Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Clinical Pathways For Pancreatic Surgery: Are They A Suitable Instrument For Process Standardization To Improve Process And Outcome Quality Of Patients Undergoing Distal And Total Pancreatectomy? - A Retrospective Cohort Study
Authors Téoule P, Römling L, Schwarzbach M, Birgin E , Rückert F, Wilhelm TJ, Niedergethmann M, Post S, Rahbari NN, Reißfelder C, Ronellenfitsch U
Received 11 May 2019
Accepted for publication 9 September 2019
Published 1 October 2019 Volume 2019:15 Pages 1141—1152
DOI https://doi.org/10.2147/TCRM.S215373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Patrick Téoule,1 Laura Römling,1 Matthias Schwarzbach,2 Emrullah Birgin,1 Felix Rückert,1 Torsten J Wilhelm,3 Marco Niedergethmann,4 Stefan Post,1 Nuh N Rahbari,1 Christoph Reißfelder,1 Ulrich Ronellenfitsch5
1Department of Surgery, Universitätsmedizin Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim 68167, Germany; 2Department of General, Visceral, Vascular, and Thoracic Surgery, Klinikum Frankfurt Höchst, Frankfurt 65929, Germany; 3Department of General and Visceral Surgery, GRN-Klinik Weinheim, Weinheim 69469, Germany; 4Department of Surgery, Alfried Krupp Hospital, Essen 45131, Germany; 5Department of Visceral, Vascular and Endocrine Surgery, University Hospital Halle, Halle, Germany
Correspondence: Ulrich Ronellenfitsch
University Hospital Halle, Department of Visceral, Vascular and Endocrine Surgery, Ernst-Grube-Street 40, Halle (Saale) 06097, Germany
Tel +49 345 557 2314
Fax +49 345 557 2551
Email [email protected]
Purpose: Pancreatic surgery demands complex multidisciplinary management, which is often cumbersome to implement. Clinical pathways (CPs) are a tool to facilitate this task, but evidence for their utility in pancreatic surgery is scarce. This study evaluated if CPs are a suitable tool for process standardization in order to improve process and outcome quality in patients undergoing distal and total pancreatectomy.
Patients and methods: Data of consecutive patients who underwent distal or total pancreatectomy before (n=67) or after (n=61) CP introduction were evaluated regarding catheter management, postoperative mobilization, pancreatic enzyme substitution, resumption of diet and length of stay. Outcome quality was assessed using glycaemia management, morbidity, mortality, reoperation and readmission rates.
Results: The usage of incentive spirometers for pneumonia prophylaxis increased. The median number of days with hyperglycemia decreased significantly from 2.5 to 0. For distal pancreatectomy, the incidence of postoperative diabetes dropped from 27.9% to 7.1% (p=0.012). The incidence of postoperative exocrine pancreatic insufficiency decreased from 37.2% to 11.9% (p=0.007). There was no significant difference in mortality, morbidity, reoperation and readmission rates between groups.
Conclusion: Following implementation of a pancreatic surgery CP, several indicators of process and outcome quality improved, while others such as mortality and reoperation rates remained unchanged. CPs are a promising tool to improve quality of care in pancreatic surgery.
Keywords: clinical pathways, pancreatic surgery, distal pancreatectomy, pancreatectomy, quality of care
Introduction
Pancreatic surgery demands complex and multidisciplinary perioperative management to mitigate the risk of potentially dangerous postoperative complications. Impaired exocrine and endocrine function after pancreatic resection can lead to malnutrition due to lipid malabsorption and diabetes mellitus. Especially after total pancreatectomy, some patients suffer from serious hypoglycemia which can be life-threatening.1 In distal pancreatectomy and pancreatic head resection, there is a considerable risk of pancreatic stump leakage or pancreatic fistula. Distal pancreatectomy is often combined with splenectomy which is related to a higher rate of infectious complications.2
One possible approach to ensure a high quality of perioperative management is the implementation of clinical pathways (CPs).3 CPs are intended to advance quality of processes and, consequently, of outcomes. They are a timeline protocol for all tasks that have to be performed in the course of a given treatment.4–6 CPs usually involve all different disciplines that are part of the treatment team and aim to translate evidence into clinical practice.7–9 CPs have shown favorable perioperative results for a number of operations in gastrointestinal surgery.10 Kennedy et al (2012), Walters et al (2012) and Van der Kolk et al (2017) evaluated CPs for pancreaticoduodenectomy.11–13 All showed a reduction in length of stay and Kennedy et al additionally reported a non-significant decrease of overall complication rates. Van der Kolk reported a significant decrease of major complications according to the Clavien-Dindo classification. There are three studies assessing CPs for distal pancreatectomy, and, to our knowledge, none for total pancreatectomy. While one of the studies for distal pancreatectomy showed no difference in outcomes and length of stay after CP implementation,14 two studies demonstrated an improvement in length of stay and short-term outcomes.15,16 Given the limited evidence, we conducted a study assessing if CPs are a suitable instrument for process standardization in order to improve process and outcome quality in patients undergoing distal or total pancreatectomy.
Patients And Methods
CP Design, Implementation, And Content
Since 2006, the Department of Surgery, Universitätsmedizin Mannheim, Medical Faculty Mannheim, Heidelberg University has performed a stepwise implementation of CPs for different surgical procedures.17–25 In February 2011, three CPs were introduced for pancreatic surgery: one for pancreaticoduodenectomy, one for distal pancreatectomy, and one for total pancreatectomy. The first mentioned CP has been assessed in a separate study.
The content of the CP is based on CPs for fast-track colorectal and bariatric surgery which had been previously established.18,19 Specific treatment steps were modified to adapt this CP for use in pancreatic surgery. Both the original colorectal CP and the pancreatic surgery CPs are based on national and international treatment and nursing recommendations, as well as on best available evidence. The design and implementation processes were carried out by an interdisciplinary (surgery, anesthesiology, physiotherapy, nutritional services) and multi-hierarchical team.
First drafts of the respective CPs were elaborated after a literature review to identify evidence on perioperative treatment elements. In a second step, pre-existent institutional standards were integrated into the CPs. In a last step, a consensus meeting with all project participants was held to agree on a final CP version. Prior to definite implementation, all staff members were trained on how to work with the CPs. After implementation, continuous efforts were made to further develop and improve the CPs based on suggestions of staff members.
Full versions of the CPs are shown in the online Supplementary material. The main elements of the CPs included the following items: hospital admission was scheduled for the day before surgery. Epidural catheter placement was stipulated for all patients. A stepwise oral analgesia scheme, with a basis medication of non-opioids and on demand medication of potent opioids, was included in both CPs. Patients were monitored postoperatively in a surgical intermediate care unit for at least one night or in an intensive care unit, if considered necessary by the surgeon and/or anesthesiologist. All patients were encouraged to drink sweetened tea until two hours prior to planned intubation. For distal pancreatectomy, pancreas enzymes in the drainage fluid were determined on days three and five after surgery. Detailed instructions on how to use an incentive spirometer were given to all patients. Pancreatic enzymes were orally substituted in case of steatorrhea. Glycaemia levels were closely monitored. In the distal pancreatectomy CP, an on-demand insulin scheme was included, whereas the total pancreatectomy CP included a fixed glycaemia-dependent insulin scheme. The designated time of discharge was day ten for distal pancreatectomy and day twelve for total pancreatectomy. Outpatient follow-up appointments were scheduled within 14 days of discharge. Patients were instructed to present at our emergency room in case of clinical abnormalities.
CPs were designed as four-page paper-based documents containing all stipulated treatment steps for the single pre- and postoperative days. They were kept with patients’ treatment charts and thus always available for all staff involved in treatment.
Study Design
The study was designed as a single center retrospective study. A research protocol had been developed before evaluation. The protocol has not been published previously. All patients undergoing elective distal or total pancreatectomy were included. The intervention group (CP group), either distal pancreatectomy (CP-D group) or total pancreatectomy (CP-T group), comprised all consecutive patients operated following the implementation of the CPs in February 2011. The control group (pre-CP group), either distal pancreatectomy (pre-CP-D group) or pancreatectomy (pre-CP-T group), comprised all patients operated in the five years (pre-CP-D) or eight years (pre-CP-T) before CP implementation, respectively. In this retrospective study, there was no formal sample size calculation. Study group sizes were determined in order to obtain equally large groups before and after CP implementation. All necessary data were retrieved by retrospective chart review.
Patients in the CP groups were treated according to the respective CP (see below), whereas the pre-CP groups were treated according to the individual judgment of and decisions taken by the treating surgeons. Although several semiformal standards for selected elements of care (e.g., epidural analgesia, early removal of catheters, early mobilization) had been in place, at that time there was no instrument covering the whole treatment continuum.
The study was approved by the competent ethical committee of the Medical Faculty of Mannheim (2015-863R-MA). Patient consent to review their medical records was not required by the ethical committee because of the retrospective nature of the study without direct patient contact and without any study-related measures which directly affected the patient. Confidentiality of patient data was ensured. The study was conducted in compliance with the Declaration of Helsinki. Neither the individual deidentified participant data, nor the specific data is intended to be shared by the authors. The clinical pathway documents will be accessible indefinitely, as online supplement data. The study has been registered at the German Clinical Trials Register (DRKS 00016749).
Surgery
In both groups (before and after CP implementation), surgery was performed by dedicated HPB surgeons with an experience of more than four years. All surgeons performed surgery according to the in-house standard. Pancreatectomy and distal pancreatectomy were performed in a uniform fashion. In case of distal pancreatectomy sharp transection was performed with a blade in a “fish-mouth” fashion. The visible main pancreatic duct was occluded by a stich and the pancreatic remnant was secured with minimal sutures.
Study Outcomes
The preoperative status of patients was assessed using the ASA (American Society of Anesthesiologists) physical status classification system.26 The study evaluated parameters of both process and outcome quality. Process and outcome quality were defined according to the model proposed by Donabedian.27,28 Process quality was defined as the adherence to treatment specifications as detailed in the CP. It reflected protocol adherence and was assessed by the following parameters: placement of central venous line and epidural catheter, day of removal of foley catheter and epidural catheter, day of first and second measurement of pancreas enzymes in the drainage fluid, substitution of pancreas enzymes and administration of somatostatin, removal of intraabdominal drainages and nasogastric tube, application of single shot antibiotics, postoperative mobilization and day of resumption of liquid and solid diet.
Outcome quality was measured through the following variables: morbidity, mortality, reoperation, length of stay stratified by the presence or absence of complications, pain levels on a numeric rating scale, day of first postoperative defecation and readmission. Morbidity was assessed according to the Clavien-Dindo classification of postoperative complications.29 The following complications were specifically assessed: postoperative pancreatic fistula (POPF), delayed gastric emptying (DGE) and postoperative pancreatic hemorrhage (PPH). For the different degrees of severity, the official definitions of the International Study Group of Pancreatic Surgery (ISGPS) were used.30–32 Other specific complications included postoperative pancreatitis, hypoglycemia (blood glucose level lower than 60mg/dl), days with at least one measured blood glucose level higher than 200 mg/dl, postoperative diabetes (fasting blood glucose level higher than 126 mg/dl), and exocrine insufficiency (repetitive substitution of pancreas enzymes). Surgical site infections were diagnosed according to the Centers for Disease Control and prevention (CDC) definition.33 Readmission was only counted as such if it took place within 14 days after initial discharge and was related to a postoperative problem.
Statistical Analysis
All study outcomes were compared between the respective CP and pre-CP groups. No imputation of missing values was performed, and missing values were not counted in the analyses. Dichotomous variables were evaluated with the chi-square test. Ordinal variables were evaluated with student’s t-test if normally distributed and the Mann–Whitney-U-test if not normally distributed. P-values <0.05 were considered to be significant. There was no adjustment for multiple testing. For all statistical analyses, the software SAS 13.2 was used.
Results
Patients’ Characteristics
During the study period, 85 patients underwent distal pancreatectomy, of which 43 were in the pre-CP-D and 42 in the CP-D group (Table 1). Thirty-three patients underwent total pancreatectomy, comprising 24 in the pre-CP-T and 19 in the CP-T group. Figure 1 summarizes the patient recruitment and group allocation. There were no relevant differences between the pre-CP and CP groups regarding demographic and clinical characteristics of patients. Likewise, the underlying condition for which resection was performed did not differ between groups for total pancreatectomy. In the CP-D group, the frequency of splenectomy was significantly higher.
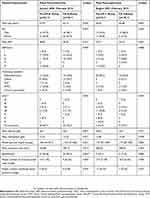 |
Table 1 Characteristics Of The Study Groups |
Process Quality
The comparison of measures of process quality is presented in Table 2. Regarding distal pancreatectomy, there was a number of significant differences between the pre-CP-D and CP-D group. The number of patients receiving a central venous catheter decreased after CP implementation. The day of the removal of the central venous catheter, arterial catheter, foley catheter, nasogastric tube and abdominal drainage did not differ for patients treated with and without CP. Likewise, the days of first intake of liquids, liquid nutritional supplement and solids did not change after CP implementation. The postoperative day of first and second determination of pancreas enzymes in drainage liquids differed significantly between groups, with more patients in the CP group receiving enzyme determination on the recommended days. Usage of incentive spirometers increased significantly following CP implementation. All patients in the CP-D group used a spirometer. Lastly, patients were mobilized later in the CP-D group.
 |
Table 2 Parameters Of Process Quality |
For total pancreatectomy, there were fewer differences between the pre-CP-T and CP-T group. The only significant finding was that patients in the CP-T group used significantly more often an incentive spirometer than those in the pre-CP-T group. In the CP-T group, all but one patient used a spirometer. Foley catheters were removed later in the CP-T than the pre-CP-T group, and the finding showed a trend towards statistical significance.
Outcome Quality
The results regarding outcome quality are outlined in Table 3. The causes of mortality are well-known complications after total pancreatectomy. In the pre-CP-T group, one patient each died due to septic multi-organ failure, post-pancreatectomy hemorrhage and an unknown cause. One patient with septic multi-organ failure suffered from a bowel leakage, the other patient from a severe atypical pneumonia and the third patient from liver failure of unknown cause. The patient with post-pancreatectomy hemorrhage had a leakage of the duodenojejunostomy, with septic bleeding from the aorta. In the CP-T group, two patients died due to multi-organ failure: one caused by postoperative hemorrhage from the portal vein and the other one caused by necrotizing pancreatitis and severe pneumonia. One patient in the CP-T group died due to liver failure caused by postoperative occlusion of the hepatic artery. After distal pancreatectomy, one patient in each group died due to septic multi-organ failure, caused by severe pneumonia. One of these patients suffered from severe COPD, the other one had POPF Grade C.
 |
Table 3 Parameters Of Outcome Quality |
There were several significant differences in patients undergoing distal pancreatectomy. After CP implementation, the median number of days with glycaemia >200 mg/dl was reduced from 2.5 to 0, and the incidence of postoperative diabetes mellitus was lower. The incidence of postoperative exocrine insufficiency was lower in the CP-D compared to the pre-CP-D group. The number of days with a relevant pain level was higher in the CP-D than the pre-CP-D group, but the difference was only borderline significant. It was not reflected by a higher number of requests for additional analgesics. Regarding postoperative morbidity and mortality, there were no differences between groups, neither for the summary measures, nor for specific complications. Length of stay did not relevantly differ between patients treated with and without CP, and the discharge goal stipulated in the CP was not met.
For total pancreatectomy only one significant difference was found. The number of days with a relevant pain level (higher than 3) increased from one in the pre-CP-T group to three in CP-T group. No difference was observed regarding additional analgesic requests. For all other measures of outcome quality, there were no relevant differences between patients treated with and without CP. In both groups, length of stay was generally longer than foreseen in the CP.
Discussion
This study evaluated the effect of CP implementation for distal and total pancreatectomy on various parameters of perioperative process and outcome quality. Pancreatic surgery is complex and should only be performed by experienced and specialized surgeons. In the last decades, perioperative mortality has dropped, but morbidity remains high.34–37 This might partly be explained by the fact that older patients with significant comorbidities or locally advanced tumors are resected, but a lack of standardization of perioperative treatment might contribute to high morbidity.38–40 Therefore, the principal aim of this study was to evaluate if CP implementation led to standardization of perioperative treatment patterns and therefore to an amelioration of process and outcome quality of patients undergoing distal and total pancreatectomy.
Parameters of process quality were regarded as key performance indicators to measure protocol adherence. We observed an improvement for a few parameters of process quality, while others remained unchanged or even deteriorated after CP implementation. The frequency of incentive spirometer usage increased significantly for both procedures so that all but one patient in the CP groups used the device. Incentive spirometers are a valuable means to lower the risk of pneumonia and therefore their increased use is an important step towards prevention of this common postoperative complication.41 For distal pancreatectomy, the timing of pancreas enzyme measurement in drainage fluid was standardized after CP implementation. This measurement is important for a timely diagnosis of a possible postoperative pancreatic fistula, the most frequent complication after distal pancreatectomy. At the same time, the risk of drain-related ascending infection or enteral fistula increases with the time of drain indwelling, so that timely drain removal is recommended once drain fluid shows no increased enzyme levels.42–44
In contrast to these encouraging findings, process quality regarding some other parameters deteriorated after CP implementation. Most importantly, mobilization after distal pancreatectomy took place significantly later in patients treated with a CP compared to those treated without. The results show that the median day of first mobilization does not differ between the two groups, but that the difference originates from a high number of outliers in the CP-D group in whom mobilization took place exceedingly late. The reasons for this finding are not fully understood. Probably, late mobilization can be at least partially explained by complications. However, the incidence of complications was the same among the two groups, and thus it remains unclear why many patients treated with the CP were mobilized late. Mobilization of patients in a limited physical status, with drains and catheters in place, is cumbersome and requires intensive efforts by staff and the patient as well as sufficient time to perform these efforts. Therefore, it is conceivable that in spite of the recommendations being clearly stated in the CP, these could not be realized due to a lack of sufficient resources. Another possible reason for delayed mobilization could be insufficient pain control. The number of days with a relevant pain level was in fact higher in the CP groups. This finding is rather surprising, because the CP contained a dedicated analgesia scheme according to recent recommendations. It included epidural catheter placement, which was carried out in the overwhelming majority of patients. Additional oral analgesics were administered in a stepwise, pain-adjusted manner, so that there is no obvious explanation for higher pain levels in patients treated according to the CP. Additional analgesic requests by patients also occurred with the same frequency as in patients treated without CP. One potential explanation, although merely hypothetical, could be that nursing staff had increased awareness for possible postoperative pain after CP implementation and tended to assess patients more meticulously regarding their pain, inciting a higher reported pain level. This would be a form of ascertainment bias. Delayed mobilization and insufficient pain control can have relevant consequences for the patient, because of an increased risk of postoperative morbidity especially with regard to pulmonary complications.45 Moreover, some studies found a significant correlation between delayed mobilization and increased length of stay.46
Regarding outcome quality, several differences between patients treated with and without CP were observed. In patients undergoing distal pancreatectomy, the number of days with at least one measurement of blood glucose higher than 200 mg/dl was significantly lower in the CP group. These findings are indicative for a much-improved glucose management by using a CP which contains a detailed and aggressive insulin scheme aiming at early postoperative normoglycemia. The safety of this scheme was demonstrated by the absence of hypoglycemia. Tight glycemic control after pancreatic surgery is crucial to prevent infectious complications.47 For total pancreatectomy, glycemic control was not different between pre-CP and CP patients. This shows that already prior to CP implementation, glycemic control had been in the focus of treatment after total pancreatectomy. Beyond improved glycemic control, the incidence of postoperative diabetes, defined as elevated fasting glucose, was also lower after CP implementation in patients undergoing distal pancreatectomy. This finding can obviously not be explained as a consequence of improved glucose management and the exact reasons remain unclear. It is possible that the surgical approach changed from one surgeon to the other and that surgeons operating after CP implementation performed less radical distal pancreatectomies, thus leaving more endocrine pancreatic tissue in situ. The same might be a potential explanation for the observed lower incidence of exocrine pancreatic failure.
of Regarding the incidence of postoperative complications and mortality, the analyses did not show a difference between patients treated before and after CP implementation. While mortality after distal pancreatectomy was in the range of what is reported from comparable series,48,49 mortality after total pancreatectomy was higher than benchmark rates found in the literature.1,50 Since the overall number of patients comprised in the present analysis is rather low, the found mortality rates are sensitive to random fluctuation and might overestimate the true mortality in our setting. Yet, the relatively low overall volume of pancreatectomy cases during the study period might have contributed to an increased mortality, as there is a well-known volume-outcome relationship for total pancreatectomy.50 Compared to other studies, also the overall postoperative complication rate in our patients seems high.1 A possible reason is the use of the Clavien-Dindo classification for postoperative complications. This classification counts every deviation from the normal postoperative process as complication, while many studies reporting complications have not used a dedicated classification or lack a detailed definition of complications.29,51 The incidence of specific complications has also not changed significantly after implementation of the CPs. The most frequent complication after distal pancreatectomy is postoperative pancreatic fistula. It is virtually impossible to influence its incidence postoperatively. However, potentially hazardous sequelae can be mitigated by dedicated drain management,52 which was realized more frequently after CP implementation.
One of the aims of CP implementation is to avoid unnecessarily long hospital stays without a clear medical reason by means of streamlining perioperative processes. In this study, length of stay did not decrease after CP implementation, and still showed a relevant variation between single patients. However, length of stay in larger series in the literature was rather in the range of what we observed before and after CP implementation than in the range of the goals of the CP.49,53 Moreover, the analyses comprised all consecutive patients including those with relevant complications, which explains the large variation and exceedingly long hospital stay in some patients.
The study has a number of methodological limitations. It is retrospective and relied on chart review for data collection. Therefore, the validity of data could be inferior to prospectively collected data. Moreover, for some variables values for single patients were not documented and not used for the analyses. This might bias the results, although there is no reason to assume that variables were selectively not recorded. During the development and implementation of the CPs, a crossover respectively contamination bias could have been occurred. Likewise, caregivers who were part of the development team could have used their knowledge of CP content prior to implementation in February 2011. To counteract these points, we carried out the actual design and implementation of CPs over a short time period of approximately three months. Therefore, a third group covering only the transition period would have been too small for meaningful analyses. Obviously, surgical technique and the skills and experience of the single surgeon do affect perioperative outcomes.35 During the study period, a number of different surgeons operated on patients and surgical performance bias cannot be excluded. Most of these limitations would have been overcome by designing the study as randomized controlled trial, which is however hardly feasible to conduct when evaluating clinical pathway usage.36
The methodological strength of our study was that patients were included according to the “intention-to-treat” principle. All consecutive patients undergoing distal or total pancreatectomy before and after CP implementation were included. Even if certain goals of the CP such as drain removal or mobilization were not met, patients were not taken “off the pathway”. Moreover, patients were analyzed regardless of possible complications. Consequently, selection bias can virtually be ruled out. We believe this approach to be the only valid way for evaluating the true clinical impact of CPs because it represents clinical reality, where CPs are meant to be a tool for treating the entirety of patients with a specific intervention or condition.
Conclusion
In conclusion, this study showed that CPs for distal and total pancreatectomy can affect several aspects of perioperative treatment. CP usage fulfilled the expectations regarding a high degree of process standardization. Drain management and the uptake of respiratory training as well as glycemic control were improved. Other expected improvements, such as earlier mobilization, better pain control, and shorter length of stay, were not realized after CP implementation. Outcome parameters such as morbidity and mortality did not differ between patients treated with and without CP. CPs in pancreatic surgery can be used to facilitate some perioperative processes, but their utility must be weighed against the expected cost and efforts of implementation. The results of this study can serve to further refine and adapt the two used CPs in our given setting, but also to inform the design of future studies assessing CPs for pancreatic surgery.
Author contributions
P. Téoule and U. Ronellenfitsch participated in the conception and design of the study. L. Römling and P. Téoule performed data collection, analyzed the data and drafted the manuscript. P. Téoule, U. Ronellenfitsch, L. Römling, M. Schwarzbach, E. Birgin, F. Rückert, TJ. Wilhelm, M. Niedergethmann, S. Post, NN. Rahbari and C. Reißfelder participated in the analysis and interpretation of data, revision of the manuscript for important intellectual content. All authors have read and approved the final manuscript and are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no proprietary or commercial interests in any products mentioned or concept discussed in this article.
References
1. Del Chiaro M, Rangelova E, Segersvärd R, Arnelo U. Are there still indications for total pancreatectomy? Updates Surg. 2016;68(3):257–263. doi:10.1007/s13304-016-0388-6
2. Werner J, Büchler MW. Resectional techniques: pancreaticoduodenectomy, segmental pancreatectomy, total pancreatectomy, and transduodenal resection of the papilla of vater. In: Jarnagin WJ, Blumgart LH (eds.). Blumgart’s Surgery of the Liver, Pancreas and Biliary Tract.
3. Ronellenfitsch U, Rössner E, Jakob J, Post S, Hohenberger P, Schwarzbach M. Clinical pathways in surgery: should we introduce them into clinical routine? A review article. Langenbecks Arch Surg. 2008;393(4):449–457. doi:10.1007/s00423-008-0303-9
4. Weiland DE. Why use clinical pathways rather than practice guidelines? Am J Surg. 1997;174(6):592–595. doi:10.1016/s0002-9610(97)00196-7
5. Glenn DM, Macario A. Do clinical pathways improve efficiency? Semin Anesth Perioper Med Pain. 1999;18(4):281–288. doi:10.1016/S0277-0326(99)80023-3
6. Pearson SD, Goulart-Fisher D, Lee TH. Critical pathways as a strategy for improving care: problems and potential. Ann Intern Med. 1995;123(12):941–948. doi:10.7326/0003-4819-123-12-199512150-00008
7. Rotter T, Kinsman L, Machotta A. et al. Clinical pathways for primary care: effects on professional practice, patient outcomes, and costs. Cochrane Database Syst Rev. 2010;(3):CD00663. doi:10.1002/14651858.CD010706
8. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31. doi:10.1186/1741-7015-8-31
9. van Zelm R, Coeckelberghs E, Sermeus W, et al. Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Int J Colorectal Dis. 2017;32(10):1471–1478. doi:10.1007/s00384-017-2863-z
10. Lemmens L, van Zelm R, Borel Rinkes I, van Hillegersberg R, Kerkkamp H. Clinical and organizational content of clinical pathways for digestive surgery: a systematic review. Dig Surg. 2009;26(2):91–99. doi:10.1159/000206142
11. Walters DM, McGarey P, LaPar DJ, et al. A 6-day clinical pathway after a pancreaticoduodenectomy is feasible, safe and efficient. HPB (Oxford). 2013;15(9):668–673. doi:10.1111/hpb.12016
12. Kennedy EP, Rosato EL, Sauter PK, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution–the first step in multidisciplinary team building. J Am Coll Surg. 2007;204(5):917–923. discussion 923–924. doi:10.1016/j.jamcollsurg.2007.01.057
13. van der Kolk M, van den Boogaard M, Becking-Verhaar F, et al. Implementation and evaluation of a clinical pathway for pancreaticoduodenectomy procedures: a prospective cohort study. J Gastrointest Surg. 2017;21(9):1428–1441. doi:10.1007/s11605-017-3459-1
14. Nussbaum DP, Penne K, Speicher PJ, et al. The role of clinical care pathways: an experience with distal pancreatectomy. J Surg Res. 2014;190(1):64–71. doi:10.1016/j.jss.2014.02.026
15. Kennedy EP, Grenda TR, Sauter PK, et al. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg. 2009;13(5):938–944. doi:10.1007/s11605-009-0803-0
16. Pecorelli N, Capretti G, Balzano G, et al. Enhanced recovery pathway in patients undergoing distal pancreatectomy: a case-matched study. HPB (Oxford). 2017;19(3):270–278. doi:10.1016/j.hpb.2016.10.014
17. Hardt J, Schwarzbach M, Hasenberg T, Post S, Kienle P, Ronellenfitsch U. The effect of a clinical pathway for enhanced recovery of rectal resections on perioperative quality of care. Int J Colorectal Dis. 2013;28(7):1019–1026. doi:10.1007/s00384-013-1650-8
18. Ronellenfitsch U, Schwarzbach M, Kring A, Kienle P, Post S, Hasenberg T. The effect of clinical pathways for bariatric surgery on perioperative quality of care. Obes Surg. 2012;22(5):732–739. doi:10.1007/s11695-012-0605-4
19. Schwarzbach M, Hasenberg T, Linke M, Kienle P, Post S, Ronellenfitsch U. Perioperative quality of care is modulated by process management with clinical pathways for fast-track surgery of the colon. Int J Colorectal Dis. 2011;26(12):1567–1575. doi:10.1007/s00384-011-1260-2
20. De Allegri M, Schwarzbach M, Loerbroks A, Ronellenfitsch U. Which factors are important for the successful development and implementation of clinical pathways? A qualitative study. BMJ Qual Saf. 2011;20(3):203–208. doi:10.1136/bmjqs.2010.042465
21. Schwarzbach M, Rössner E, Schattenberg T, Post S, Hohenberger P, Ronellenfitsch U. Effects of a clinical pathway of pulmonary lobectomy and bilobectomy on quality and cost of care. Langenbecks Arch Surg. 2010;395(8):1139–1146. doi:10.1007/s00423-010-0600-y
22. Schwarzbach M, Bönninghoff R, Harrer K, et al. Effects of a clinical pathway on quality of care in kidney transplantation: a non-randomized clinical trial. Langenbecks Arch Surg. 2010;395(1):11–17. doi:10.1007/s00423-009-0551-3
23. Schwarzbach MHM, Ronellenfitsch U, Wang Q, et al. Effects of a clinical pathway for video-assisted thoracoscopic surgery (VATS) on quality and cost of care. Langenbecks Arch Surg. 2010;395(4):333–340. doi:10.1007/s00423-009-0507-7
24. Ronellenfitsch U, Vargas Hein O, Uerlich M, Dahmen A, Tuschy S, Schwarzbach M. Klinische pfade als instrument zur qualitätsverbesserung in der perioperativen medizin. Perioper Medizin. 2009;1(3):164–172. doi:10.1016/j.periop.2009.06.002
25. Ronellenfitsch U, Schwarzbach M. [clinical pathways in surgery]. Zentralbl Chir. 2010;135(2):99–101.
26. Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2(3):281–284. doi:10.1097/00000542-194105000-00004
27. Donabedian A. The quality of care. how can it be assessed? Jama. 1988;260(12):1743–1748. doi:10.1001/jama.260.12.1743
28. Donabedian A, Bashshur R. An Introduction to Quality Assurance in Health Care. New York: Oxford University Press; 2003. Available from: https://trove.nla.gov.au/version/45740523. Accessed August 21, 2019.
29. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
30. Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20–25. doi:10.1016/j.surg.2007.02.001
31. Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–768. doi:10.1016/j.surg.2007.05.005
32. Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8–13. doi:10.1016/j.surg.2005.05.001
33. (uk) NCC for W and CH. Definitions, Surveillance and Risk Factors. RCOG Press; 2008. Available from: https://www.ncbi.nlm.nih.gov/books/NBK53724/. Accessed July 28, 2018.
34. Bentrem DJ, Yeh JJ, Brennan MF, et al. Predictors of intensive care unit admission and related outcome for patients after pancreaticoduodenectomy. J Gastrointest Surg. 2005;9(9):1307–1312. doi:10.1016/j.gassur.2005.09.010
35. Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242(4):540–544. discussion 544–547. doi:10.1097/01.sla.0000184190.20289.4b
36. Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–579.
37. Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi:10.1056/NEJMsa012337
38. Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476–e485. doi:10.1016/S1470-2045(13)70172-4
39. Nentwich MF, Bockhorn M, König A, Izbicki JR, Cataldegirmen G. Surgery for advanced and metastatic pancreatic cancer–current state and trends. Anticancer Res. 2012;32(5):1999–2002.
40. Burdelski CM, Reeh M, Bogoevski D, et al. Multivisceral resections in pancreatic cancer: identification of risk factors. World J Surg. 2011;35(12):2756–2763. doi:10.1007/s00268-011-1263-8
41. Lawrence VA, Cornell JE, Smetana GW; American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of physicians. Ann Intern Med. 2006;144(8):596–608. doi:10.7326/0003-4819-144-8-200604180-00011
42. Villafane-Ferriol N, Shah RM, Mohammed S, et al. Evidence-based management of drains following pancreatic resection: a systematic review. Pancreas. 2018;47(1):12–17. doi:10.1097/MPA.0000000000000961
43. Pedrazzoli S, Liessi G, Pasquali C, Ragazzi R, Berselli M, Sperti C. Postoperative pancreatic fistulas: preventing severe complications and reducing reoperation and mortality rate. Ann Surg. 2009;249(1):97–104. doi:10.1097/SLA.0b013e31819274fe
44. Niedergethmann M, Bludau F, Dusch N, Nowak K, Post S. [Significance of drains in surgery]. Chirurg. 2011;82(12):1079–1084. doi:10.1007/s00104-011-2115-7
45. Stiller KR, Munday RM. Chest physiotherapy for the surgical patient. Br J Surg. 1992;79(8):745–749. doi:10.1002/bjs.1800790807
46. Yip VS, Dunne DFJ, Samuels S, et al. Adherence to early mobilisation: key for successful enhanced recovery after liver resection. Eur J Surg Oncol. 2016;42(10):1561–1567. doi:10.1016/j.ejso.2016.07.015
47. Eshuis WJ, Hermanides J, van Dalen JW, et al. Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg. 2011;253(4):739–744. doi:10.1097/SLA.0b013e31820b4bfc
48. Fabre JM, Houry S, Manderscheid JC, Huguier M, Baumel H. Surgery for left-sided pancreatic cancer. Br J Surg. 1996;83(8):1065–1070. doi:10.1002/bjs.1800830810
49. Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229(5):693–698. discussion 698–700. doi:10.1097/00000658-199905000-00012
50. McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246(2):246–253. doi:10.1097/01.sla.0000259993.17350.3a
51. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
52. Fujino Y. Perioperative management of distal pancreatectomy. World J Gastroenterol. 2015;21(11):3166–3169. doi:10.3748/wjg.v21.i11.3166
53. Nazzani S, Preisser F, Mazzone E, et al. In-hospital length of stay after major surgical oncological procedures. Eur J Surg Oncol. 2018;44(7):969–974. doi:10.1016/j.ejso.2018.05.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

