Back to Journals » Journal of Pain Research » Volume 15
Clinical Outcomes of Amniotic Membrane/Umbilical Cord Particulate in Spinal Disorders: A Retrospective Study
Authors Ross A, Gambrill V, Main C
Received 18 May 2022
Accepted for publication 21 November 2022
Published 16 December 2022 Volume 2022:15 Pages 3971—3979
DOI https://doi.org/10.2147/JPR.S375201
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sudhir Diwan
Amanda Ross, Vikki Gambrill, Chris Main
Midwest Bone & Joint Center, Macon, MO, USA
Correspondence: Chris Main, Midwest Bone & Joint Center, Macon, MO, USA, Email [email protected]
Background: Musculoskeletal spinal disorders significantly impact patient populations from everyday workers to military soldiers. Effective treatment is critical to minimize the time between injury and returning to work and daily activities. Injection of amniotic membrane/umbilical cord (AMUC) tissue has demonstrated great potential in reducing patients’ pain and has become an increasingly popular treatment option for painful orthopedic disorders.
Methods: A single-center, retrospective study was conducted on patients diagnosed with musculoskeletal spinal disorders and subsequently treated with AMUC via epidural and facet injections. Demographics and outcomes related to pain were assessed. Pain was verbally reported by the patient on a scale of 0– 10 where 0 indicated no pain and 10 indicated worst imaginable pain. Complications and adverse events were also reported.
Results: A total of 52 patients (average age 40.8 ± 9.6 years) were included in the analysis with diagnoses of spondylosis (n = 44), intervertebral disc degeneration (n = 31), radiculopathy (n = 18), stenosis (n = 2), or other conditions. The cohort’s average baseline pain score was 4.9 ± 2.2 with a mean duration of symptoms for 54.2 months (range: 1– 300 months). After AMUC injection, pain significantly decreased to 3.4 ± 2.3 at two weeks (p < 0.0001) and 3.5 ± 2.2 at 3– 4 weeks (p = 0.0023). For the mean follow-up period of 10.6 ± 5.4 weeks, pain was reduced to 2.8 ± 2.1 (p < 0.0001 vs baseline). No significant complications or adverse events were reported.
Conclusion: Use of an injectable AMUC, such as CLARIX FLO, may alleviate pain in patients with painful spinal indications of various pathologies. This study provides further evidence of its safety and efficacy in epidural and facet injections. Further studies are warranted to verify these promising results.
Keywords: spinal disorders, chronic pain, treatment, amniotic membrane/umbilical cord, regenerative medicine, injection
Introduction
Musculoskeletal disorders are the second leading cause of disability worldwide, and they have a substantial impact on physical activity level, depression, cognitive impairment, and sleep quality.1 Back pain is one of the most common musculoskeletal complaints and is the leading cause of disability in the developed world with an incidence of 245.9 million cases/year worldwide and a lifetime prevalence of 75–84%.2,3 Approximately 8% (65 million) of all US adults experience chronic back pain, which is defined as pain that persists for ≥3 months.4,5 Back pain can occur due to many issues, but the most common pain generators in the lumbar spine are the facet joints, representing 15–45% of the patients.6 Facet joint syndrome or spondylosis is generally caused by natural weathering or abnormal body mechanics from repetitive stress and/or cumulative low-level trauma which cause spinal joint degeneration over time due to the release of inflammatory mediators.6,7
First-line therapy consists of conservative multimodal management such as physical therapy, non-steroidal anti-inflammatory medication, non-narcotic analgesics, narcotic analgesics including opioids, activity modification, and/or lumbar/cervical orthosis, followed by steroid injections. Conventional pain management treatments are short-lived in relieving symptoms and can have deleterious side effects.8 For example, opioids have been found to have similar effectiveness as placebo in reducing chronic pain symptoms and are associated with many complications including nausea, vomiting, constipation, somnolence and respiratory depression.9–11 Despite the lack of effectiveness, opioids are commonly used. In fact, the prescription of opioids for chronic musculoskeletal pain has increased in recent decades.12 Fifteen percent of the patients with knee, hip and spine osteoarthritis reported occasional use of opioids and an additional 15% reported daily use of prescription opioids.13 In a study examining treatment for chronic back pain, opioids were prescribed up to 66% of the time.10 Unfortunately, when people are prescribed a high dose of opioids, it can lead to addiction.10 Prevalence of substance use disorders in the patient population with chronic back pain from the same study ranged from 36% to 56%.10 In 2020, 188 Americans died each day from opioid-related drug overdose.14 Furthermore, nearly 25% of the opioid overdose deaths in the US were attributable to prescription opioids.14
An alternative, non-opioid, regenerative treatment option for orthopedic spinal pathologies manifesting pain could be amniotic membrane and umbilical cord (AMUC) tissue which has been clinically used for decades with reported effectiveness in reducing patients’ pain15–26 due to its anti-inflammatory, anti-scarring, and pro-regenerative properties.8,27–29 In particular, injection of micronized AMUC matrix has demonstrated effectiveness by reducing pain and improving function in many musculoskeletal conditions including plantar fasciitis,16 knee osteoarthritis,30 wrist osteoarthritis,27 and articular cartilage damage.29 Studies have also shown benefit of intradiscal or facet injection of AMUC for patients with spine facet osteoarthritis9 and discogenic pain.28 However, these studies were limited to small sample size and no study has reported the outcomes of epidural AMUC injection or effect of repeat injection of AMUC. As such, the main objective of this study was to retrospectively analyze real-world data on patients treated with AMUC for the repair, reconstruction, replacement, or supplementation of a variety of spinal musculoskeletal disorders manifesting pain to generate real-world evidence of patient safety and effectiveness in routine clinical practice.
Methods
This is a single-center, retrospective chart review of patients with spinal indications of musculoskeletal disorders that were subsequently treated with micronized AMUC between March 1, 2020 and July 1, 2021. Medical records were reviewed from a private physician practice (Midwest Bone & Joint Center, PC) and all patients were treated by the same medical provider. All eligible patients enrolled in the retrospective study had to be older than 18 years of age, have a spinal indication of musculoskeletal disorder (eg, osteoarthritis, spinal stenosis, herniated discs, radiculopathy), received AMUC injection, and have had at least one follow-up and baseline pain score. Data was collected without patient identifying information. Sterling IRB reviewed the study protocol before research began, and a category 4 DHHS non-human subjects research exemption request was granted on July 20, 2021 (9106-CMain).
The AMUC (CLARIX FLO; Amniox; Miami, FL) is comprised of amniotic membrane and amniotic membrane from the umbilical cord derived from donated human placental tissue following healthy, live, caesarian section, full-term births. The AMUC has been lyophilized, micronized, and terminal sterilized. The AMUC does not contain any living cells and comes as a 100mg powder within a vial. At the facility, the medical provider generally performs a series of AMUC injections for patients with spinal indications: epidural injection at initial presentation, bilateral facet injection at 2 weeks, a second epidural injection at 4 weeks, and another facet injection at 6 weeks.
Full discussion of the risks, benefits, complications, alternatives, and anticipated outcome from this procedure was discussed with the patient and written consent recorded. Risks and complications included infection, allergic or other inflammatory reactions, increased pain, and, in rare circumstances, nerve damage. Benefits and the anticipated outcome included decreased pain, increased range of motion, increased sensation in affected dermatomes, reduction of radicular symptoms, independence from other treatments, and return to daily activities and work. Alternatives to AUMC injection include corticosteroid injections, physical therapy, NSAIDS, chiropractic treatment, and orthosis, among others. As explained to patients, it is common to experience a side effect of increased pain or soreness for 24–48 hours following the injection due to the inflammatory and regenerative nature of the injection, but the pain quickly and substantially dissipates with results seen within 7–10 days from the injection. Patients’ heart rate, blood pressure, and demeanor were closely monitored before, during, and after the procedure.
The epidural and facet injections are performed as follows:
Epidural Injection
The spinal-level interspace was determined after visualizing fluoroscopically in the A/P and lateral projections. Once the appropriate vertebral level was determined, the skin entry point was marked with a skin marker. Then, the skin was prepped in aseptic fashion with chlorhexidine for 30 seconds, followed by a betadine solution three times. While the solution was allowed to dry, the procedure tray was prepared, and sterile drapes were applied. A 27-gauge needle was then used to inject 3 mL of 1% lidocaine to the subcutaneous skin and deep tissues.
Lumbar
An 18- or 20-gauge Tuohy needle was introduced into the skin and subcutaneous tissue, and orientation was confirmed with fluoroscopic guidance in the A/P projection. Using a loss of resistance technique, the Tuohy needle was advanced slowly to the epidural space with the fluoroscopy machine positioned in the lateral projection. Following negative aspiration for heme or CSF, 1 mL of contrast dye was injected without evidence of vascular intake or intrathecal injection and spread of contrast was confirmed in the lateral and A/P projections. A solution of CLARIX-FLO 100 mg in 2–4 mL preservative free 0.9% was then injected without paresthesia. The needle was then discontinued, and rapid hemostasis was achieved at the skin level.
Cervical
A 20-gauge Tuohy needle was introduced into the skin and subcutaneous tissue, and orientation confirmed with fluoroscopic guidance. Using a hang-drop and loss of resistance technique, the Tuohy needle was advanced slowly to the epidural space with the fluoroscopy machine positioned in the lateral or contralateral projection. Following negative aspiration for heme or CSF, 1 mL of contrast dye was injected without evidence of vascular intake or intrathecal injection and spread of contrast was confirmed in the lateral and A/P projections. A solution of CLARIX-FLO 100 mg in 2–4 mL preservative free 0.9% was then injected without paresthesia. The needle was then discontinued, and rapid hemostasis was achieved at the skin level.
Facet Injection
The bilateral facet joints were identified, and skin entry points were marked with a skin marker. The patient was prepped with chlorhexidine followed by a betadine solution and sterile drapes were applied. The skin entry points were then localized with a 27-gauge needle; then, a 22-gauge, spinal needle was advanced through the skin and subcutaneous tissue, and needle orientation was confirmed with fluoroscopy. The needles were advanced to contact the peri-articular ligament. Following negative aspiration for heme or CSF, a solution containing CLARIX-FLO 100 mg was diluted into 2 mL preservative free 0.9% normal saline and 25mg was injected through each of four needles (injections commonly done at two levels of facet joints at a time with 25 mg/facet joint). The needles were then discontinued, and rapid hemostasis was achieved at the skin level.
Demographics were recorded in all cases including age, gender, smoking status, and diagnosis, as displayed in Tables 1 and 2. The primary clinical outcome was change in pain from baseline to follow-up at 2, 3–4, 6, or 8 weeks. Secondary outcomes include increased range of motion, return to activities of daily living, return to full work activities, increased sensation, lack of radicular nerve pain, and decreased use of non-narcotic and narcotic analgesics. Other possible negative secondary outcomes were monitored such as increased or equal pain sensation, changes in blood pressure or heart rate, infection, or allergic responses. The Numerical Pain Rating Scale was used to document patients’ pain in which patients verbally reported their average severity of pain on a scale, ranging from “no pain” at 0 to “worst imaginable pain” at 10. The health provider explained this to the patient at the beginning of each appointment, encouraged an honest answer, and recorded their response accordingly. This type of pain rating scale is commonly used and has been proven to be an effective marker of pain, especially when comparing within a patient over time.31,32
 |
Table 1 Patient Diagnoses |
 |
Table 2 Patient Demographics |
Statistical Analysis
After careful review and collection of data from patients’ medical records, summary data was assembled. A total of four patients were excluded from the study. Of these, three did not return after their first injection for unknown reasons and one, while they reported improvement, did not have a baseline pain score on file. A total of 52 injection sets met the eligibility criteria. Patients were divided between cervical and lumbar pathologies and could be included in both sets of data if they received injections in both areas. Patients’ change in pain score was assessed with Student t-test comparison of post-injection pain scores with baseline. Student 2-sided t-tests were conducted between baseline and each of the follow-up timepoints after an injection set: 2, 3–4, 5–6, and 8 weeks. In addition, a Student t-test was conducted between the overall mean baseline score and the overall mean value of the last follow-up pain score that was collected for each patient. A p-value less than 0.05 was considered statistically significant. Descriptive statistics were used to characterize the data and were reported as mean ± standard deviation.
Results
A total of 52 injection sets met the eligibility criteria and were included in this analysis. The most common patient diagnoses were spondylosis or inflammatory spondylopathy (84.6%), intervertebral disc disorder (59.6%), and radiculopathy (34.6%). Patients often received multiple diagnosis depending on the presentation of their symptoms. These results are summarized in Table 1. Table 2 illustrates patient demographic characteristics. The patients (35 male, 17 female) had an average age of 40.8 years, and 39 patients were current (21.2%) or former (51.9%) smokers. Mean duration of symptoms was 54.2 months. Additional treatment characteristics are shown in Table 3. Patients received different numbers of injections in some cases depending on the location and severity of their symptoms. Patients could receive both lumbar and cervical injections if necessary as well as both epidural and facet type injections, depending on the presentation of their symptoms. Patients could receive two injections of the same type 4 weeks apart. Patients received a mean of 3.5 AMUC injections throughout the follow-up period.
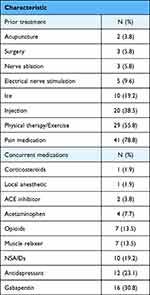 |
Table 3 Patient Additional Treatment Characteristics |
The patients’ pain score significantly decreased as early as two weeks post-injection. Compared to a mean baseline of 4.9 ± 2.2, pain scores significantly reduced to 3.4 ± 2.3 at 2 weeks (p < 0.0001) and 3.5 ± 2.2 at 3–4 weeks (p = 0.0023). While pain scores were also reduced at week 6 (3.2 ± 2.3, p = 0.6667) and week 8 (3.1 ± 1.2, p = 0.08), the results are not significant due to the small sample size because patients were not specifically requested to return for follow-up visits after 4 weeks. For the mean follow-up period of 10.6 ± 5.4 weeks, reported pain was significantly reduced from baseline (2.8 ± 2.1, p < 0.0001). Table 4 and Figure 1 depict these promising results.
 |
Table 4 Patient Baseline and Follow-Up Numerical Pain Scores by Weeks After Last Injection |
A common side effect noticed by patients from the injection was pain or soreness near the injection site. This reaction is common in epidural and facet injections. Some patients required acetaminophen or other pain medication in the few days after the injection while the medication took effect. Patients noticed improvement within 5–7 days after the injection. No significant complications or adverse events were noted over the average 10.6 weeks of follow-up. All patients reported improvements in pain, range of motion, or sensation.
When stratified by gender as in Table 5, the results continue to demonstrate significant improvement in pain rating over time. The average baseline pain score for the 35 men included in the study was 4.69/10 and 5.47/10 for the 17 women. The mean pain score at the end of the follow-up period was 2.61 for men and 3.04 for women, both significantly decreasing from baseline scores. While the baseline score for women was higher than for men, the women experienced a greater improvement in pain ratings (−2.43 versus −2.08) over the course of the study.
 |
Table 5 Pain Scores Stratified by Gender Over Time |
Discussion
For back-related pain, first-line therapy generally consists of conservative multimodal management such as pain medication (acetaminophen, nonsteroidal anti-inflammatory drugs, muscle relaxants, narcotic and non-narcotic analgesics), physiotherapy, acupuncture, and, if necessary, psychotherapy.21,33 When conservative measures fail, interventional procedures are considered to reduce pain, improve functionality and reduce side effects from systemic medications.21 Interventional procedures generally include intraarticular or medial branch injections34 of steroids such as methylprednisolone, triamcinolone, and betamethasone for the lumbar area, and dexamethasone for cervical or thoracic areas.22,25 However, the Agency for Healthcare Research and Quality 2015 review reported there was limited evidence for facet joint corticosteroid injections (intra-articular, periarticular, or MBB) versus placebo interventions because the studies that were reviewed found no clear differences between the interventions.35 Furthermore, corticosteroids also have potential complications of suppression of the pituitary-adrenal axis, hyperadrenocorticism, Cushing syndrome, osteoporosis, avascular necrosis of bone, steroid myopathy, epidural lipomatosis, weight gain, fluid retention, and hyperglycemia.34 Moreover, long-term use of narcotic pain medication is ineffective and can lead to substance use disorders along and cause vomiting, constipation, somnolence, and respiratory depression.9–11
As an alternative, use of injectable AMUC tissue products such as CLARIX FLO significantly decreases pain and symptoms in patients with limiting spinal indications. These preliminary safety and effectiveness data are consistent with prior studies in which AMUC was beneficial in patients with various musculoskeletal injuries.17,19–22,36–38 The benefits of AMUC injections in musculoskeletal pathologies might be based on their reported therapeutic actions in reducing inflammation, inhibiting scar tissue formation, and supporting stem cell function. AMUC contains, growth factors, cytokines and peptide complexes including HC-HA/PTX3, which has been shown to promote apoptosis of pro-inflammatory cells24 and suppress fibrosis.25 These actions create a biological regenerative response that facilitates accelerated wound healing, in this case repairing the discs within the spinal joints.
In this study, injectable AMUC significantly reduced pain as early as two weeks post-injection in patients that had moderate-to-severe pain (average pain score of 4.9 ± 2.2) despite failing prior pain relief therapies for 54.2 months. While some patients experienced a minor side effect of soreness at the injection site in the days following the injection, no significant adverse reactions or complications occurred. Injections were effective in cervical, lumbar, and lumbosacral pathologies of spondylosis, intervertebral disc disorder, and radiculopathy among others. Differences in pain improvement and baseline scores between genders are also noted. Both women and men experienced significant decreases in pain scores throughout the study. The baseline and final pain scores for men were lower than those for women, but women experienced an larger average decrease in pain score than men over time.
AMUC particulate injections are a promising alternative to current therapies for acute and chronic back pain. Evidence provided illustrates the dangers of the long-term use of opioids and corticosteroid injections as well as the lack of supporting evidence for their effectiveness in the long term.9–11,34,35 This study supports the use of AMUC injections for reducing pain caused by cervical and lumbar spinal pathologies, allowing patients to return to activities of daily living without pain.
Conclusion
Overall, this study provides additional evidence for the safe use of amniotic membrane/umbilical cord (AMUC) particulate in the treatment of back pain. Epidural and facet injections of AMUC in the lumbar and cervical regions significantly reduced pain from 4.9 out of 10 to 2.8 out of 10 within two weeks. The average follow-up period of 10.6 weeks allows a preview into the use of these injections for pain management and symptom relief. Further interventional studies or clinical trials may be worthwhile to investigate this promising treatment option for back pain. Future studies should include additional pain and functional outcomes in the long term.
Disclosure
Dr Chris Main reports grants, non-financial support from TissueTech, during the conduct of the study. Miss Amanda Ross reports grants from TissueTech, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Storheim K, Zwart JA. Musculoskeletal disorders and the Global Burden of Disease study. Ann Rheum Dis. 2014;73(6):949–950. doi:10.1136/annrheumdis-2014-205327
2. Thiese MS, Hegmann KT, Wood EM, et al. Prevalence of low back pain by anatomic location and intensity in an occupational population. BMC Musculoskelet Disord. 2014;15:283. doi:10.1186/1471-2474-15-283
3. Mattiuzzi C, Lippi G, Bovo C. Current epidemiology of low back pain. J Hosp Manag Health Policy. 2020;4:1. doi:10.21037/jhmhp-20-17
4. Chronic back pain. Health policy institute. Available from: https://hpi.georgetown.edu/backpain/.
5. Low back pain fact sheet. Available from: https://www.ninds.nih.gov/health-information/patient-caregiver-education/fact-sheets/low-back-pain-fact-sheet.
6. Perolat R, Kastler A, Nicot B, et al. Facet joint syndrome: from diagnosis to interventional management. Insights Imaging. 2018;9(5):773–789. doi:10.1007/s13244-018-0638-x
7. Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine. 2004;29(19):2091–2095. doi:10.1097/01.brs.0000141265.55411.30
8. Cooke M, Tan EK, Mandrycky C, He H, O’Connell J, Tseng SCG. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care. 2014;23(10):465–476. doi:10.12968/jowc.2014.23.10.465
9. Bennett DS. Cryopreserved amniotic membrane and umbilical cord particulate for managing pain caused by facet joint syndrome: a case series. Medicine. 2019;98(10):e14745. doi:10.1097/MD.0000000000014745
10. Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–127. doi:10.7326/0003-4819-146-2-200701160-00006
11. Baldini A, Korff MV, Lin EHB. A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Prim Care Companion CNS Disord. 2012;14(3):27252. doi:10.4088/PCC.11m01326
12. Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP Study. Pain. 2008;138(2):440–449. doi:10.1016/j.pain.2008.04.027
13. Power JD, Perruccio AV, Gandhi R, et al. Factors associated with opioid use in presurgical knee, hip, and spine osteoarthritis patients. Arthritis Care Res. 2019;71(9):1178–1185. doi:10.1002/acr.23831
14. National Institute on Drug Abuse. Overdose death rates. National Institute on Drug Abuse; 2022. Available from; https://nida.nih.gov/drug-topics/trends-statistics/overdose-death-rates.
15. Frost CØ, Hansen RR, Heegaard AM. Bone pain: current and future treatments. Curr Opin Pharmacol. 2016;28:31–37. doi:10.1016/j.coph.2016.02.007
16. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151–158. doi:10.1177/1071100714552824
17. Covell DJ, Cohen B, Ellington JK, Jones CP, Davis WH, Anderson RB. The use of cryo-preserved umbilical cord plus amniotic membrane tissues in the resection of tarsal coalition. Foot Ankle Orthop. 2016;1(1):2473011416S0031. doi:10.1177/2473011416S00311
18. Sakarya Y, Sakarya R, Yildirim A. Sutureless amniotic membrane fixation with fibrin glue in symptomatic bullous keratopathy with poor visual potential. Eur J Ophthalmol. 2010;20(1):249. doi:10.1177/112067211002000142
19. John T, Tighe S, Sheha H, et al. Corneal nerve regeneration after self-retained cryopreserved amniotic membrane in dry eye disease. J Ophthalmol. 2017;2017:1–10. doi:10.1155/2017/6404918
20. Morkin MI, Hamrah P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul Surf. 2018;16(1):132–138. doi:10.1016/j.jtos.2017.10.003
21. Park JH, Jeoung JW, Wee WR, Lee JH, Kim MK, Lee JL. Clinical efficacy of amniotic membrane transplantation in the treatment of various ocular surface diseases. Contact Lens Anterior Eye. 2008;31(2):73–80. doi:10.1016/j.clae.2007.11.004
22. Kheirkhah A. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126(8):1059. doi:10.1001/archopht.126.8.1059
23. Huang H, Sheha Y. Self-retained amniotic membrane transplantation for recurrent corneal erosion. J Clin Exp Ophthalmol. 2013;4:2. doi:10.4172/2155-9570.1000272
24. Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5(5):645–661. doi:10.1586/eop.10.63
25. Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51–77. doi:10.1016/j.survophthal.2003.10.004
26. Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. 2004;2(3):201–211. doi:10.1016/S1542-0124(12)70062-9
27. Kim SR. Successful treatment of scapholunate advanced collapse: a case report. Clin Case Rep. 2019;7(6):1230–1232. doi:10.1002/ccr3.2201
28. Buck D. Amniotic umbilical cord particulate for discogenic pain. J Osteopath Med. 2019;119(12):814–819. doi:10.7556/jaoa.2019.138
29. Williams GK. Articular cartilage restoration with adjunctive use of cryopreserved amniotic membrane and umbilical cord particulate. ICRS Summit. 2019;98:5.
30. Castellanos R, Tighe S. Injectable amniotic membrane/umbilical cord particulate for knee osteoarthritis: a prospective, single-center pilot study. Pain Med Malden Mass. 2019;20(11):2283–2291. doi:10.1093/pm/pnz143
31. Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine. 2000;25(24):3140–3151. doi:10.1097/00007632-200012150-00009
32. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15(Suppl 1):S17–S24. doi:10.1007/s00586-005-1044-x
33. Ellington JK, Ferguson CM. The use of amniotic membrane/umbilical cord in first metatarsophalangeal joint cheilectomy: a comparative bilateral case study. Surg Technol Int. 2014;25:63–67.
34. Argoff CE. Managing Chronic Pain, an Issue of Medical Clinics of North America. Elsevier Health Sciences; 2016.
35. Berliner E. Multisociety letter to the agency for healthcare research and quality: serious methodological flaws plague technology assessment on pain management injection therapies for low back pain: multisociety letter to AHRQ. Pain Med. 2015. doi:10.1111/pme.12934
36. Espana EM, Grueterich M, Sandoval H, et al. Amniotic membrane transplantation for bullous keratopathy in eyes with poor visual potential. J Cataract Refract Surg. 2003;29(2):279–284. doi:10.1016/S0886-3350(02)01525-0
37. Georgiadis NS, Ziakas NG, Boboridis KG, Terzidou C, Mikropoulos DG. Cryopreserved amniotic membrane transplantation for the management of symptomatic bullous keratopathy. Clin Experiment Ophthalmol. 2008;36(2):130–135. doi:10.1111/j.1442-9071.2008.01696.x
38. Finger PT. Finger’s amniotic membrane buffer technique: protecting the cornea during radiation plaque therapy. Arch Ophthalmol. 2008;126(4):531. doi:10.1001/archopht.126.4.531
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

