Back to Journals » Journal of Pain Research » Volume 14
Clinical Outcomes of Fracture Haemorrhage Aspiration for Percutaneous Vertebroplasty in Treating Osteoporotic Vertebral Compression Fractures
Authors Peng J, Qin J , Huang T, Luo X, Zhong W, Quan Z
Received 22 October 2021
Accepted for publication 21 December 2021
Published 31 December 2021 Volume 2021:14 Pages 3951—3959
DOI https://doi.org/10.2147/JPR.S345760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Junmu Peng,1,2,* Jie Qin,1,* Tianji Huang,1 Xiaoji Luo,1 Weiyang Zhong,1 Zhengxue Quan1
1Department of Orthopedic Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, 400016, People’s Republic of China; 2Department of Orthopedic Surgery, The Ninth People’s Hospital of Chongqing, Chongqing, 400799, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiyang Zhong Email [email protected]
Background: A retrospective study aimed to introduce a new method for improving the diffusion degree of bone cement and to observe its clinical efficacy in percutaneous vertebroplasty treating osteoporotic vertebral compression fractures (OVCFs).
Methods: From January 2019 to March 2020, a total of 83 patients were enrolled and reviewed. The patients were divided into two groups according to the operation method. The clinical and radiographic parameters were recorded and compared between these two groups. Those who received percutaneous vertebroplasty with haemorrhage aspiration were recorded as group A (n=42). In group A, the haemorrhage in the vertebral fracture was aspirated compared with conventional percutaneous vertebroplasty. Patients who underwent conventional percutaneous vertebroplasty were classified as group B (n=41).
Results: Visual analogue scale (VAS) values and Oswestry Disability Index (ODI) scores showed no significant difference between the two groups preoperatively, postoperatively or at the final follow-up (FU) (P> 0.05). The intraoperative VAS score (bone cement injection) in group A was significantly lower than that in group B (3.83± 0.79 vs 5.44± 1.32, P < 0.01). The local kyphotic angle (LKA) (final follow-up), LKA loss, fractured vertebral anterior height loss (FVAHL) and anterior vertebral height loss ratio (AVHLR) were significantly lower in group A than in group B. The anterior vertebral height ratio (AVHR) at the final FU in group A was higher than that in group B (P=0.013). The distribution of bone cement was significantly different (P=0.034). By analysing the distribution pattern of bone cement, it was found that the values of LKA loss, FVAHL and AVHLR were superior in the type A bone cement distribution to those in types B and C.
Conclusion: Compared with traditional surgical methods, bone haemorrhage aspiration could improve the diffusion degree of bone cement and reduce the height loss and deformity of injured vertebrae. This method provides a feasible new scheme for improving the dispersion of bone cement.
Keywords: osteoporotic vertebral compression fractures, percutaneous vertebroplasty, bone cement distribution, fracture haemorrhage aspiration
Background
Osteoporotic vertebral compression fractures (OVCFs) are the most common fractures in senior citizens, and the incidence of OVCFs increases with age.1–4 OVCFs cause back pain, neurological symptoms, functional limitations, malformations and a reduction in quality of life. Percutaneous vertebroplasty (PV) is a commonly used surgical treatment for patients with OVCFs. This treatment can effectively strengthen the compressed vertebral body, relieve the pain caused by thoracolumbar fractures, improve the quality of life of patients, and avoid the complications caused by long-term bedrest.5–7 Recent studies have found that the distribution of bone cement may affect vertebral body height restoration and local kyphosis, and a better dispersion distribution may indicate better clinical recovery.1,3,8,9 However, the existing articles do not reveal the factors affecting bone cement distribution patterns of the vertebral body, and we believe that bleeding inside the fracture occupies part of the space in the vertebral body. Therefore, during PV surgical puncture, the fracture haemorrhage was aspirated and then filled with bone cement to improve the distribution pattern of bone cement. Additionally, this method was compared with traditional surgical methods.
Methods
Patient Selection
The study was approved by the Institutional Review Board of The First Affiliated Hospital of Chongqing Medical University and was conducted according to the principles of the Declaration of Helsinki. All the patients provided their written informed consent to participate in our study prior to the storage of their data in the hospital database. Patients who received PV treatment from January 2019 to March 2020 were reviewed. The inclusion criteria were as follows: the average bone mineral density (BMD) (T score<-2.5) preoperatively, single thoracic or lumbar vertebral fractures, without history of PK or neurological symptoms, failed to conservative treatment (bed rest, anti-osteoporosis treatments, non-steroidal anti-inflammatory drugs etc), the bone cement was injected through the pedicles bilaterally, the follow-up (FU) time was at least than 1 year, no history of trauma during the FU. The exclusion criteria were as follows: pathological vertebral lesions such as vertebral metastatic carcinoma etc, fractures of adjacent vertebral bodies, patients who were lost FU. 83 consecutive patients who underwent PV treatment for OVCFs were investigated and were randomly assigned to choose one of the two treatments. Those who received PV with haemorrhage aspiration were recorded as group A (n=42). Patients who underwent conventional PV were classified as group B (n=41). All patients were admitted 1 day before surgery for preoperative preparation and management. During this time, All patients were treated with non-steroidal anti-inflammatory drugs (NSAIDs) for symptomatic relief and calcium (1000 to 1500 mg per day) and vitamin D (400 to 1200 IU per day) supplement.
Surgical Technique
All operations were performed by the same experienced spine surgeon. The procedure was performed with the patient under local anaesthesia with adequate preoperative analgesia. A C-arm X-ray was used to capture standard anteroposterior and lateral images for the operative vertebral bodies, and safety puncture techniques for the vertebral body (baseline positioning, puncture point positioning, puncture direction adjustment, step-by-step insertion) were used. The bilateral pedicle puncture approach was used to reach the front of the posterior margin of the vertebral bodies for approximately 3 mm guide wires, expansion cannulae, and working cannulae were then sequentially utilized. Two working 10mL empty needles are connected to the working cannula, one of the empty needles is used for blood aspiration, and the other is kept under pressure without blood aspiration. Approximately 3–5 mL of haemorrhage in the fractured vertebra were aspirated from one pedicle with a needle in the experimental group but not in the control group (Figure 1). The prepared bone cement was slowly injected into the vertebral body through the working cannulas, accompanied by perspective monitoring until the vertebral body was filled with bone cement. The cannula was then pulled out. The patients were allowed to walk 4 hours postoperatively. Standard anti-osteoporosis treatment was performed after the operation. X-ray films were rechecked within 1 day after the operation to assess the filling of bone cement, and X-ray films were rechecked 1 month, 6 months and 12 months after the operation.
Assessed Parameters
The study indicators were divided into three parts: treatment information, curative effect assessment and radiologic assessment. Treatment information: hospitalization duration, intraoperative blood loss, operation time, and bone cement volume. Preoperative and postoperative treatment information of the patients was recorded. Clinical outcomes were assessed using the Oswestry disability index (ODI) and visual analogue scale (VAS). ODI scores were assessed before and after surgery as well as at the final follow-up, and VAS scores were assessed before the operation, during the operation (after cementing), after the operation and at the final FU. Radiologic assessments: fractured vertebral anterior height (FVAH); and anterior vertebral height ratio (AVHR)= FVAH/(sum of vertebral anterior height of adjacent vertebral body) ×2. The anterior vertebral height recovery ratio (AVHRR) was defined as postoperative AVHR - preoperative AVHR. The anterior vertebral height loss ratio (AVHLR) was defined as postoperative AVHR - final FU AVHR. Local kyphotic angle (LKA)/region kyphotic angle (RKA) recovery=preoperative LKA/RKA- postoperative LKA/RKA, LKA/RKA loss=the final FU LKA/RKA- postoperative LKA/RKA. Fractured vertebral anterior height recovery (FVAHR)= postoperative FVAH - preoperative FVAH. Fractured vertebral anterior height loss (FVAHL)=postoperative FVAH-final FU FVAH. The distribution pattern of bone cement was evaluated as follows. The upper and lower endplates of the injured vertebrae were used as a reference to evaluate the longitudinal distribution of bone cement, and the midline of the coronal position of the radiologic film was used as a reference to evaluate the horizontal distribution of bone cement. A: Bone cement is connected to the upper and lower endplates on both sides of the midline. B: Bone cement is connected to the upper and lower endplates on one side of the midline. C: Bone cement is not connected to the upper or lower endplates on either side of the midline (Figure 2).
The assessment of studies was performed by 2 independent assessors, with any disagreement between assessors was resolved through discussion.
Statistical Analysis
SPSS 23.0 software was used to analyse the data. Continuous variables are expressed as the mean ± standard deviation, and independent sample t-tests or variance analyses (ANOVAs) were used. The chi-square test was adapted to analyse the categorical variables. Significant differences were defined as p < 0.05.
Results
A total of 83 patient medical records were included in this study; there were 42 patients in experimental group A and 41 patients in control group B. There were no significant differences in age, sex, BMI or follow-up time between the two groups (P>0.05) (Table 1). There was no significant difference in the hospitalization duration, intraoperative blood loss (do not contain blood loss in aspiration) or operation time between the two groups (P > 0.05). The bone cement dosage in group B was 5.98±1.45 mL, which was significantly lower than that in group A (4.80±1.14mL, P<0.01).
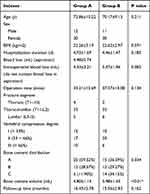 |
Table 1 Basic Information of the Patients |
The VAS and ODI scores showed no significant difference between the two groups preoperatively, postoperatively or at the final FU (P>0.05). The intraoperative VAS score (bone cement injection) in group A was significantly lower than that in group B (3.83±0.79 vs 5.44±1.32, P<0.01). The LKA (final follow-up), LKA loss, RKA loss, FVAHL and AVHLR were significantly lower in group A than in group B (11.53±6.23 vs 14.95±7.12, p=0.022; 1.66±2.97 vs 3.32±3.07, p=0.014; 0.95±3.40 vs 2.62±4.14, p=0.048; 0.94±1.74 vs 2.69±2.45, p<0.01; 2.86±6.58 vs 10.00±9.48, p<0.01). The AVHR (final follow-up) in group A was higher than that in group B (79.38±17.38 vs 70.83±12.74, P=0.013) (Table 2). The distribution of bone cement was significantly different (P=0.034). By analysing the distribution pattern of bone cement, it was found that the LKA loss, FVAHL and AVHLR were superior in the type A bone cement distribution to those in types B and C (Table 3).
 |
Table 2 Analysis of Outcomes Between Different Groups |
 |
Table 3 Analysis of Bone Cement Distribution Group |
Case Presentation
Case 1 A 63-year-old female underwent lumbar fracture (L1) percutaneous vertebroplasty of bone cement distribution type: A, with no significant recompression of the fractured vertebral body at the last follow-up. (FVAH (pre-op): 21.37mm; FVAH (24h post-op): 21.95mm; FVAH (final FU): 21.57mm) (Figure 3)
Case 2 A 64-year-old female underwent thoracic vertebra fracture (T12) percutaneous vertebroplasty of bone cement distribution type: B, with slight recompression of the fractured vertebral body at the last follow-up. (FVAH (pre-op): 16.09mm; FVAH (24h post-op): 18.69mm; FVAH (final FU): 17.01mm) (Figure 4)
Case 3 A 68-year-old female underwent lumbar fracture (L1) percutaneous vertebroplasty of bone cement distribution type: C, with obvious recompression of the fractured vertebral body at the last follow-up. (FVAH (pre-op): 16.04mm; FVAH (24h post-op): 19.34mm; FVAH (final FU): 16.22mm) (Figure 5)
Discussion
OVCFs are a major public health problem that has afflicted most elderly people. Bone cement strengthens the compressed vertebral body to treat OVCFs, and the prognosis of PVP is affected by BMD, bone cement distribution, etc.1,2,9,10 It was reported that the patients with thoracic and lumbar compressed fractures according to the type of diffusion of bone cement and found that the vertebral body with poor diffusion of bone cement may have unstable spines, leading to postoperative re-compression of the fractured vertebral body and aggravating clinical symptoms.11 Previous studies have pointed out that preoperative severe kyphotic deformities, solid lump cement distribution patterns, and larger reduction angles are risk factors for the development of recollapse; among these risk factors, the solid lump cement distribution pattern is the most significant. Due to the lack of or rare occurrence of contiguous interdigitation with surrounding cancellous bones, the stress may concentrate on the surrounding fragile cancellous bone, resulting in recollapse.12 Once the bone cement diffuses to the surrounding cancellous bone, the stress load can be transmitted from the upper endplate to the lower endplate via the bone cement. However, the weaker cancellous bone was not loaded in series as it was if the bone cement mass was not dispersive but consisted of 1 or 2 solid masses, resulting in greater height loss and kyphotic deformity.13,14
The distribution pattern of bone cement has been studied previously. It was conducted grouping studies on the distribution of bone cement according to whether the bone cement touched the upper and lower endplates and found that when the bone cement touched the upper and lower endplates, the vertebral strength could better recover to maintain the height of the vertebral body and reduce the risk of vertebral recompression.10,11 It has been reported that when there is bone cement distribution around the upper and lower endplates, the rate of vertebral recompression may be lower than that of endplates without bone cement distribution.15 In a finite element analysis of bone cement distribution, it was found that if only one side of the endplate was surrounded by bone cement, the vertebral stiffness only increased 2 fold, with almost no change in strength. However, when bone cement contacted the upper and lower endplates, the vertebral stiffness and strength could increase by a maximum of 8 fold and 11 fold, respectively.16,17
Although previous studies have shown that simultaneous contact of bone cement with upper and lower endplates reduces the compression of fractured vertebrae and is beneficial for improving prognosis,17 obtaining a better distribution of bone cement when PV is performed remains a problem for surgeons. There are still few studies concerning how to improve the distribution mode of bone cement. Some scholars believe that increasing the amount of bone cement injected may improve the distribution of bone cement, while others point out that increasing the amount of bone cement injected may increase the risk of bone cement leakage. Many studies proposed that if the intraoperative diffusion of bone cement on one side of the endplate was not satisfactory, the puncture position could be adjusted for secondary injection to improve the diffusion of bone cement.8–11 In a cadaver study, researchers administered lumbar lavage to reduce fat and bone marrow in the vertebrae for decompression within the vertebrae to reduce cement leakage and improve cement distribution.11–18 Obviously, this technique of removing fat and bone marrow from part of the vertebral body is not suitable in the clinic, but reducing the internal pressure of the vertebral body provides guidance. Based on this, we proposed a novel ideal to improve the distribution of bone cement by reducing internal haemorrhage in the fractured vertebral body, which removes the barriers of diffusion. In this study, the distribution of bone cement was significantly different (P =0.034). And the distribution rate of type A as well as the FVAHL, AVHR and AVHLR in the experimental group were all superior to those in the control group (P<0.05). After analysis of the distribution mode of bone cement, it was found that once the bone cement contacted the upper and lower endplates, its LKA loss, RKA loss, FVAHL and AVHLR were significantly better than those of other distribution modes (P<0.05), which supports the results of previous studies.
Some scholars have found that the distribution pattern of bone cement has no significant effect on the short-term relief of clinical symptoms in patients. We believed that this is because the thermal effect of bone cement leads to the degeneration and necrosis of teleneurons in the vertebral body,17–19 while vertebral body recompression has not yet occurred, so the clinical symptoms of patients are well relieved in a short postoperative period. Patients with better cement distribution were found to obtain significant clinical relief during long-term FU. In this study, there was no significant difference in VAS or ODI scores between the experimental group and the control group preoperatively, postoperatively or at the final follow-up. However, a short follow-up may not powerfully reflect the long-term improvement of prognosis. Besides, the intraoperative VAS score in the experimental group was significantly lower than that in the control group. One reason to explain this phenomenon is that the internal pressure of the vertebral body was reduced when blood haemorrhage was aspirated from the fractured vertebral body when bone cement perfusion occurred. Therefore, the VAS score in the experimental group was better than that in the control group intraoperatively. The VAS (last follow-up) and ODI (last FU) scores of the type A distribution mode were better than those of the other distribution modes.
The bone cement dosage affects prognosis. Increasing the bone cement perfusion measure optimizes injured vertebral height restoration and improves the degree of bone cement dispersion, but more leakage may occur. In addition, the injection amount of bone cement was not associated with pain relief, so increasing the amount of bone cement to improve the diffusion of bone cement is not an ideal method.19–21 Based on biomechanical research in the laboratory, it was found that the stiffness and strength of vertebrae recovered to 70% and 64%, respectively, when the perfusion amount of bone cement reached 2 mL, and they recovered to 94% and 100%, respectively, when the perfusion amount reached 6 mL.22,23 Other studies have shown that large bone cement dosages do not show greater benefits and lead to asymmetrical cement distribution and excessive vertebral stiffness.6,8,20,21 In some cases, even if the injection volume of bone cement was increased, bone cement was an uncertainly connected endplate, indicating that the amount of bone cement is not the decisive factor in improving the distribution pattern.23,24 Based on these findings, we explored a new approach. In our study, the experimental group obtained better diffusion of bone cement. It is believed that the diffusion of bone cement could be improved by reducing blood accumulation in fractured vertebrae without increasing the amount of bone cement and that the bone cement leakage caused by excessive amounts of bone cement could be avoided. Albers et al25 reported that the vertebral body lavage reduces hemodynamic response to vertebral body augmentation with PMMA, most likely resulting from decreased amounts of bone marrow substance displaced into the circulation thereby decreasing the risk of pulmonary fat embolism syndrome. However, our team tried the technique of vertebral body lavage but we are still improving related tools to achieve a higher success rate.
Conclusions
Compared with traditional surgical methods, bone haemorrhage aspiration can improve the diffusion degree of bone cement and reduce the height loss and deformity of injured vertebrae. This method provides a feasible new scheme for improving the dispersion of bone cement. This study is a single-centre retrospective study with a short follow-up time. We will continue to follow these patients, and a large sample multicentre study will solve this problem.
Abbreviations
OVCFs, osteoporotic vertebral compression fractures; PV, percutaneous vertebroplasty; VAS, visual analogue scale; ODI, Oswestry Disability Index; PK, percutaneous kyphoplasty; PMMA, polymethylmethacrylate; BMD, bone mineral density; BMI, body mass index; MRI, magnetic resonance imaging; FU, follow-up; FVAH, fractured vertebral anterior height; AVHR, anterior vertebral height ratio; AVHRR, anterior vertebral height recovery ratio; AVHLR, anterior vertebral height loss ratio; LKA, local kyphotic angle; RKA, region kyphotic angle; FVAHR, fractured vertebral anterior height recovery; FVAHL, fractured vertebral anterior height loss.
Data Sharing Statement
The datasets generated and/or analysed during the current study are not publicly available due to the data is confidential patient data but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University approved this study and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before their data were stored in our hospital database and used for study purposes.
Author Contributions
WYZ designed the study. JMP collected the data. TJH, JQ, XJL and ZXQ performed the statistical analysis and drafted and revised the manuscript. JMP wrote the manuscript and WYZ revised the manuscript. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the hospital training funding(PYJJ2019-08) and the study was supported by the medical programme of Chongqing health and science, technology commission (2021MSXM285). The funding body has not been involved in the design, data collection, analysis, interpretation or the writing of the manuscript.
Disclosure
The authors declare that they have no competing interests.
References
1. Lin J, Qian L, Jiang C, et al. Bone cement distribution is a potential predictor to the reconstructive effects of unilateral percutaneous kyphoplasty in OVCFs: a retrospective study. J Orthop Surg Res. 2018;13(1):140. doi:10.1186/s13018-018-0839-5
2. Martikos K, Greggi T, Faldini C, et al. Osteoporotic thoracolumbar compression fractures: long-term retrospective comparison between vertebroplasty and conservative treatment. Eur Spine J. 2018;27(Suppl 2):244–247. doi:10.1007/s00586-018-5605-1
3. Chen Q, Liu L, Liang G. Distribution characteristics of bone cement used for unilateral puncture percutaneous vertebroplasty in multiple planes. Orthopade. 2018;47(7):585–589. doi:10.1007/s00132-018-3527-6
4. Inose H, Kato T, Ichimura S, et al. Risk factors of nonunion after acute osteoporotic vertebral fractures: a prospective multicenter cohort study. Spine. 2020;45(13):895–902. doi:10.1097/BRS.0000000000003413
5. Rustagi T, Bourekas E, Mendel E. Floating vertebral body cement ball after high-viscosity-cement vertebroplasty for lytic defect: report of 2 cases. Int J Spine Surg. 2020;14(4):594–598. doi:10.14444/7079
6. Wang D, Li Y, Yin H, et al. Three-dimensional finite element analysis of optimal distribution model of vertebroplasty. Ann Palliat Med. 2020;9(3):1062–1072. doi:10.21037/apm-20-955
7. Garnon J, Doré B, Auloge P, et al. Efficacy of the vertebral body stenting system for the restoration of vertebral height in acute traumatic compression fractures in a non-osteoporotic population. Cardiovasc Intervent Radiol. 2019;42(11):1579–1587. doi:10.1007/s00270-019-02265-y
8. Goldstein CL, Chutkan NB, Choma TJ, Orr RD. Management of the elderly with vertebral compression fractures. Neurosurgery. 2015;77(Suppl 4):S33–S45. doi:10.1227/NEU.0000000000000947
9. McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician. 2016;94(1):44–50.
10. Liang X, Zhong W, Luo X, Quan Z. Risk factors of adjacent segmental fractures when percutaneous vertebroplasty is performed for the treatment of osteoporotic thoracolumbar fractures. Sci Rep. 2020;10(1):399. doi:10.1038/s41598-019-57355-1
11. Zhong W, Liang X, Luo X, Quan Z. Vertebroplasty and vertebroplasty in combination with intermediate bilateral pedicle screw fixation for OF4 in osteoporotic vertebral compression fractures: a retrospective single-Centre cohort study. BMC Surg. 2019;19(1):178. doi:10.1186/s12893-019-0646-x
12. Park JS, Park YS. Survival analysis and risk factors of new vertebral fracture after vertebroplasty for osteoporotic vertebral compression fracture. Spine J. 2021;21:1355–1361. doi:10.1016/j.spinee.2021.04.022
13. Tan L, Wen B, Guo Z, et al. The effect of bone cement distribution on the outcome of percutaneous Vertebroplasty: a case cohort study. BMC Musculoskelet Disord. 2020;21(1):541. doi:10.1186/s12891-020-03568-9
14. Inose H, Kato T, Ichimura S, et al. Predictors of residual low back pain after acute osteoporotic compression fracture. J Orthop Sci. 2021;26(3):453–458. doi:10.1016/j.jos.2020.04.015
15. Yu W, Xiao X, Zhang J, et al. Cement distribution patterns in osteoporotic vertebral compression fractures with intravertebral cleft: effect on therapeutic efficacy. World Neurosurg. 2019;123:e408–e415. doi:10.1016/j.wneu.2018.11.181
16. Kim MJ, Lindsey DP, Hannibal M, et al. Vertebroplasty versus kyphoplasty: biomechanical behavior under repetitive loading conditions. Spine. 2006;31(18):2079–2084. doi:10.1097/01.brs.0000231714.15876.76
17. Kweh BTS, Lee HQ, Tan T, et al. The role of spinal orthoses in osteoporotic vertebral fractures of the elderly population (age 60 years or older): systematic review. Global Spine J. 2021;11(6):975–987. doi:10.1177/2192568220948036
18. Chevalier Y, Pahr D, Charlebois M, et al. Cement distribution, volume, and compliance in vertebroplasty: some answers from an anatomy-based nonlinear finite element study. Spine. 2008;33(16):1722–1730. doi:10.1097/BRS.0b013e31817c750b
19. Tokeshi S, Eguchi Y, Suzuki M, et al. Relationship between skeletal muscle mass, bone mineral density, and trabecular bone score in osteoporotic vertebral compression fractures. Asian Spine J. 2021;15(3):365–372. doi:10.31616/asj.2020.0045
20. Chen XS, Jiang JM, Sun PD, et al. How the clinical dosage of bone cement biomechanically affects adjacent vertebrae. J Orthop Surg Res. 2020;15(1):370. doi:10.1186/s13018-020-01906-0
21. Molloy S, Mathis JM, Belkoff SM. The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine. 2003;28(14):1549–1554. doi:10.1097/01.BRS.0000076831.38265.8D
22. Prost S, Pesenti S, Fuentes S, et al. Treatment of osteoporotic vertebral fractures. Orthop Traumatol Surg Res. 2021;107(1S):102779. doi:10.1016/j.otsr.2020.102779
23. Ren HL, Jiang JM, Chen JT, et al. Risk factors of new symptomatic vertebral compression fractures in osteoporotic patients undergone percutaneous vertebroplasty. Eur Spine J. 2015;24(4):750–758. doi:10.1007/s00586-015-3786-4
24. Cazzato RL, Bellone T, Scardapane M, et al. Vertebral augmentation reduces the 12-month mortality and morbidity in patients with osteoporotic vertebral compression fractures. Eur Radiol. 2021;31:8246–8255. doi:10.1007/s00330-021-07985-9
25. Albers CE, Schott PM, Ahmad SS, et al. Vertebral body lavage reduces hemodynamic response to vertebral body augmentation with PMMA. Global Spine J. 2019;9(5):499–504. doi:10.1177/2192568218803106
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





