospital admission registry database was interrogated to exclude hospital episodes with a diagnosis of pneumonia at hospital admission, age younger than 18 years, or survival time less than 72 hours. The hospital prescription database was also accessed to exclude hospital episodes with a treatment duration of vancomycin or linezolid that was less than 48 hours. To control the heterogeneity associated with the highly varied admission diagnoses, the study cohort only included hospital episodes with admission diagnoses ranked in the top ten in distribution for the two antibiotics.
Data extraction
All eligible hospital episodes were identified within the hospital administrative databases, and the necessary information was extracted. The hospital admission registry database was used to extract patient demographic information, including age, gender, health insurance plan, hospital admission diagnosis, hospital admission and discharge dates, and vital status at hospital discharge. The hospital radiological examination database was used to collect information on the radiological signs for pneumonia. The hospital drug prescription database was used to extract information on specified antibiotics between the first diagnosis of HAP and initial administration of vancomycin or linezolid, as well as information on dosage and treatment duration for vancomycin and linezolid, antibiotics combined with vancomycin or linezolid for treating HAP, and any additional use of antibiotics after the treatment with vancomycin or linezolid. The hospital discharge summary database was used to obtain information on comorbidities, the primary intervention for the hospital admission diagnosis, type of hospital ward at the time of diagnosis of HAP, the use of mechanical ventilation prior to and after a diagnosis of HAP, signs and symptoms of pneumonia, pneumonia-related complications, and cause of death. The hospital daily management database was used to extract information on body temperature, pulse rate, breath frequency, blood pressure, and arterial oxygen saturation during treatment with vancomycin or linezolid. The hospital laboratory database was used to extract white blood cell counts, bacteria Gram stain and culture test results, and bacteria antibiotic resistance test results obtained during treatment. The hospital billing summary database was used to extract billing summary information for medications, medical examinations, medical supplies, and other unclassified expense items. Unit prices and dosage information associated with vancomycin and linezolid were extracted from the hospital prescription databases to calculate the drug costs for these two antibiotics.
Diagnosis of HAP
In this study, HAP was defined as any pneumonia diagnosed after hospital admission. The diagnosis of HAP was established using a combination of radiological signs seen on chest radiographs or computed tomography examination and at least one of the following signs or symptoms prior to initiation of treatment with vancomycin or linezolid: auscultatory findings of pneumonia, dyspnea, tachypnea, or hypoxemia in the hospital discharge summary; body temperature >37.8°C; respiratory rate >30 breaths/minute; systolic blood pressure <90 mmHg; pulse rate ≥120 beats/minute; arterial oxygen saturation <90%; elevated total peripheral white blood cell count >10,000 cells/mm3; and/or positive bacterial test from cultures of respiratory tract, sputum, or blood samples.
Outcome measures
The primary clinical outcome measures used were the clinical responses at the end of treatment. Clinical responses were further classified as clinical cure, clinical improvement, and treatment failure. Clinical cure was defined as the resolution of the baseline clinical signs and symptoms of pneumonia with improvement or lack of progression of radiographic findings. Clinical improvement was defined if a clinical cure was not achieved but any improvements of the baseline signs and symptoms of pneumonia were seen at the end of treatment. Treatment failure was defined as the persistence or progression of the baseline signs and symptoms of pneumonia and/or administration of another effective antibiotic because of lack of clinical improvement from the treatment with either of the two antibiotics of interest. Secondary clinical outcome measures included any occurrences of pneumonia-related complications (pulmonary edema, sepsis, infection shock, and/or respiratory failure), pneumonia-related mortality (defined as any death caused by pneumonia-related complications), and all-cause mortality at the end of treatment and at hospital discharge, respectively. The primary cost outcome measures used were the hospital costs that were classified into the acquisition costs of vancomycin or linezolid, other medication costs, medical examination costs, medical supply costs, and other unclassified costs.
Data analysis
Patient baseline characteristics associated with each hospital episode were summarized using descriptive statistical methods. Student’s t-test for continuous variables and chi square test for proportional variables were performed to identify unbalanced patient baseline characteristics associated with the antibiotic treatment groups. Multiple logistic regression analysis was conducted to identify those baseline characteristics independently predicting the treatment selection for HAP in this tertiary care hospital. Patients with the same primary hospital admission diagnosis were included to perform propensity score matching and identify the best matched pairs (1:1) for the two antibiotics using the greedy approach.10 The matched pairs for the top ten hospital admission diagnoses were pooled to create matched treatment groups for adjusted comparisons on clinical effectiveness and resource consumption and costs associated with the antibiotics.
Paired t-test and McNemar’s test were used for adjusted comparisons on clinical responses, pneumonia-related complications, pneumonia-related mortality, and all-cause mortality. Wilcoxon signed-rank test was performed in the adjusted comparisons on the median hospital costs because the distribution of hospital costs was often skewed.11 To further adjust residual imbalance of patient baseline characteristics after matching, multiple logistic regression analyses using the generalized estimating equation were performed to adjust those unbalanced baseline characteristics12 and estimate the risk differences in treatment failure at the end of treatment – pneumonia-related in-hospital mortality and all-cause in-hospital mortality, respectively. Multiple linear regression analyses using the generalized estimating equation were also performed to explore any differences in the common logarithm of categorized hospital costs between the two antibiotics after adjustment of unbalanced baseline characteristics between the propensity score matched treatment groups. Statistical significance was defined as a two-sided P-value less than 0.05. The data analyses were performed using the SAS version 9.2 statistical package (SAS Institute Inc., Cary, NC, USA).
Results
The initial screening of the hospital prescription database identified 3,708 hospital episodes with vancomycin prescription records and 273 hospital episodes with linezolid prescription records. After further excluding hospital episodes without radiological evidence for pneumonia (n=1,841), patients with a diagnosis of pneumonia at hospital admission (n=93), those younger than 18 years (n=22), those with a survival time less than 72 hours (n=13), or those with less than 48 hours of treatment with vancomycin or linezolid (n=402), 1,610 patients were included. These were then ranked by hospital admission diagnoses and the 681 hospital episodes that were associated with the top ten most common admission diagnoses (621 for vancomycin and 60 for linezolid) were included to create the study cohort (Figure 1).
  | Figure 1 Flow chart to identify eligible hospitalized patients receiving empirical treatment with vancomycin or linezolid for hospital-acquired pneumonia. |
Patient’s baseline characteristics and predictors of empirical treatment selection
The study cohort of patients with a study-specified criteria case of HAP consisted of 621 and 60 cases who were administered vancomycin and linezolid, respectively. These patients had a mean age of 56.7 years and were more likely to be men (70.6%). The most common hospital admission diagnoses among this restricted cohort were highly related to heart diseases (72.8%), including coronary heart disease (23.0%), multiple valve diseases (19.7%), aortic aneurysm and dissection (14.4%), rheumatic mitral valve diseases (8.8%), and non-rheumatic aortic valve disorders (6.9%). Over four out of five patients (82.5%) included in this cohort received heart-related surgery, and mechanical ventilation was previously or currently used in more than half of the study cohort (55.8%). In addition, 95.7% of patients developed HAP 48 hours or longer after hospital admission and 83.7% of HAP cases were diagnosed using a chest radiograph. The most frequently used first-line antibiotics for HAP in this cohort were cephalosporins (42.8%) and other β-lactams (45.2%). HAP causative pathogen and antibiotics resistance were not routinely assessed during treatment in the study cohort.
Some patient baseline characteristics were not balanced between the two antibiotic treatment groups (Table 1). When compared to the linezolid group, the vancomycin group was significantly younger (mean age: 56.4 versus 60.2 years, respectively; P=0.035), more likely to have comorbid heart failure (23.4% versus 10.0%, respectively; P=0.015), have a hospital admission diagnosis of multiple valve diseases (20.0% versus 3.3%, respectively; P=0.001), have heart-related surgery (84.4% versus 36.7%, respectively; P<0.001), and have been treated initially with cephalosporins for HAP (43.8% versus 23.3%, respectively; P=0.002). However, the linezolid group had significantly higher proportions of hospital admission diagnoses concerning lung or digestive system cancers (40.0% versus 9.2%, respectively; P<0.001), chronic obstructive pulmonary disease (5.0% versus 1.0%, respectively; P=0.037), surgery related to the liver (11.7% versus 2.9%, respectively; P=0.004) or stomach (16.7% versus 2.6%, respectively; P<0.001), symptom control management (15% versus 3.5%, respectively; P=0.001), and current use of mechanical ventilation (46.7% versus 26.3%; P<0.001). Multiple regression analysis further confirmed that no current use of mechanical ventilation (odds ratio [OR]: 0.200; 95% confidence interval [CI]: 0.099–0.404; P<0.001), heart-related surgery (OR: 8.183; 95% CI: 2.538–26.388; P<0.001), and previous treatment with cephalosporins (OR: 3.747; 95% CI: 1.562–8.987; P=0.003) were significantly and independently associated with the selection of vancomycin versus linezolid for HAP empirical treatment.
Creating propensity score matched treatment groups for vancomycin versus linezolid
One-to-one propensity score matching of the vancomycin to the linezolid cases resulted in 60 best-matched pairs from the initial study cohort (Figure 2). The average dosages of vancomycin and linezolid in the matched patients were 1,485 and 1,111 mg/day, respectively. Treatment duration was slightly longer (11.8 versus 9.3 days, respectively; P=0.319) in the matched vancomycin group. In addition, the matched vancomycin treatment group was associated with a significantly higher proportion of combination treatment with cephalosporins (43.3% versus 18.3%, respectively; P=0.007). After matching, vancomycin versus linezolid group imbalance persisted for prevalence of current (16.7% versus 46.7%, respectively; P=0.001) and previous use (46.7% versus 63.3%, respectively; P=0.033) of mechanical ventilation and the front-line antibiotic treatment with cephalosporins (51.7% versus 23.3%, respectively; P=0.001) and other β-lactams (36.7% versus 56.7%, respectively; P=0.029).
Comparing vancomycin versus linezolid for clinical outcomes
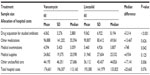
A comparison of the clinical responses at the end of treatment between vancomycin and linezolid showed an almost identical clinical cure rate (30.0% versus 31.7%, respectively; OR: 0.923; P=0.847) associated with the two antibiotics and slightly higher treatment failure rate (55.0% versus 45.0%, respectively; OR: 1.494; P=0.289) associated with vancomycin in the created 60 matched pairs (Table 2). The hospital stay length associated with vancomycin was slightly longer than that for linezolid without statistical significance (46.0 versus 39.1 days, respectively; P=0.311). No significant differences in the occurrences of pneumonia-related complications, pneumonia-related death, and all-cause death at the end of treatment were identified between the two matched treatment groups. However, higher rates of pneumonia-related mortality (10.0% versus 1.7%, respectively; OR: 6.425; P=0.059) and all-cause mortality (18.3% versus 3.3%, respectively; OR: 6.564; P=0.013) at hospital discharge were observed in the matched linezolid treatment group. With further adjusting for the unbalanced baseline characteristics between the two matched treatment groups, the risk difference of treatment failure rate associated with the two antibiotics was further reduced (OR: 1.139; 95% CI: 0.887–1.461; P=0.308). The risk differences of pneumonia-related mortality and all-cause mortality at hospital discharge were also reduced and the lower risk of all-cause mortality associated with vancomycin was approaching statistical significance (OR: 0.186; 95% CI: 0.033–1.039; P=0.055) (Figure 3).
Comparing vancomycin versus linezolid for the allocation of hospital costs
The median total hospital costs associated with the study cohort exceeded RMB 85,033 (or USD 13,471), the gross domestic product per capita in Shanghai in 2012.13 Directly comparing the allocation of hospital costs in the matched patients indicated that vancomycin was associated with significantly lower median drug acquisition cost (RMB 2,880 versus RMB 8,194, respectively; median difference: −RMB 5,314; P<0.001; 1 RMB =0.16 USD), medical examinations (RMB 3,059 versus RMB 3,807, respectively; median difference: −RMB 748; P=0.042), and other unclassified care (RMB 27,686 versus RMB 44,826, respectively; median difference: −RMB 17,141; P=0.006) when compared to linezolid (Table 3). However, this study only observed a strong but nonsignificant trend indicating lower median total hospital costs associated with vancomycin in the comparison with linezolid (RMB 113,160 versus RMB 133,825, respectively; median difference: −RMB 20,665; P=0.076). After further adjustment of unbalanced baseline characteristics between the two matched treatment groups, vancomycin was associated with significantly lower common logarithm of acquisition cost (coefficient: −0.826; 95% CI: −1.088 to −0.564; P<0.001) but similar common logarithm of hospital costs for other medications (coefficient: 0.072; 95% CI: −0.260–0.404; P=0.671), medical examinations (coefficient: −0.061; 95% CI: −0.348–0.227; P=0.679), medical supplies (coefficient: −0.102; 95% CI: −0.552–0.348; P=0.657), and other unclassified care (coefficient: −0.101; 95% CI: −0.381–0.178; P=0.478) when compared to linezolid.
Discussion
To the authors’ knowledge, this is the first real-world study describing the clinical characteristics and management associated with a large cohort of patients with difficult HAP in a Chinese tertiary care hospital. This study confirmed that the causative pathogen was not routinely identified for HAP in current clinical practice in the People’s Republic of China. However, the patients in the study cohort were treated using vancomycin or linezolid when HAP did not respond to first-line antibiotic treatment. Although the initial search identified approximately 4,000 eligible hospital episodes, highly varied hospital admission diagnoses and the relatively small number of patients receiving linezolid substantially reduced the sample size of the study cohort used in the data analyses. After adjusting for possible confounding effects with propensity score methods and conventional regression methods, the two antibiotics were found to have comparable clinical responses at the end of treatment. However, the in-hospital mortality rate associated with linezolid was five times higher than that for vancomycin in the study cohort. Even though vancomycin was associated with significantly lower acquisition costs and the higher in-hospital mortality associated with the linezolid group increased utilization of health resources,14 the longer length of hospital stay associated with vancomycin treatment likely led to more consumption of health resources and reduced total hospital cost saving in patients receiving vancomycin for their HAP.
Identification of the causative pathogen for HAP was not routinely conducted in the study cohort as only five of the 681 included patients had laboratory records for bacterial culture testing. Thus, the treatment with vancomycin and linezolid was largely empirical. When compared with published randomized trials comparing vancomycin versus linezolid for HAP caused by Gram-positive organisms15 or MRSA,16 the current study observed that the clinical cure rates associated with the two antibiotics were almost reduced by half. Because Gram-negative organisms are the predominant causative pathogens for HAP in Chinese tertiary hospitals,10 it was suspected that MRSA was not the main pathogen associated with HAP in the current study cohort, and the clinical responses associated with the two antibiotics were substantially diminished as a result. In addition, linezolid was reported to increase mortality when compared to vancomycin, oxacillin, or dicloxacillin when used for the treatment of patients infected with Gram-negative bacteria alone or in patients infected with both Gram-positive and Gram-negative bacteria.17 It was suspected that the high risk of Gram-negative infection in the current study cohort was a factor that increased the in-hospital mortality associated with treatment with linezolid. The possible high risk of HAP caused by Gram-negative bacteria in the study cohort also explained why the previously reported superiority of linezolid in clinical response was not observed.18 According to the latest randomized trial comparing linezolid and vancomycin in patients with HAP caused by MRSA, the clinical cure rate associated with linezolid was only increased by 11%.13 Such small increase on clinical response could be easily diluted if half of the study cohort were infected by Gram-negative bacteria.
Health care resource utilization associated with this study cohort was considerable, with the median total hospitalization costs associated with the two antibiotics exceeding the local gross domestic product per capita. This study did not differentiate between hospital costs attributable to medical care related to HAP and other causes; however, it did demonstrate that the lower acquisition cost of vancomycin likely led to the observed nonsignificant trend showing lower total hospital cost associated with vancomycin. It is also suspected that the lower in-hospital mortality associated with vancomycin likely extended the hospital stay length, which likely increased hospital resources utilization19 and neutralized the saved acquisition costs associated with vancomycin and reduced the likelihood of observing significant difference in total hospital costs between the two antibiotics.
This study has several strong implications for clinical practice and future research. It confirmed that HAP causative pathogens were not routinely assessed for after the initial empirical use of antibiotics in Chinese tertiary care hospitals. Because MRSA is unlikely to be the predominant causative pathogen for HAP in Chinese tertiary care hospitals, current empirical treatment strategy without guidance by identified HAP causative pathogens unlikely optimizes health outcomes but may cause harm and unnecessary usage of health care resources associated with the misuse of antibiotics. In addition, the increased mortality associated with the empirical use of linezolid for difficult cases of HAP further addressed the need for HAP causative pathogen assessment to avoid the treatment with linezolid in HAP caused by Gram-negative bacteria. Finally, this study suggested that empirical use of vancomycin to treat difficult HAP was cost-effective by having lower in-hospital mortality but likely costing less than linezolid. Future clinical practice guidelines could use this evidence to further support the established first-line treatment with vancomycin for difficult HAP.20
This retrospective cohort study had several limitations that need to be considered when interpreting the study results. Although this study initially identified approximately 4,000 hospital episodes, the small number of patients receiving linezolid and strict propensity score matching dramatically reduced the sample size of patients to 60 matched pairs in the adjusted comparisons. Thus, the study results were based on a highly selected study cohort with a relatively small sample size and the risk of selection bias is high. The data sources used for the study were hospital administrative databases that did not contain information on disease severity, contraindications of two antibiotics (vancomycin was not likely used in patients with renal failure21 and linezolid was not likely used in patients receiving select serotonergic agents22), or the socioeconomic status of the patient, which could substantially confound both clinical and cost outcomes. In addition, incomplete or missing information in the hospital administrative databases was common. Although this study used several indicators to measure clinical response assessment, approximately 10% of patients did not have a clinical response assessment due to missing information. The study did not adjust for the hospital admission year in the cost analyses. Because linezolid was launched in the People’s Republic of China in 2007, the number of patients receiving linezolid was not likely to be similarly well distributed by hospital admission year as those receiving vancomycin, a medication that has been available and widely used in hospital practice for many years. In addition, the high currency inflation rates occurring in the People’s Republic of China during the study observation time period could lead to an overestimation of hospital costs associated with linezolid.
Conclusion
This retrospective observational cohort study suggested that the empirical use of vancomycin and linezolid was associated with comparable clinical responses for HAP that failed with first-line antibiotic treatment in a Chinese tertiary care hospital. However, the study showed a higher in-hospital mortality rate associated with linezolid treatment, possibly due to the lack of the treatment guidance by HAP causative pathogen assessment. Although the acquisition cost of vancomycin was significantly lower, the longer length of hospital stay associated with vancomycin likely increased the use of health resources and discounted total hospital costs saved by vancomycin. Thus, vancomycin was considered more cost-effective than linezolid by leading to lower in-hospital mortality but likely costing less for empirically treating difficult HAP in Chinese tertiary care hospitals where causative pathogens for HAP were not routinely assessed.
Acknowledgments
This study was funded by an unrestricted Health Outcomes Research grant of Eli Lilly China.
Author contributions
Y Song and Y Yang formulated the research idea and developed the study protocol. Y Song, Y Yang, W Chen, W Liu, K Wang, X Li, K Wang, M Papadimitropoulos and W Montgomery contributed to the study design, data analysis and interpretation of the results. Y Song supervised the data collection from the hospital administrative database. All authors participated in the drafting and review of the manuscript and agree to be accountable for all aspects of the work.
Disclosure
Y Song received honorariums for travels and expenses on the study. W Liu, K Wang, X Li, M Papadimitropoulos, and W Montgomery are employees of Eli Lilly. Contributions from Y Yang and K Wang were made whilst they were employees of Eli Lilly. W Chen received a project consulting fee from Eli Lilly to compensate for his time on this project.
References
American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. | |
Chawla R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am J Infect Control. 2008;36(Suppl 4):S93–S100. | |
Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. | |
Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004–2009. Am J Infect Control. 2012;40(5):396–407. | |
Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol. 2012;33(3):250–256. | |
Zhang Y. [A two-year prospective survey on nosocomial infections]. Zhonghua Yi Xue Za Zhi. 1991;71(5):253–256. Chinese. | |
Liu MD, Cheng P. [Analysis of nosocomial infection in hospitalized critical and serious patients]. Zhonghua Liu Xing Bing Xue Za Zhi. 1995;16(4):231–233. Chinese. | |
Hou SR, Xu XJ, Yi P. [Nosocomial pneumonia: a report of 372 cases]. Zhonghua Nei Ke Za Zhi. 1992;31(6):338–340. Chinese. | |
Liu YN, Cao B, Wang H, et al. [Adult hospital acquired pneumonia: a multicenter study on microbiology and clinical characteristics of patients from 9 Chinese cities]. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35(10):739–746. Chinese. | |
Rosenbaum PR. Observational Studies. 2nd edition. New York, NY: Springer-Verlag; 2002. | |
Shaw B, Marshall AH. Modeling the health care costs of geriatric inpatients. IEEE Trans Inf Technol Biomed. 2006;10(3):526–532. | |
D’Agostino RB Jr, D’Agostino RB Sr. Estimating treatment effects using observational data. JAMA. 2007;297(3):314–316. | |
[2012 Statistical Communiqué of the provinces on National Economic and Social Development] [webpage on the Internet]. TJCN; 2014. Available from: http://www.tjcn.org/plus/view.php?aid=26117. Accessed July 2, 2014. Chinese. | |
Steiner CA, Friedman B. Hospital utilization, costs, and mortality for adults with multiple chronic conditions, Nationwide Inpatient Sample, 2009. Prev Chronic Dis. 2013;10:E62. | |
Rubinstein E, Cammarata S, Oliphant T, Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32(3):402–412. | |
Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629. | |
Wilcox MH, Tack KJ, Bouza E, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a Phase III study. Clin Infect Dis. 2009;48(2):203–212. | |
Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124(5):1789–1797. | |
Macedo-Vinas M, De Angelis G, Rohner P, et al. Burden of meticillin-resistant Staphylococcus aureus infections at a Swiss University hospital: excess length of stay and costs. J Hosp Infect. 2013;84(2):132–137. | |
Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008;19(1):19–53. | |
Spapen HD, Janssen van Doorn K, Diltoer M, et al. Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care. 2011;1(1):26. | |
Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother. 2013;47(3):388–397. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.