Back to Journals » Cancer Management and Research » Volume 10
Clinical outcomes of stereotactic body radiotherapy for de novo pulmonary tumors in patients with completely resected early stage non-small cell lung cancer
Authors Zhao Q , Chen G, Ye L, Zeng Z , Shi S, He J
Received 16 July 2018
Accepted for publication 19 October 2018
Published 28 November 2018 Volume 2018:10 Pages 6391—6398
DOI https://doi.org/10.2147/CMAR.S180345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Rituraj Purohit
Qianqian Zhao, Gang Chen, Luxi Ye, Zhaochong Zeng, Shiming Shi, Jian He
Department of Radiation Oncology, Zhongshan Hospital, Fudan University, Shanghai, China
Purpose: Following surgery for early stage non-small-cell lung cancer (NSCLC), de novo pulmonary tumors are common. This study aimed to assess the efficacy, patterns of failure, and toxicity of stereotactic body radiotherapy (SBRT) in the treatment of de novo pulmonary tumors following curative resection of early stage NSCLC.
Patients and methods: We reviewed the medical data of patients who had received definitive intent SBRT for small lung cancer at Zhongshan Hospital, Fudan University, between June 2011 and December 2017. Patients who had experienced complete resection for prior early stage NSCLC before SBRT were identified for further analysis. Incidences of locoregional recurrence (LR) and distant metastasis (DM) were evaluated using the alternative cumulative incidence competing risk method. The probability of survival was estimated using the Kaplan–Meier method.
Results: A total of 33 patients with 36 lesions were eligible and included in this study. The median follow-up time was 32 months. Estimated incidences of LR and DM were 37.62% and 15.92%, respectively, at 1 year and 48.02% and 21.23%, respectively, at 2 years. The progression-free survival and overall survival of all patients were 62.40% and 90.30%, respectively, at 1 year and 52.00% and 69.90%, respectively, at 2 years. In all, 26 patients experienced grade 1 SBRT-related toxicity, 11 patients experienced grade 2 SBRT-related toxicity, and three patients experienced grade 3 toxicity. There were no grade 4/5 toxicities or SBRT-related deaths during the follow-up period.
Conclusion: SBRT appears to be a safe and potentially effective alternative therapeutic option for de novo pulmonary tumors following early stage NSCLC radical resection, despite impaired pulmonary reserve.
Keywords: stereotactic body radiotherapy, de novo pulmonary tumors, surgical resection, clinical outcomes
Introduction
Lung cancer is one of the leading causes of cancer worldwide. Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, representing ~85% of all cases.1 Surgery is the preferred treatment modality for patients with resectable NSCLC and results in 5-year overall survival (OS) rates ranging from 24% to 73%.2 Extended survival outcomes after curative resection of early stage NSCLC come with an increased risk for development of novel pulmonary tumors, which are associated with a particularly poor prognosis.3–7 Surgical resection remains the preferred modality for those patients who developed pulmonary tumors following curative resection and has yielded considerable survival achievement;7–11 however, a large proportion of cases are not suited for repeated pulmonary resection due to inadequate postoperative pulmonary reserve, and there are few other options for further treatment.12–14
Stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), has been recommended as a standard alternative treatment to surgery for early stage NSCLC patients who are not surgical candidates. This type of radiotherapy (RT) has been shown to achieve local control rates (LCRs) comparable to those seen with radical resection in multiple prospective trials.15–20 Moreover, SBRT yields better clinical outcomes for early stage NSCLC than conventional fractionated RT.6,7,20,21 In addition, several reports support the efficacy of SBRT for multiple primary lung cancers (MPLCs).16,17,22 However, the efficacy and safety of SBRT in patients with de novo pulmonary tumors after lung resection remain unclear. The pulmonary reserve of this patient population is impaired, which limits the ability of these patients to tolerate further antitumor therapy. Therefore, we conducted this retrospective study to evaluate our experience regarding the therapeutic effectiveness, feasibility, and safety of SBRT for de novo pulmonary tumors in patients with completely resected early stage NSCLC.
Patients and methods
Patient population
A review was conducted of the medical data of patients who had received definitive intent SBRT for small lung cancer between June 2011 and December 2017 at Zhongshan Hospital, Fudan University. Patients who had experienced complete resection for the prior early stage NSCLC before SBRT were identified for further analysis. A multidisciplinary tumor board team, including at least a thoracic radiologist, thoracic surgeon, pulmonologist, and pathologist, determined the diagnosis and treatment strategy of the de novo pulmonary tumors. Inclusion criteria were as follows: 1) margin-negative radical resection (R0) for the prior lung cancer with postoperative pathological confirmation; 2) a clinical presentation consistent with malignant tumor based on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) scan before SBRT in patients without pathological confirmation of de novo pulmonary nodules; 3) three or less lesions confined to the lung; 4) no evidence of regional lymph node metastases, mediastinal spread, or systemic metastases at the time of SBRT; 5) treatment with curative intent; 6) no antitumor therapy other than SBRT until the disease progression; and 7) at least 6 months of follow-up. Patients who had other malignancies in other sites or had an Eastern Cooperative Oncology Group (ECOG) performance status of >2 were excluded.
A total of 33 patients with 36 lesions met the eligibility criteria for inclusion in this study. Medical records were reviewed to obtain each patient’s baseline characteristics and clinical and therapeutic data. During the data screening process, patients with >5% missing data were excluded. We staged every lesion for each patient independently according to the tumor, node, and metastasis system based on the American Joint Committee on Cancer (AJCC) seventh edition and retrospectively coded the lesions according to the AJCC eighth edition.14 The interval between the two lesions was defined as the time between the date of surgery for the first tumor and the date of radiological diagnosis of the de novo pulmonary lesion.17,23
This retrospective study was approved by the Zhongshan Hospital Ethics Committee. Written informed consent was obtained from each patient for the use of his or her clinical data in clinical studies.
SBRT techniques
Our institution’s SBRT treatment planning and delivery method has been previously described.24 Briefly, each patient was immobilized in the supine position with arms overhead using a customized vacuum cushion. All patients underwent respiration-correlated helical four-dimensional (4D)-computed tomography (CT) scans with a 3 mm slice thickness under free quiet respiration using a 16-slice CT scanner (Siemens Somatom CT, Sensation Open; Siemens Healthcare, Munich, Germany). A breath-hold or respiration-gated technique was considered for cases with tumor movement of >1 cm in any direction. The gross tumor volume (GTV) was defined as a lesion visible in the lung window on CT and/or PET/CT. There was no clinical target volume (CTV) construction. The internal target volume (ITV) was created based on the maximum-intensity projection image obtained in the 4D-CT. The planning target volume (PTV) was generated by adding a uniform 5 mm margin expansion to the ITV for setup uncertainty. Dose constraints for the organs at risk (OAR) were based on the Radiation Therapy Oncology Group (RTOG) 0236 guidelines.19 All SBRT treatments were administered using a Helical Tomotherapy (HT) Hi-Art Treatment System (Accuray, Madison, WI, USA). Daily image-guided RT was performed with a megavoltage computed tomography (MVCT) scan before each treatment, and automatic adjustments were made to confirm the position of the tumor throughout the course of treatment. Dose/fractionation schedules were applied depending on the tumor size, tumor location, and lung function parameters. A total dose of 50–65 Gy in five to 10 fractions was delivered. Treatments were performed over 5–14 days (median 10 days).
Follow-up evaluations
Follow-up evaluations after SBRT were conducted based on regular CT scans of the chest and clinical examinations that were obtained from the medical records. These evaluations were acquired every 3 months during the first 2 years, then every 6 months for another 3 years, and annually thereafter. An 18F-FDG PET/CT scan was performed if clinical recurrence or metastasis was suspected. The follow-up started on the first date of SBRT and ended on May 31, 2018. Clinical response to therapy was assessed 6 months after SBRT based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Locoregional recurrence (LR) after SBRT was defined as a new lesion reappearance in the radiation field, in the same lobe, or in the ipsilateral hilar/mediastinal lymph nodes. All other sites of failure were considered as distant metastasis (DM). The follow-up time was calculated from the initial date of SBRT to the date of the last follow-up visit. OS was calculated from the initial date of SBRT to the date of death from any cause. Progression-free survival (PFS) was calculated from the initial date of SBRT to the date of disease progression or the date of death. Patients were censored at the date of the last available follow-up if alive. A radiation oncologist or pulmonologist diagnosed radiation pneumonitis (RP) based on clinical symptoms and radiographic changes that occurred during the first 12 months after completion of SBRT and if there was no evidence indicating other competing diagnoses. The National Cancer Institute’s Common Toxicity Criteria (CTC) Version 3.0 was used for grading adverse events.25
Statistical analyses
Continuous variable data were summarized as medians and ranges. Categorical variable data were expressed as percentages. The median follow-up time was calculated using the reverse Kaplan–Meier method. OS and PFS were calculated using the Kaplan–Meier method. LR and DM rates were evaluated using the alternative cumulative competing risk method, with death as a competing risk. Statistical analyses were performed using SPSS statistical software (version 23.0; IBM Corporation, Armonk, NY, USA) and R statistical software (version 3.4.4) using the cmprsk and survival packages (R Foundation, Vienna, Austria).
Results
Patients’ characteristics
A total of 33 patients with 36 lesions were eligible and included in this study. There were three patients with two intrapulmonary lesions, and all received SBRT for both lesions. Only one tumor had marginal recurrences to atypical resections, whereas other tumors were de novo pulmonary tumors apart from the surgical sites. All patients completed the course of SBRT without unscheduled interruption. The median follow-up was 32 months (range, 8–84 months) for all patients and 36 months for living patients (range, 8–84 months). In 17 patients, the time between the first tumor and the second novel lesion was less than 2 years, and in the remaining 16 patients, it was longer than 2 years. At the end of the follow-up period, 15 patients developed recurrence or metastasis. Baseline demographics, clinical characteristics, and treatment characteristics are summarized in Table 1.
Local control and patterns of failure
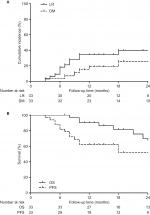
Six months after the completion of SBRT, complete response (CR) was reported for five (13.89%) lesions, partial response (PR) for 14 (38.89%) lesions, stable disease (SD) for 10 (27.78%) lesions, and progression disease (PD) for 7 (19.44%) lesions. The tumor response rate was 52.78%. By the end of the follow-up periods, five (15.15%) patients developed LR combined with DM, eight (24.24%) patients developed LR alone, and two (6.06%) patients developed DM alone. The 1- and 2-year cumulative incidences of LR were 37.62% (95% CI, 36.09%–39.15%) and 48.02% (95% CI, 46.00%–50.04%), respectively. The corresponding incidences of DM were 15.92% (95% CI, 15.06%–16.78%) and 21.23% (95% CI, 19.92%–22.54%), respectively (Figure 1A). Among patients with LR, six (18.18%) developed LR within the radiation field or the involved lobe and seven (21.21%) developed regional lymph node recurrence. Bone (two cases, 6.06%) and liver (two cases, 6.06%) were the most common sites of DM, followed by the contralateral lung (one case, 3.03%), brain (one case, 3.03%), adrenal gland (one case, 3.03%), and celiac lymph nodes (one case, 3.03%). All patients with PD received further treatment for the recurrence or metastasis.
Survival
In all, 25 patients were alive at the time of the last follow-up. Of these patients, 18 were alive with no evidence of disease. Among the eight deaths, four patients died as a result of systemic spread, two patients died of local control failure, and two patients died of other cancer-related pathology. The median PFS was 53 months (95% CI, 5.60–100.40 months) for all patients, whereas the median estimated OS was not reached. The 1-year and 2-year PFS rates for all patients were 62.40% (95% CI, 45.54%–79.26%) and 52.00% (95% CI, 32.79%–71.21%), respectively, and the 1-year and 2-year OS rates were 90.30% (95% CI, 79.90%–100.69%) and 69.90% (95% CI, 50.30%–89.50%), respectively (Figure 1B).
Toxicity evaluation
Adverse events were carefully recorded during the treatment and follow-up period (Table 2). In all, 26 patients experienced grade 1 SBRT-related toxicity, 11 patients experienced grade 2 toxicity, and three patients experienced grade 3 toxicity (Table 2). There were no grade 4/5 toxicities or SBRT-related deaths during the follow-up period.
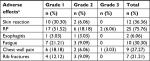  | Table 2 Adverse effects after SBRT Note: aAdverse effects were graded according to the National Cancer Institute’s CTC Version 3.0.26 Abbreviations: CTC, Common Toxicity Criteria; RP, radiation pneumonitis; SBRT, stereotactic body radiotherapy. |
Discussion
With the development of diagnostic modalities and treatment innovations, survival outcomes in patients with resected early stage NSCLC have improved. However, the risk of developing a new lesion/disease recurrence in this population is also steadily increasing.5,26,27 Although a surgical approach remains the frontline treatment for de novo tumors after lung resection, a significant proportion of cases do not qualify for additional surgery due to the impaired pulmonary reserve.8,9 The results presented here demonstrate that SBRT is a safe and potentially effective alternative therapeutic option for de novo pulmonary tumors following lung cancer surgical resection. Over the course of the study, with a median follow-up of 32 months, there were no grade 4/5 toxicities or SBRT-related deaths. The 2-year OS and PFS rates of all patients were 66.90% and 52.00%, respectively, with a tumor response rate of 52.78% at 6 months.
There are currently no systematic or authoritative guidelines for the treatment of de novo pulmonary tumors in patients with completely resected early stage first lung cancer. Zuin et al assessed clinical outcomes for different operation methods in the treatment of second primary lung cancer (SPLC) in patients who had received pulmonary resection.23 They reported that the overall 5-year survival rate following the second surgery was 42%. Additionally, Stella et al28 described the surgical management for pulmonary metastasis and SPLC after curative resection for the index lung cancer. The 2-year OS rate after the second resection of pulmonary metastasis was 29%, whereas the corresponding survival rate of SPLC was 81%, with a 2-year OS rate of ~60%. The overall 2-year survival rate of 33 patients after SBRT in our cohort was 69.90%, which appeared to be slightly higher than that of surgical resection.28 Although the subjects in our study were medically inoperable, these data suggest that clinical outcomes of SBRT for de novo pulmonary tumors after curative resection were not inferior to those of additional surgery. Previous retrospective studies of the role of SBRT in patients with early stage MPLC found 2-year OS rates of 73.2%, 56.0%, and 58.5%, which were slightly higher or comparable to that of our patients.17,22,29 However, some of the de novo tumors in our patients were pulmonary metastases from the prior lung cancer, which tend to have poorer prognosis than MPLC.30,31 Therefore, the present data and prior reports lead us to believe that SBRT can be a safe and effective alternative to further pulmonary resection for de novo pulmonary tumors after NSCLC radical resection when repeated surgery is not possible due to poor pulmonary reserve.
Of note, LR and DM rates in this current analysis were inferior to those reported previously in patients undergoing SBRT for early stage NSCLC.27,32 In the series of Horne et al,33 the 2-year LCR of SBRT for residual/recurrent and new primary NSCLC was 78.4%. The LR and DM rates that we observed here may be attributable to several factors, including poor pulmonary function capacity, stage III or IV disease de novo tumors due to pulmonary recurrences or metastases, and because almost 40% of our patients received a biologically effective dose (BED) of <100 Gy. As the radiation dose is crucial for local tumor control and a BED of >100 Gy to the target volume is needed to achieve optimal local control,19,34 we compared the survival of patients who did and did not receive ≥100 Gy. We did not detect any difference in OS (P=0.667) or PFS (P=0.603) between these two groups. The reason for this phenomenon may be attributed to the small sample size of our patients. In addition, there were six patients in the current study who developed disease progression within 6 months following SBRT. This short interval suggests that microscopic metastatic disease may have been present at the time of SBRT. All patients in our study received SBRT delivered via HT, which is associated with excellent clinical outcome and normal tissue sparing because of its sharp dose gradient. HT-based SBRT for primary lung cancer showed improved LCR for primary lung cancer. The 2-year LCR was 97% for primary lung cancer using HT, whereas that for linac-based SBRT was ~87%.35 In our previous report, 3-year LCR was >90% for stage I lung cancer via HT.25 It is essential to accurately differentiate SPLC from pulmonary metastases or recurrence before performing SBRT because the prognosis and treatment are markedly different.35 MPLC was first described by Beyreuther36 in 1924. The most recent widely used and recognized criteria for diagnosing MPLC are still those outlined by Martini and Melamed in 1975.37 These criteria have recently been revised and updated in the American College of Chest Physicians Lung Cancer Guideline. The update extended the time interval, further differentiated pathological subtypes, and added clinical assessment of molecular genetic characteristics to improve the diagnostic accuracy.38 In clinical practice, there is still ambiguity in distinguishing a new primary lesion from intrapulmonary metastasis or a satellite lesion derived from the prior index cancer for tumors with the same pathological diagnosis. In addition, with the development of newer scanning techniques, such as thin-section CT, and treatment for early stage NSCLC, the detection of small-sized lung cancer in patients who have undergone surgical resection for early stage lung cancer has increased. However, a substantial proportion of these lesions are not suitable for biopsy confirmation due to associated medical issues, perceived risk of potential complications, or the relatively too small lesion to obtain pathologic specimen. Therefore, establishing a diagnosis of MPLC based on a comprehensive clinical and radiological assessment would be particularly important. Matsunaga et al39 proposed a set of new simple radiological criteria for MPLC based on prognosis. They suggested that a tumor with a ground-glass opacity and clinical N0 should not be diagnosed as MPLC because its prognosis is satisfactory. Ono et al distinguished MPLC from intrapulmonary by analyzing differential protein expression profiles.31 Further research on accurate selection of radiological criteria for MPLC is necessary to provide clinical guidelines in early detection of multiple pulmonary lesions, especially metachronous lesions.
Despite limited pulmonary reserve after previous pulmonary resection of the patients in this study, SBRT was well tolerated with an acceptable toxicity profile (Table 2). Results from the RTOG 0236 trail analyzing patients treated with SBRT indicate that poor baseline pulmonary function test does not correlate with pulmonary toxicity and OS following SBRT in medically inoperable, early stage NSCLC.19 No significant changes in pulmonary function test were observed in the RTOG 0236 trial; therefore, a poor baseline pulmonary function test alone should not be used to exclude patients with early stage lung cancer from treatment with SBRT, a conclusion supported by other reports.40,41
RP was the most common SBRT-related toxicity in our study. Three of the 33 patients in our study (9.67%) developed grade 3 SBRT-related toxicity, which was at a lower rate than the 12.7% reported in RTOG 0236.19 During the follow-up period of our study, there were no grade 4/5 toxicities or SBRT-related deaths. The rate and severity of other toxicities were considered acceptable and comparable to those previously published for SABR for early stage NSCLC.12,18,23,27
Our study did have several limitations. First, because of the retrospective nature and relatively small sample size, we were unable to draw conclusions regarding risk factors for any form of treatment failure. Second, several patients did not have pathological confirmation of de novo pulmonary nodules due to the difficulty and perceived risk in obtaining a pathologic specimen from a small lesion. Third, we could not distinguish between an SPLC and metastasis because of the limited patient data. Fourth, there were 13 patients who received a BED of <100 Gy, 10 patients who received 96 Gy (~100 Gy), and three patients who received 75 Gy in our study. The patients in our study were old (median age: 68 years) and had undergone pulmonary resection, which diminished their pulmonary reserve and physical conditions and limited their ability to tolerate high doses (>100 Gy) of BED. However, all patients in our clinical study received SBRT via HT, which is associated with excellent clinical outcomes. Each tumor was sliced based on HT and received a high dose within few minutes, which is in contrast to the CyberKnife or intensity-modulated radiotherapy with linear accelerator, which takes dozens of minutes. The radiobiology may be different among the RT equipment, but the results warrant further study. In our prior clinical experience, the 3-year LCR could reach 90% for stage I lung cancer with BED=96 Gy via HT.24 Additional studies are needed to validate our conclusions.
Conclusion
We reported on a series of de novo tumors after curative resection treated with SBRT in our institution. Our data demonstrate that SBRT is a reasonable and potentially effective alternative therapeutic option for de novo tumors in patients with previous lung resection and has clinically acceptable treatment toxicity. Additional large, randomized prospective trials are needed to confirm our results and help refine treatment recommendations for de novo tumors in patients with completely resected early stage initial NSCLC.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Yang HX. Long-term survival of early-stage non-small cell lung cancer patients who underwent robotic procedure: a propensity score-matched study. Chin J Cancer. 2016;35(1):66. | ||
Rea F, Zuin A, Callegaro D, Bortolotti L, Guanella G, Sartori F. Surgical results for multiple primary lung cancers. Eur J Cardiothorac Surg. 2001;20(3):489–495. | ||
Rahn RD 3rd, Thakur S, Makani S, Sandhu A. Stereotactic body radiation therapy (SBRT) for multiple primary lung cancers (MPLC): a review and case series. J Radiosurg SBRT. 2013;2(2):135–140. | ||
Yu YC, Hsu PK, Yeh YC, et al. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg. 2013;96(6):1966–1974. | ||
Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol. 2013;22(3):156–161. | ||
Subotic D, Van Schil P, Grigoriu B. Optimising treatment for post-operative lung cancer recurrence. Eur Respir J. 2016;47(2):374–378. | ||
Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer. 2011;73(2):237–242. | ||
Usuda J, Ichinose S, Ishizumi T, et al. Management of multiple primary lung cancer in patients with centrally located early cancer lesions. J Thorac Oncol. 2010;5(1):62–68. | ||
van Bodegom PC, Wagenaar SS, Corrin B, Baak JP, Berkel J, Vanderschueren RG. Second primary lung cancer: importance of long term follow up. Thorax. 1989;44(10):788–793. | ||
Rosengart TK, Martini N, Ghosn P, Burt M. Multiple primary lung carcinomas: prognosis and treatment. Ann Thorac Surg. 1991;52(4):773–778. | ||
Tanvetyanon T, Robinson L, Sommers KE, et al. Relationship between tumor size and survival among patients with resection of multiple synchronous lung cancers. J Thorac Oncol. 2010;5(7):1018–1024. | ||
Okada M, Tsubota N, Yoshimura M, Miyamoto Y. Operative approach for multiple primary lung carcinomas. J Thorac Cardiovasc Surg. 1998;115(4):836–840. | ||
Cai XW, Xu LY, Wang L, et al. Comparative survival in patients with postresection recurrent versus newly diagnosed non-small-cell lung cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(4):1100–1105. | ||
Yano T, Okamoto T, Fukuyama S, Maehara Y. Therapeutic strategy for postoperative recurrence in patients with non-small cell lung cancer. World J Clin Oncol. 2014;5(5):1048–1054. | ||
Rahn DA 3rd, Thakur S, Makani S, Sandhu A. Stereotactic body radiation therapy (SBRT) for multiple primary lung cancers (MPLC): a review and case series. J Radiosurg SBRT. 2013;2(2):135–140. | ||
Chang JY, Liu YH, Zhu Z, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer. 2013;119(18):3402–3410. | ||
Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. | ||
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. | ||
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–5159. | ||
Jeppesen SS, Schytte T, Jensen HR, Brink C, Hansen O. Stereotactic body radiation therapy versus conventional radiation therapy in patients with early stage non-small cell lung cancer: an updated retrospective study on local failure and survival rates. Acta Oncol. 2013;52(7):1552–1558. | ||
Griffioen GH, Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Treatment of multiple primary lung cancers using stereotactic radiotherapy, either with or without surgery. Radiother Oncol. 2013;107(3):403–408. | ||
Zuin A, Andriolo LG, Marulli G, et al. Is lobectomy really more effective than sublobar resection in the surgical treatment of second primary lung cancer? Eur J Cardiothorac Surg. 2013;44(2):e120–e125. | ||
He J, Huang Y, Shi S, Hu Y, Zeng Z. Comparison of effects between central and peripheral stage I lung cancer using image-guided stereotactic body radiotherapy via helical tomotherapy. Technol Cancer Res Treat. 2015;14(6):701–707. | ||
Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. | ||
Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014;90(3):603–611. | ||
Sun B, Brooks ED, Komaki RU, et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: Results of a phase 2 clinical trial. Cancer. 2017;123(16):3031–3039. | ||
Stella F, Luciano G, Dell’Amore A, et al. Pulmonary Metastases from NSCLC and MPLC (Multiple Primary Lung Cancers): Management and Outcome in a Single Centre Experience. Heart Lung Circ. 2016;25(2):191–195. | ||
Creach KM, Bradley JD, Mahasittiwat P, Robinson CG. Stereotactic body radiation therapy in the treatment of multiple primary lung cancers. Radiother Oncol. 2012;104(1):19–22. | ||
Arai J, Tsuchiya T, Oikawa M, et al. Clinical and molecular analysis of synchronous double lung cancers. Lung Cancer. 2012;77(2):281–287. | ||
Ono K, Sugio K, Uramoto H, et al. Discrimination of multiple primary lung cancers from intrapulmonary metastasis based on the expression of four cancer-related proteins. Cancer. 2009;115(15):3489–3500. | ||
Verstegen NE, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol. 2011;101(2):250–254. | ||
Horne ZD, Dohopolski MJ, Clump DA, Burton SA, Heron DE. Thoracic reirradiation with SBRT for residual/recurrent and new primary NSCLC within or immediately adjacent to a prior high-dose radiation field. Pract Radiat Oncol. 2018;8(3):e117–e123. | ||
Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7(5):295–301. | ||
Matthiesen C, Thompson JS, De La Fuente Herman T, Ahmad S, Herman T. Use of stereotactic body radiation therapy for medically inoperable multiple primary lung cancer. J Med Imaging Radiat Oncol. 2012;56(5):561–566. | ||
Beyreuther H. Multiplicität von Carcinomen bei einem Fall von sog. “Schneeberger” [Multiplicity of carcinomas in a case of so-called “Schneeberger” lung cancer with tuberculosis]. Lungenkrebs mit Tuberkulose. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 1924;250(1–2):230–243. German. | ||
Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg. 1975;70(4):606–612. | ||
Shen KR, Meyers BF, Larner JM, Jones DR; American College of Chest Physicians. Special treatment issues in lung cancer: ACCP Evidence-based clinical practice guidelines (2nd Edition). Chest. 2007;132(3 Suppl):290S–305S. | ||
Matsunaga T, Suzuki K, Takamochi K, Oh S. New simple radiological criteria proposed for multiple primary lung cancers. Jpn J Clin Oncol. 2017;47(11):1073–1077. | ||
Klement RJ, Belderbos J, Grills I, et al. Prediction of early death in patients with early-stage NSCLC-Can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11(7):1132–1139. | ||
Guckenberger M, Allgäuer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol. 2013;8(8):1050–1058. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.