Back to Journals » Cancer Management and Research » Volume 11
Clinical relevance and significance of programmed death-ligand 1 expression, tumor-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma
Authors Quan H, Yan L, Wang S, Wang S
Received 14 January 2019
Accepted for publication 10 April 2019
Published 9 May 2019 Volume 2019:11 Pages 4335—4345
DOI https://doi.org/10.2147/CMAR.S201568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Chien-Feng Li
Huatao Quan,1 Li Yan,1 Shuyi Wang,2 Shengzi Wang1
1Department of Radiation Oncology, Eye and ENT Hospital of Fudan University, Shanghai, People’s Republic of China; 2Department of Pathology, Eye and ENT Hospital of Fudan University, Shanghai, People’s Republic of China
Purpose: Immunotherapy may be a potential alternative for patients with sinonasal squamous cell carcinoma (SNSCC). Data regarding potential immunotherapy targets, such as programmed death-ligand 1 (PD-L1) and tumor-infiltrating lymphocytes (TILs), in SNSCC are limited. In this study, we assessed the prevalence and prognostic value of PD-L1 expression and TILs in p16-negative and p16-positive SNSCC.
Patients and methods: Tissues from 96 patients with SNSCC were stained using immunohistochemistry against PD-L1, CD8, and Foxp3 to assess the immune environment. The correlations between PD-L1 expression, TILs, and p16 status were analyzed. Additionally, PD-L1, CD8, and Foxp3 expressions, as well as p16 status, were analyzed in relation to patient clinicopathological variables and prognosis.
Results: Twenty-nine (30.2%) patients with SNSCC showed PD-L1 expression in >5% of tumor cells. PD-L1 expression was significantly correlated with poor differentiation and a high level of TILs. PD-L1 expression and the CD8+ and Foxp3+ T-cell infiltrates in p16-negative patients (n=78, 81.2%) and p16-positive patients (n=18, 18.8%) were not significantly different. PD-L1 expression and p16 status were not associated with overall survival (OS) and disease-free survival (DFS). Patients with high CD8+ or Foxp3+ cell infiltration had better clinical outcomes. A multivariate analysis confirmed that CD8 TILs were a significant independent and favorable prognostic factor for OS (p=0.023) and DFS (p=0.008).
Conclusion: TILs can play a prognostic role in SNSCC. We did not find differences in immune marker expression between p16-positive and p16-negative SNSCC tissues. The high correlation between PD-L1 expression and TILs indicates that the PD-1/PD-L1 pathway is a promising immunotherapeutic target for SNSCC.
Keywords: immunotherapy, squamous cell carcinoma, sinonasal cancer, prognosis, biomarker
Introduction
Sinonasal squamous cell carcinoma (SNSCC) is a rare malignant epithelial neoplasm originating from the nasal cavities or paranasal sinuses. It accounts for fewer than 1% of all malignant tumors and approximately 3% of head and neck cancers.1 Because of the lack of specific symptoms in the early stages of the disease, a majority of SNSCC patients are diagnosed at an advanced stage.2 The main treatment modality for SNSCC is surgery combined with radiotherapy, with some multimodal approaches also including chemotherapy.3,4 Despite improvements in surgery, radiotherapy, and systemic therapy, the prognosis for SNSCC remains poor, with a 5-year survival rate of approximately 30–50%.5,6 Moreover, SNSCC tumors often invade adjacent structures such as the orbits, oral cavity, and skull base. Radical treatments often result in severe functional and esthetic defects.7 Thus, there is a considerable need for new therapeutic options for SNSCC treatment.
Immunotherapy, especially immune checkpoint inhibitors, may be an option for SNSCC treatment. Tumor cells can be recognized and attacked by activated T-cells. However, tumors can express programmed death-ligand 1 (PD-L1), a co-inhibitory molecule that binds to its receptor, programmed cell death protein 1 (PD-1), on T lymphocytes, resulting in escape from T-cell attack.8 Specific monoclonal antibodies (mAb) can block the PD-1/PD-L1 axis and enhance the antitumor activity of the immune system.8 In 2016, the Food and Drug Administration (FDA) approved the use of nivolumab and pembrolizumab (an anti-PD-1 mAb) for recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). The effect of PD-1 checkpoint inhibitors on locally advanced HNSCC is currently being explored in several clinical trials, and preliminary results have shown good efficacy and tolerance of anti-PD-1 or anti-PD-L1 antibodies.9 However, only a portion of patients respond to the treatment; the response rate of recurrent or metastatic HNSCC was found to be 13.3–22% in previous clinical trials.10 Previous studies have shown that PD-L1 expression and tumor-infiltrating lymphocytes (TILs) are vital biomarkers for predicting the clinical efficacy of immunotherapy.11 However, few studies have investigated PD-L1 expression and its relation to TILs in SNSCC.
The protein p16 is an important tumor-suppressor protein, which can be used as a surrogate marker for human papillomavirus (HPV) infection.12 High p16 expression has been found to be associated with better prognosis of head and neck cancer, especially in oropharyngeal squamous cell carcinoma (OPSCC).13,14 Several studies have found that PD-L1 expression is associated with p16 status in HNSCC.15,16 An association between p16 expression and immune cell infiltrate has also been identified.17,18 However, the association between p16 status and the tumor immune microenvironment in SNSCC is unclear.
The aim of this study was to evaluate the prevalence of PD-L1 expression and TILs in p16-negative and p16-positive SNSCC and to analyze their correlation with patient clinicopathological characteristics and prognosis.
Materials and methods
Patient cohort
We included 96 patients with SNSCC in this study, whose diagnosis had been confirmed by pathological analysis. Enrolled patients were those who underwent radical treatment at the Eye and ENT Hospital of Fudan University, Shanghai, China, between 2010 and 2016. Patients with a second primary tumor or those without tumor blocks for histopathological analyses were excluded. Written informed consent was obtained from all patients. Ethical approval was granted by the Institutional Review Committee of the Eye and ENT Hospital of Fudan University, and experiments were conducted in accordance with the Declaration of Helsinki. The clinical and pathological characteristics of the 96 patients in this study are summarized in Table 1. All of the tumors were staged according to the 7th edition of the American Joint Committee on Cancer Staging Manual.
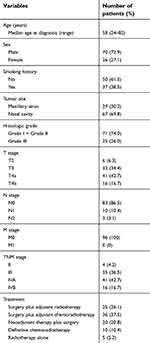 | Table 1 Clinicopathologic characteristics of 96 patients with sinonasal squamous cell carcinoma |
Immunohistochemistry
The primary tumor samples obtained from biopsy or surgery were fixed with formalin and embedded in paraffin. The formalin-fixed paraffin-embedded tissue blocks were cut into 4 μm-thick sections for immunohistochemical staining. The primary antibodies used were as follows: anti-PD-L1 (rabbit mAb, 13684, CST, Danvers, MA,, USA, 1:200), anti-Foxp3 (rabbit mAb, 98377, CST, Danvers, MA, USA, 1:200), anti-CD8 (rabbit mAb, ab93278, Abcam, Cambridge, UK, 1:500), and anti-p16 (rabbit mAb, ab108349, Abcam, Cambridge, UK, 1:100). The tissue sections were deparaffinized in xylene and rehydrated in graded concentrations of ethanol. Antigen retrieval was performed according to the manufacturers’ recommendations for the respective antibodies. After serial blockade in 0.3% hydrogen peroxide and 10% normal serum, sections were incubated with the primary antibody at 4°C overnight. After washing, sections were incubated with anti-rabbit secondary antibodies (Dako EnVision, Copenheagen, Denmark) at room temperature for 30 mins. Finally, staining signals were revealed using 3,3‘-diaminobenzidine staining followed by hematoxylin counterstaining. Human tonsils were used as positive controls for CD8, Foxp3, and PD-L1. A clinically diagnosed HPV-associated tonsil squamous cell carcinoma was used as a positive control in the p16 assay. Negative control was performed by omitting the primary antibody.
Immunohistochemical analysis
PD-L1 staining was scored into four classes: 0%, 1–5%, 5–50%, and 50–100% membranous staining of tumor cells. The tumors were then classified as negative or positive using a 5% cutoff.19 The sample was considered positive for p16 if strong and diffuse nuclear and cytoplasmic staining was detected in ≥75% of the tumor. The levels of CD8 and Foxp3 lymphocyte infiltration were evaluated by counting the number of cells in 10 randomly selected images acquired at high magnification (400×) from each sample, using ImageJ software (NIH, USA). The average infiltration value was calculated for each sample. Because there is no standardized cutoff point for the quantification TILs, the median was used to divide the patient cohort into two groups (low and high) of CD8 and Foxp3 expression. All of the assessments were evaluated independently by two pathologists (HL and SYW), who were blinded to the clinical and follow-up data. Discrepancies between the observers were resolved by a consensus review.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA). Categorical data were analyzed using a Chi-square test. The differences in CD8 and Foxp3 expression levels between subgroups were analyzed using a Mann–Whitney U test, as the variables had a non-normal distribution. The relationship between the CD8 and Foxp3 lymphocyte infiltrates was assessed using a Pearson’s correlation coefficient. Overall survival (OS) was calculated from the date of the end of treatment to the date of death, or the date of the last follow-up. Disease-free survival (DFS) was defined as the period between the completion of primary treatment and the detection of residual disease, recurrent disease, or death. The survival rates were calculated using the Kaplan–Meier method and compared using a log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards model. All of the statistical tests performed were two-tailed, with p-values <0.05 considered statistically significant.
Results
Follow-up
The median follow-up period of the 96 patients was 45 months (range: 2–98 months). Local recurrence (n=26) was the main cause of death, and distant metastases were observed in 10 patients (10.4%). Regional recurrence was observed in two patients (2.1%). The overall 3- and 5-year survival rates of the cohort were 68.8% and 59.3%, respectively.
PD-L1, p16, CD8, and Foxp3 expression
The membranous PD-L1 staining on tumor cells was scored using the following cutoff values: >1% staining, found in 48 patients (50%); >5% staining, found in 29 (30.2%); and >50% staining, found in 16 (16.7%) patients. PD-L1 staining in the tumor-infiltrating immune cells was observed in 44 (45.8%) of SNSCC cases. Overall, positive PD-L1 expression was observed in 29 (30.2%) patients. PD-L1 expression was correlated with poor differentiation, but was not associated with age, smoking status, primary tumor site, or stage (Table 2).
 | Table 2 Correlation of PD-L1, p16, CD8, and Foxp3 with clinicopathologic parameters |
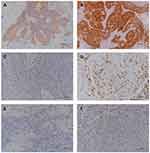
Among the patient cohort, 18 (18.8%) were found to be p16 positive. The average value of CD8+ T-cells at 400× magnification was 2–132, with a median of 32. The number of Foxp3+ T-cells at 400× magnification was 0–123, with a median of 8. High CD8+ T-cell infiltration was associated with poor differentiation and lymph node metastasis. High Foxp3+ T-cell infiltration was also associated with poor differentiation (Table 2). Figure 1 shows representative images of immunohistochemical staining for PD-L1, p16, CD8, and Foxp3 in SNSCC tissue.
Correlation between PD-L1 expression, p16 status, and TILs
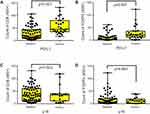
Positive PD-L1 expression was strongly associated with high lymphocytic CD8 (p<0.001) and Foxp3 (p<0.001) expression (Figure 2A and B). PD-L1 expression was not associated with p16 status. The CD8+ and Foxp3+ T-cell infiltrates were not significantly different between p16-negative and p16-positive patients (p=0.523 and p=0.903, respectively; Figure 2C and D). Evaluation by Pearson’s correlation coefficient indicated a strong and significant correlation between CD8 and Foxp3 expression (r=0.518, p<0.001; Figure 3).
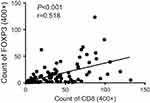 | Figure 3 Correlation between Foxp3+-infiltrating lymphocytes and CD8+-infiltrating lymphocytes in SNSCC. Abbreviation: SNSCC, sinonasal squamous cell carcinoma. |
Survival analysis
Results of the Kaplan–Meier analysis indicated that there was no significant difference between the OS and DFS of PD-L1-positive and PD-L1-negative patients (p=0.760 and p=0.233, respectively; Figure 4A and B). An analysis using multiple cutoff values of PD-L1 expression also showed no significant differences in DFS and OS. Further analysis of the prognostic value of PD-L1 expression for p16 status and CD8 expression did not achieve statistical significance. The OS and DFS of the p16-positive group were no better than those of the p16-negative group (Figure 4C and D). However, patients with high levels of CD8 or Foxp3 infiltration were associated with significantly higher rates of OS and DFS. The accumulated 5-year OS and DFS of patients with high levels of CD8 infiltration were 74.1% and 77.4%, vs 53.7% and 42.1% for those with low levels of CD8 infiltration (p=0.003 and p<0.001, respectively; Figure 4E and F). The 5-year OS and DFS rates of patients with high levels of Foxp3 infiltration were 76.2% and 75.3% vs 51.8% and 43.2% for those with low Foxp3 infiltration (p=0.013 and p=0.001, respectively; Figure 4G and H). A univariate analysis revealed that OS was not significantly associated with clinical variables, such as age, sex, smoking status, histological grade, tumor site, tumor extent, and lymph node metastasis. However, the univariate analysis indicated a significant association between a high histological grade and favorable DFS (Table 3).
 | Table 3 Univariate analysis of clinicopathological features with overall survival, and disease-free survival in sinonasal squamous cell carcinoma |
A multivariate analysis for OS and DFS was performed and included age, sex, histological grade, T classification, N classification, smoking status, p16 status, PD-L1 expression, CD8 expression, and Foxp3 expression. Only CD8 TILs were found to be a significant independent favorable prognostic factor for OS and DFS (Table 4).
 | Table 4 Multivariate analysis of clinicopathological features with overall survival and disease-free survival in sinonasal squamous cell carcinoma |
Discussion
SNSCCs are aggressive tumors with a generally poor prognosis. In cases of advanced or recurrent SNSCC, treatment options are often limited. With their low toxicity and encouraging efficacy, PD-1/PD-L1 immune checkpoint inhibitors could be potential alternatives for the treatment of SNSCC. To use these forms of immunotherapy, it is essential to clarify the immune environment present in SNSCC, in particular, the PD-L1 expression profile and its relationship to TILs.
In this study, PD-L1 expression was evaluated in 96 SNSCC cases. Membranous PD-L1 staining in >5% of tumor cells was observed in 29 patients (30.2%). A review of the literature resulted in little data for comparison, with the exception of a study by Riobello et al, which evaluated PD-L1 expression in 53 cases of SNSCC.19 The frequency of PD-L1 expression in our cohort was similar to that observed by Riobello et al. We found that high PD-L1 expression was associated with poor SNSCC differentiation, which suggested that PD-L1 expression could be involved in SNSCC tumor progression. Other studies have demonstrated an association between high PD-L1 expression and lymph node metastasis in head and neck cancers.20,21 In this study, PD-L1-positive SNSCC cases tended to have a higher lymph node metastasis rate than PD-L1 negative SNSCC (20.7% vs 10.4%); however, no statistical difference was observed (p=0.178; Table 2). High PD-L1 expression contributes to the process of immune evasion, and it has been found be an indicator of poor survival in many different types of malignancies.22 However, we found no prognostic significance of PD-L1 in SNSCC in the present study. In the study by Riobello et al, PD-L1 expression was associated with lower DFS, although it lost statistical significance following multivariate analysis.19 In the present study, the PD-L1 positive cohort tended to have better DFS rates (p=0.233). This discrepancy may be related to the treatment modalities used. All patients in our study received radiotherapy, and some studies have found that high PD-L1 expression is associated with radiosensitivity.23–25 Even though a relatively large cohort was analyzed in our study, the bias arising from retrospective analysis may also confound the finding of a prognostic value of PD-L1 expression. Thus, the prognostic value of PD-L1 expression in SNSCC requires further study.
TILs are a group of immune cells with higher specific immunological reactivity in the tumor environment. They are considered to have prognostic value, and play a vital role in the therapeutic outcomes of immunotherapy.26 CD8+ cytotoxic T-cells and Foxp3+ regulatory T-cells (Tregs) are the most important subsets of TILs. CD8+ lymphocytes are the main effector T-cells that act against tumors, and they are predictive of favorable outcomes in a variety of malignancies. In contrast, Foxp3+ Tregs play a key role in immune escape and are often associated with poor prognosis.27,28 Consistent with the findings of previous studies, the level of CD8 infiltration was a significant independent favorable prognostic factor for SNSCC in our patient cohort. However, high Foxp3+ Treg infiltrates also seemed to be associated with favorable OS and DFS in SNSCC. A meta-analysis of 15,512 cancer cases suggested that the prognostic role of Foxp3+ Tregs was highly influenced by tumor site.29 The meta-analysis found that high Foxp3+ Treg infiltration predicted worse survival rates in the majority of solid tumors, but better outcomes in colorectal, head and neck, and esophageal cancers. The discrepancy of prognostic value of Foxp3+ Tregs may be related to their conventional regulatory function.27 We also found that high levels of CD8 infiltration were related to poor differentiation and lymph node metastasis. The paradoxical coexistence of high numbers of CD8+ TILs and tumor progression indicates the existence of complex crosstalk between tumor and infiltrating inflammatory cells.30 A significant correlation between CD8 and Foxp3 expression in SNSCC was observed in our study, and it is known that the function of intratumoral CD8 T-cells is controlled by Foxp3 Tregs. Thus, the positive correlation between CD8+ and Foxp3+ cells suggested that deletion of Foxp3+ regulatory T-cells could be an effective immunotherapy strategy for SNSCC.
It is well established that patients with p16-positive OPSCC have a more favorable prognosis compared with those with p16-negative OPSCC.31 However, the prognostic significance of p16 expression in nonoropharyngeal HNSCC is less pronounced.14 In this study, we did not find a survival advantage in the p16-positive SNSCC group. Since the number of p16-positive SNSCC cases in our study was small, more trials with a sufficient number of patients are required to clarify the prognostic significance of p16 expression in SNSCC. Chen et al found that p16 positive was associated with high PD-L1 expression in nonoropharyngeal HNSCC.15 Ryu et al found that p16 expression patterns were associated with immune cell infiltration in HNSCC.17 In our study, no association was found between PD-L1 expression and p16 status in SNSCC cases. There was no significant difference in CD8+ and Foxp3+ T-cell infiltrates between p16-negative patients and p16-positive patients. The inconsistent result between our study and previous studies may be related to anatomical site and HPV status. Several studies demonstrated that p16 as a clinical biomarker of HPV infection seemed much less specific outside of the oropharynx.32,33 Based on our study, p16 expression is not a good marker to identify immune phenotypes of SNSCC.
In this study, we found that PD-L1 expression was strongly associated with the levels of CD8+ and Foxp3+ T-cell infiltration in SNSCC. The correlation between PD-L1 expression and TILs in HNSCC is not definite. One study observed an inverse correlation between PD-L1 and TILs in oral squamous cell carcinoma, whereas another study found a positive correlation between PD-L1 and TILs in laryngeal cancer.34,35 PD-L1 expression in tumor cells is regulated by two major mechanisms. First, tumor cells can overexpress PD-L1 through an “intrinsic” mechanism, such as genetic alterations or aberrant oncogenic signaling pathways. Second, “extrinsic” regulatory mechanisms may be involved, such as the adaptive upregulation of PD-L1 in tumor cells by the inflammatory cytokines produced by TILs.36 In some cancers, such as lung cancer, oncogenes may be the main drivers of tumor PD-L1 expression; however, in other cancers, such as melanoma, PD-L1 expression is more likely to be induced by inflammatory stimulation.37,38 The high correlation between PD-L1 expression and the TILs in SNSCC suggested that PD-L1 upregulation in SNSCC may be primarily caused by a TIL-mediated antitumor inflammatory response. The tumor microenvironment has been proposed to be divided into four types according to the presence or absence of TILs and PD-L1 expression. A microenvironment that is PD-L1 positive with a high level of TILs was found to be the group that responds most effectively to treatment with a PD-1 checkpoint inhibitor.37 Given that a large proportion of PD-L1 positive SNSCCs possess high levels of TILs, the PD-1/PD-L1 pathway could be a promising immunotherapeutic target for SNSCC.
This study has certain limitations. First, it was a retrospective study with a potential selection bias. Second, TILs consist of many subtypes of immune cells, and we have not fully accounted for or analyzed the subtypes involved. However, a review of the literature suggested that this study is the first attempt to clarify SNSCC immune phenotypes in a large patient cohort.
In summary, TILs play a prognostic role in SNSCC. In particular, high CD8 infiltration is a favorable independent prognostic indicator of SNSCC. In this study, we fail to find a difference in immune marker expression between p16-positive and p16-negative SNSCC. The high correlation between PD-L1 expression and the TILs in SNSCC indicated that the PD-1/PD-L1 pathway is a promising immunotherapeutic target for SNSCC.
Acknowledgments
We wish to thank pathologist Li Hui, from the Department of Pathology, Fudan University, for assisting with the pathological analysis. This study was supported by The Science and Technology Commission Foundation of Shanghai (12JC1402102).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(6):877–885. doi:10.1002/hed.21830
2. Jegoux F, Metreau A, Louvel G, Bedfert C. Paranasal sinus cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130(6):327–335. doi:10.1016/j.anorl.2012.07.007
3. Lopez F, Lund VJ, Suarez C, et al. The impact of histologic phenotype in the treatment of sinonasal cancer. Adv Ther. 2017;34(10):2181–2198. doi:10.1007/s12325-017-0605-9
4. Robin TP, Jones BL, Gordon OM, et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer-Am Cancer Soc. 2017;123(16):3040–3049.
5. Vazquez A, Khan MN, Blake DM, Patel TD, Baredes S, Eloy JA. Sinonasal squamous cell carcinoma and the prognostic implications of its histologic variants: a population-based study. Int Forum Allergy Rhinol. 2015;5(1):85–91. doi:10.1002/alr.21418
6. Dubal PM, Bhojwani A, Patel TD, et al. Squamous cell carcinoma of the maxillary sinus: a population-based analysis. Laryngoscope. 2016;126(2):399–404. doi:10.1002/lary.25601
7. Kang JH, Cho SH, Kim JP, et al. Treatment outcomes between concurrent chemoradiotherapy and combination of surgery, radiotherapy, and/or chemotherapy in stage III and IV maxillary sinus cancer: multi-institutional retrospective analysis. J Oral Maxillofac Surg. 2012;70(7):1717–1723. doi:10.1016/j.joms.2011.06.221
8. Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4(14):261. doi:10.21037/atm.2016.04.05
9. Moskovitz J, Moy J, Ferris RL. Immunotherapy for head and neck squamous cell carcinoma. Curr Oncol Rep. 2018;20(2):22. doi:10.1007/s11912-018-0654-5
10. Cavalieri S, Rivoltini L, Bergamini C, Locati LD, Licitra L, Bossi P. Immuno-oncology in head and neck squamous cell cancers: news from clinical trials, emerging predictive factors and unmet needs. Cancer Treat Rev. 2018;65:78–86. doi:10.1016/j.ctrv.2018.03.003
11. Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–876. doi:10.1016/j.ctrv.2015.11.001
12. Sano D, Oridate N. The molecular mechanism of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma. Int J Clin Oncol. 2016;21(5):819–826. doi:10.1007/s10147-016-1005-x
13. Smith EM, Wang D, Kim Y, et al. P16INK4a expression, human papillomavirus, and survival in head and neck cancer. Oral Oncol. 2008;44(2):133–142. doi:10.1016/j.oraloncology.2007.01.010
14. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of non-oropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930–3938. doi:10.1200/JCO.2013.54.5228
15. Chen SC, Chang PM, Wang HJ, Tai SK, Chu PY, Yang MH. PD-L1 expression is associated with p16(INK4A) expression in non-oropharyngeal head and neck squamous cell carcinoma. Oncol Lett. 2018;15(2):2259–2265. doi:10.3892/ol.2017.7564
16. Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1: PD-L1pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73(6):1733–1741. doi:10.1158/0008-5472.CAN-12-2384
17. Ryu HJ, Kim EK, Heo SJ, Cho BC, Kim HR, Yoon SO. Architectural patterns of p16 immunohistochemical expression associated with cancer immunity and prognosis of head and neck squamous cell carcinoma. Apmis. 2017;125(11):974–984. doi:10.1111/apm.12744
18. Castaneda CA, Castillo M, Torres-Cabala C, et al. Relationship between tumor-associated immune infiltrate and p16 staining over clinicopathological features in acral lentiginous melanoma. Clin Transl Oncol. 2019. doi:10.1007/s12094-019-02033-x
19. Riobello C, Vivanco B, Reda S, et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018;40(4):818–827. doi:10.1002/hed.25067
20. Straub M, Drecoll E, Pfarr N, et al. CD274/PD-L1 gene amplification and PD-L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget. 2016;7(11):12024–12034. doi:10.18632/oncotarget.7593
21. An HJ, Ko GH, Lee JH, et al. programmed death-ligand 1 expression and its correlation with lymph node metastasis in papillary thyroid carcinoma. J Pathol Transl Med. 2018;52(1):9–13. doi:10.4132/jptm.2017.07.26
22. Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One. 2015;10(6):e131403.
23. Lee VH, Lo AW, Leung CY, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PLoS One. 2016;11(6):e157969.
24. Fiedler M, Weber F, Hautmann MG, et al. Biological predictors of radiosensitivity in head and neck squamous cell carcinoma. Clin Oral Investig. 2018;22(1):189–200. doi:10.1007/s00784-017-2099-x
25. Fukushima Y, Someya M, Nakata K, et al. Influence of PD-L1 expression in immune cells on the response to radiation therapy in patients with oropharyngeal squamous cell carcinoma. Radiother Oncol. 2018;129(2):409–414. doi:10.1016/j.radonc.2018.08.023
26. Badalamenti G, Fanale D, Incorvaia L, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2018. doi:10.1016/j.cellimm.2018.01.013
27. De Meulenaere A, Vermassen T, Aspeslagh S, Vandecasteele K, Rottey S, Ferdinande L. TILs in head and neck cancer: ready for clinical implementation and why (not)? Head Neck Pathol. 2017;11(3):354–363. doi:10.1007/s12105-016-0776-8
28. Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. doi:10.1038/bjc.2017.220
29. Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi:10.1038/srep15179
30. Cancer: MA. Inflaming metastasis. Nature. 2009;457(7225):36–37. doi:10.1038/457036b
31. Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi:10.1200/JCO.2010.29.2904
32. Belobrov S, Cornall AM, Young RJ, et al. The role of human papillomavirus in p16-positive oral cancers. J Oral Pathol Med. 2018;47(1):18–24. doi:10.1111/jop.12649
33. Ilardi G, Russo D, Varricchio S, et al. HPV virus transcriptional status assessment in a case of sinonasal carcinoma. Int J Mol Sci. 2018;19(3):883. doi:10.3390/ijms19030883
34. Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22(3):704–713. doi:10.1158/1078-0432.CCR-15-1543
35. Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47(12):1148–1153. doi:10.1016/j.oraloncology.2011.08.007
36. Concha-Benavente F, Srivastava RM, Trivedi S, et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFN-gamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–1043. doi:10.1158/0008-5472.CAN-15-2001
37. Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi:10.1158/0008-5472.CAN-15-0255
38. Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol. 2014;25(10):1935–1940. doi:10.1093/annonc/mdu242
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.