Back to Journals » Clinical Ophthalmology » Volume 15
Clinical Outcomes After Bilateral Implantation of a Trifocal Presbyopia-Correcting Intraocular Lens in an Indian Population
Authors Ramamurthy D, Vasavada A, Padmanabhan P , Reddy JC, Shetty N, Dey A, Sudhir RR
Received 27 August 2020
Accepted for publication 15 November 2020
Published 22 January 2021 Volume 2021:15 Pages 213—225
DOI https://doi.org/10.2147/OPTH.S279001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dandapani Ramamurthy,1 Abhay Vasavada,2 Prema Padmanabhan,3 Jagadesh C Reddy,4 Naren Shetty,5 Arindam Dey,6 Rachapalle Reddi Sudhir3
1The Eye Foundation, Coimbatore, Tamil Nadu, India; 2Raghudeep Eye Hospital, Ahmedabad, Gujarat, India; 3Medical Research Foundation, Chennai, Tamil Nadu, India; 4LV Prasad Eye Institute, Hyderabad, Telangana, India; 5Narayana Nethralaya Eye Hospital, Rajajinagar, Bangalore, India; 6Alcon Laboratories (India) Private Ltd, Bangalore, India
Correspondence: Dandapani Ramamurthy
The Eye Foundation, Diwan Bahadur Road, Coimbatore, Tamil Nadu 641002, India
Tel +91 422 4242000
Email [email protected]
Purpose: To evaluate the effectiveness and safety of a presbyopia-correcting trifocal intraocular lens (IOL), AcrySof® IQ PanOptix® (TFNT00), in an Indian population.
Patients and Methods: This prospective, multicenter, observational, single-arm, post-marketing study included 67 patients undergoing cataract surgery with bilateral implantation of TFNT00 across five Indian sites. Postoperative outcomes were assessed at 3 months after second eye surgery. Effectiveness outcomes included: mean binocular and monocular visual acuity (VA) at distance (4 m), intermediate (60 cm), and near (40 cm); binocular defocus curve; manifest refraction; and subjective symptom questionnaire evaluation. Safety outcomes included the rate of ocular adverse events and mesopic contrast sensitivity.
Results: Mean binocular and monocular distance-corrected and uncorrected VAs of 0.1 logMAR or better (approximately 20/25 Snellen) were achieved at distance, intermediate, and near. Overall, ≥ 70% of patients achieved binocular 0.1 logMAR vision or better across all distances. TFNT00 maintained a mean VA of 0.1 logMAR or better at the defocus range of +0.5 diopters (D) to – 2.5 D (200 cm to 40 cm). The subjective symptom questionnaire-assessed frequency of halo visual disturbances was low at Month 3; halos were reported “none of the time” to “only some of the time” in 86.6% of patients. The large majority of patients (98.5%) were “satisfied” or “very satisfied” with their near, intermediate, and distance vision at Month 3, and ≥ 94.0% of patients reported spectacle independence for tasks at all distances. The adverse event rate was low; no patients discontinued due to an adverse event.
Conclusion: TFNT00 provided a continuous range of vision of 20/25 or better for distance to near and performed effectively at an intermediate functional distance of 60 cm, resulting in high levels of spectacle independence and patient satisfaction. TFNT00 demonstrated a good safety profile and a low post-operative frequency of halo visual disturbances.
Keywords: PanOptix, multifocal, cataract surgery, non-apodized, intraocular lens, IOL
Plain Language Summary
Cataract surgery involves the surgical removal of the cloudy natural lens from an eye and its replacement with an artificial intraocular lens (IOL). In many cases, patients receive a monofocal IOL, which is primarily designed to correct distance vision, requiring them to use spectacles for near and intermediate vision correction. In contrast, trifocal IOLs, such as TFNT00, are designed to provide patients with a continuous range of vision, which reduces the need for spectacles. Unlike other trifocal IOLs that commonly target an intermediate focal point of 80 cm, TFNT00 provides an intermediate focal point of 60 cm. This aims to increase patient satisfaction by making intermediate vision more comfortable because most intermediate distance work, such as computer use, is performed at 60 to 70 cm for the average person.
The purpose of this study was to evaluate TFNT00 in 67 Indian patients, namely for its efficacy in terms of visual outcomes and spectacle independence, and its safety. Our results show that at 3 months post-surgery, TFNT00 provided a functional continuous range of vision of 20/25 or better for farther (4 meters) to nearer (40 cm) distances, and performed effectively at an intermediate distance of 60 cm. Most patients receiving TFNT00 did not require spectacles at 3 months after surgery. Additionally, TFNT00 demonstrated a good safety profile and the number of patients seeing bright rings around a light source (known as “halos”) was low. This study provides the first clinical data on this lens in an Indian population.
Introduction
Visual demands have changed considerably over the last few decades, and patients today seek spectacle independence at all distances following cataract surgery and presbyopia-correcting intraocular lens (IOL) implantation. Monofocal IOLs focus light on a single focal point providing distance vision but do not provide near and intermediate vision,1 thereby increasing spectacle dependency at these distances.1–4 In contrast, multifocal IOLs are designed with refractive or diffractive optical properties that focus light at multiple foci, providing vision over a range of distances,2,3,5–7 thus multifocal IOLs provide greater spectacle independence than monofocal IOLs.2,3,7 However, these advances have often come at the cost of reduced contrast sensitivity or increased photic phenomena.1,2,4–6,8,9 Additionally, multifocal IOLs with only two focal points (bifocal) provide sub-optimal intermediate visual acuity (VA).10
Diffractive trifocal IOLs have been shown to provide functional distance, intermediate, and near vision, and are designed with three optical focal points.11,12 AcrySof® IQ PanOptix® presbyopia-correcting IOL model TFNT00 (Alcon Vision LLC) is a non-apodized, ultraviolet- and blue light-filtering, hydrophobic acrylic, diffractive IOL.13–15 TFNT00 comprises quadrifocal technology; however, in terms of function, it acts as a trifocal.13–15 The IOL uses a proprietary optical technology, ENLIGHTEN™, to manipulate the quadrifocal design and redistribute the incoming light from the focal point at 120 cm to the distance focal point.14 This creates an enhanced distance add power, together with the intermediate +2.17 diopters (D; 60 cm) and +3.25 D near (40 cm) add powers,14–16 providing a continuous range of vision. The 4.5 mm non-apodized, diffractive zone allows high light utilization, transmitting 88% of light to the retina at a 3.0 mm pupil size, 50% of which is allocated to distance, with 25% to intermediate and 25% to near;14,15,17 thus providing optimized performance in a wide range of lighting conditions due to low dependence on the pupil size.13,14 Patient demand for functional intermediate vision has grown with the rising popularity of handheld devices and increasing use of computers in daily life. Unlike other trifocal IOLs that commonly target an intermediate focal point of 80 cm, the novel diffractive structure of TFNT00 provides an optimum intermediate focal point of 60 cm (arm’s length).16,18 This design aims to increase patient satisfaction by making intermediate vision more comfortable, because most intermediate distance work is performed at 60 to 70 cm for the average person.19
The popularity of cataract surgery and IOL implantation has increased exponentially in India over the past two decades.20–22 Demand for multifocal IOLs is expected to surge globally due to: the large number of cataract surgeries (∼26 million cataract surgeries performed in 2017); changing socioeconomic frameworks; the world’s aging population; improvements in healthcare sectors and expenditure; increased demand for spectacle independence; access to innovative, advanced IOLs; and increased patient awareness.15 Although previous clinical investigations have demonstrated a continuous range of vision from near to distance with TFNT00,14,23–28 to date, no clinical studies have been conducted in Indian patients. The purpose of this study was to evaluate the 3-month postoperative effectiveness and safety of TFNT00 in providing a range of vision (distance, intermediate, and near) in an Indian population.
Methods
Study Design
This was a prospective, single-arm, multicenter, unmasked, observational cohort study to evaluate the effectiveness and safety of in-the-bag implantation of TFNT00 for the visual correction of aphakia in adult patients. The study was carried out between January 15, 2019, and January 18, 2020. The study was approved by the institutional ethics committees (The Eye Foundation, Iladevi Cataract & IOL Research Center, Medical Research Foundation, LV Prasad Eye Institute and Narayana Nethralaya Eye Hospital), conducted in accordance with the Declaration of Helsinki and registered on ClinicalTrials.gov (NCT03706066). Written informed consent was obtained from all patients. Patients included in the study were ≥18 years of age with bilateral cataracts, had an IOL power calculation of between +16.0 and +26.0 D, and preoperative regular keratometric astigmatism of <1.0 D in both eyes. Exclusion criteria included: clinically significant corneal abnormality, disease, or degeneration; history of, predisposition to, or concurrent retinal conditions; glaucoma; ocular trauma; patients with previous refractive surgery or corneal transplant; any ocular pathology, conditions, or degenerative disorders that could affect postoperative visual outcomes; and patients with rubella, congenital, traumatic, or complicated cataracts. IOL power was calculated using the Barrett Universal II formula29 and an optical A-constant of 119.1.
Study Procedures
The first surgical eye was defined as the eye with the worse preoperative best-corrected distance VA (BCDVA). If the BCDVA was the same in both eyes, the right eye was the first surgical eye. The second eye implant occurred within 7‒90 days of the first eye implant. Repeated screening of some parameters was conducted if patients had their second eye surgery >40 days after their initial screening prior to first eye implantation. A total of eight scheduled visits were planned: one pre-operative visit, two operative visits, and postoperative visits at Days 1–2 and Month 1 (after each surgery) and Month 3 (after second surgery).
VA assessments were performed under photopic conditions with chart background luminance of approximately 85 cd/m2. Distance VA (4 m) and defocus were measured using CSV-1000 (Vector Vision Inc.) Charts 1, 2, and R; intermediate (60 cm) and near (40 cm) VA were measured using Early Treatment Diabetic Retinopathy Study Near Card Charts 1 and 2. For monocular assessments, the contralateral eye was occluded.
Binocular contrast sensitivity was measured using a CSV-1000HGT contrast sensitivity unit (Vector Vision Inc.). For assessments under mesopic conditions, participants were fitted with neutral density filters to reduce their perception of the chart luminance to approximately 3 cd/m2. Subjective posterior capsular opacification (PCO) was assessed during slit-lamp examination and, if present, clinically graded as nonsignificant, clinically significant, or clinically significant requiring neodymium-doped yttrium aluminum garnet (Nd:YAG) treatment. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0.
Effectiveness and Safety Outcomes
All primary and secondary outcomes were assessed at Month 3 after bilateral implantation. The primary effectiveness outcome was mean photopic binocular BCDVA (4 m). Secondary effectiveness outcomes included mean photopic monocular BCDVA; mean photopic binocular and monocular distance-corrected VAs at intermediate and near (DCIVA [60 cm] and DCNVA [40 cm], respectively); mean photopic binocular and monocular uncorrected VAs at distance, intermediate, and near (UCDVA, UCIVA, and UCNVA, respectively); mean binocular defocus curve; manifest refraction; and subjective symptom questionnaire post-implantation. The safety outcomes were the rate of ocular AEs (including secondary surgical interventions [SSIs]), device deficiencies, subjective PCO assessment, posterior capsulotomy, and the mean binocular contrast sensitivity with and without glare for mesopic conditions.
Patient-Reported Outcomes
Patients were proactively asked to subjectively rate their quality of vision and experience of visual disturbances using a subjective symptom questionnaire developed by Alcon. This questionnaire consisted of 11 questions derived from the Spectacle Independence Lens Vision Evaluation and Repurchase (SILVER)30,31 and Assessment of Photic Phenomena and Lens EffectS (APPLES)31,32 questionnaires. The questions related to each patient’s quality of vision according to their: satisfaction and need for spectacles for near, intermediate, and distance vision; frequency and severity of halos; difficulty driving at night; and overall cataract surgery satisfaction. Responses were reported on a 4- or 5-point categorical scale ranging from “very dissatisfied” to “very satisfied” for satisfaction items, from “none of the time” to “all of the time” for frequency items, and from “none” to “severe” for severity items.
Sample Size Calculation
There was no hypothesis testing in this study; therefore, the sample size chosen was not based on power calculations. The required sample size of 80 participants was determined based on a drop-out rate of 20%. This sample was considered sufficient to ensure that at least 64 eligible participants completed the study. With 64 participants, a two-sided 95% confidence interval for mean binocular BCDVA will extend 0.044 logMAR from the observed mean, assuming that the standard deviation (SD) is known to be 0.18 logMAR (conservative estimate based on previous IOL studies33,34), and the confidence interval is based on the large sample z statistic.
Statistical Methods
The all-implanted analysis set (all eyes with successful implantation plus at least one postoperative visit) was the primary analysis set for all effectiveness endpoints, except defocus, and included all patients implanted. The safety analysis set (all eyes with attempted implantation) was the primary analysis set for all safety endpoints, except contrast sensitivity, and included all eyes that had attempted study IOL implantation (successful or aborted after contact with the eye). The best-case analysis set (all eyes successfully implanted with ≥1 postoperative visit) was the primary analysis set for defocus and contrast sensitivity analyses. It included all eyes successfully implanted with the study IOL that had at least: one postoperative visit; no previous surgery for the correction of refractive errors; no preoperative ocular pathology or macular degeneration at any time; and no major protocol deviations. There was no hypothesis testing in this study; therefore, data were summarized using descriptive statistics.
Results
Patient Disposition and Demographics
A total of 80 participants were enrolled across five sites in India, of which 7 patients discontinued prior to IOL implantation (Figure 1). Of these, 68 eligible patients were successfully implanted bilaterally with TFNT00 and 5 patients were implanted unilaterally. Of the 68 patients who received TFNT00 implanted bilaterally, 1 discontinued from participation after implantation of the study IOLs and was considered lost to follow-up; therefore, 67 patients completed the study (Figure 1). Study patients were all of Indian race (Table 1). Their overall mean age was 58.5 ± 11.46 years; approximately two-thirds of patients were aged <65 years (65.8%), and 53.4% of patients were female (Table 1). Baseline characteristics were largely similar between first and second eyes; mean preoperative monocular BCDVA was 0.43 logMAR for first eyes and 0.27 logMAR for second eyes; mean pupil size (4 mm), axial length, anterior chamber depth, corneal thickness, and lens thickness were also comparable between eyes (Table 1).
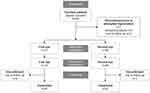 |
Figure 1 Patient disposition. |
 |
Table 1 Baseline Demographics and Clinical Characteristics of Patient Study Population (All-Implanted Analysis Set) |
Visual Outcomes
Distance-Corrected and Uncorrected Visual Acuity
Patients achieved a mean ± standard deviation binocular BCDVA of <0.0 logMAR at Month 3 (–0.052 ± 0.09 logMAR) (Table 2) and 92.5% of patients achieved a binocular BCDVA of 0.1 logMAR or better (Figure 2A). Binocular DCIVA and DCNVA of <0.1 logMAR were also achieved (0.039 ± 0.11 and 0.061 ± 0.11 logMAR, respectively) (Table 2), with 84.8% and 78.8% of patients, respectively, achieving 0.1 logMAR or better VA (Figure 2A).
 |
Table 2 Descriptive Statistics for Photopic Binocular VAs at 3 Months (logMAR) (All-Implanted Analysis Set) |
Similar results were achieved for binocular uncorrected VA at Month 3; patients demonstrated a mean binocular UCDVA of ≤0.0 logMAR (0.011 ± 0.11 logMAR) and a mean binocular UCIVA and UCNVA of <0.1 logMAR (0.055 ± 0.10 and 0.092 ± 0.13 logMAR) (Table 2). Overall, 83.6%, 80.3%, and 69.7% of patients achieved 0.1 logMAR or better UCDVA, UCIVA, and UCNVA, respectively (Figure 2B). Mean monocular distance and uncorrected VAs at distance, intermediate, and near were ≤0.1 logMAR in first and second eyes (Online Table 1).
Defocus Curve
Binocular defocus testing was consistent with the VA results. TFNT00 maintained a mean VA of 0.1 logMAR (20/25 Snellen) or better at the defocus range of +0.5 D to –2.5 D (200 cm to 40 cm) (Figure 3A), indicating that TFNT00 provides better than functional vision across a full range of defocus. Additionally, this continuous range of vision was observed for all pupil sizes (~0.1 logMAR or better) (Figure 3B).
 |
Figure 3 (A) Mean binocular defocus curve with 95% confidence intervals at 3 months (logMAR); (B) by postoperative pupil size category (best-case analysis set). |
Refractive Outcomes
At Month 3, the mean manifest refraction spherical equivalent was approximately –0.1 D for first and second eyes (Online Table 2). The majority (≥94%) of first and second eyes were within 0.5 D or less of the target refractive error at Month 3 (Online Table 2), representing the good refractive predictability of TFNT00, because the target refraction was emmetropia (±0.5 D).
Patient-Reported Outcomes
The large majority of patients (66/67 patients, 98.5%) were “satisfied” or “very satisfied” with their near, intermediate, and distance vision at Month 3, and ≥94% of patients reported spectacle independence for tasks at all distances (Figure 4A and B). The subjective symptom questionnaire-assessed frequency of halo visual disturbances was low at Month 3; halos were reported “none of the time” to “only some of the time” in 86.6% of patients (Figure 4C) and only 1 patient reported halos as “severe” (Figure 4D). Additionally, 88.6% of patients reported no difficulty driving at night (data not shown), and all but one individual were either “satisfied” or “very satisfied” with their surgery results (data not shown).
Safety Outcomes
Adverse Events
The most common ocular AEs were PCO (4 events, 5.5%) and macular fibrosis (3 events, 4.1%) in the first eye and PCO (2 events, 2.9%) in the second eye (Table 3), although no participants discontinued due to AEs. No eyes underwent Nd:YAG laser posterior capsulotomy during the study (Table 3); however, subjective assessment indicated that of the 6 events coded to PCO, three first eyes and one second eye of 3 patients had clinically significant PCO by the end of the study, of which two first eyes were assessed to require neodymium-doped: yttrium aluminum garnet (Nd:YAG) laser treatment. Of these four eyes with clinically significant PCO, two eyes were described to have eccentric PCO or capsular plaques within 2 weeks of surgery and the PCO in the other two eyes of the same patient was described as capsular folds. The macular fibrosis first eye events included inner retina cystic spaces, internal limiting membrane wrinkling and epiretinal membrane events. One serious ocular AE of macular hole was reported, although this was assessed as unrelated to the IOL; the patient’s UCDVA in the right eye was 0.34 logMAR and BCDVA was 0.28 logMAR, which indicate reduced VA. One adverse device event of mild blurred vision was reported during the study. No patients required SSI (Table 3). Additionally, no device deficiencies were reported during the study (data not shown).
 |
Table 3 Adverse Events and Secondary Surgical Interventions (Safety Analysis Set) |
Contrast Sensitivity
Mean mesopic binocular contrast sensitivity with and without glare at 3 months was >1.5 log units at spatial frequencies of 1.5–6 cycles per degree (cpd), and the values were within the range set for the measuring device.35 As expected, only slight reductions were observed with glare as a function of spatial frequency (Figure 5).
 |
Figure 5 Mean binocular mesopic contrast sensitivity with or without glare (log units) (best-case analysis set). |
Discussion
Unlike traditional multifocal IOLs, which provide good near vision but may not function effectively at intermediate distances, TFNT00 is functionally a trifocal IOL that provides a continuous range of vision from near to intermediate to distance. TFNT00 is based on non-sequential diffractive optics, in which light has been redistributed to create a trifocal with an enhanced distance power, and +2.17 D intermediate (60 cm) and +3.25 D near (40 cm) add powers.14,16,18 Optimum visual quality at intermediate distance is crucial to performing many normal daily activities (eg, use of a computer, tablet, laptop, personal computer, mobile phone).18 Although other trifocal IOLs have an intermediate focal point of 80 cm,16 TFNT00 targets an intermediate focal point of 60 cm (relaxed arm’s length), which is a distance commonly associated with a more natural and comfortable distance to perform routine daily activities. In contrast, 80 cm is the approximate arm length of a person ∼205 cm (6 feet 8 inches) tall and thus represents a distance further away for the vast majority of patients to reach comfortably. This is of particular significance for Indian patients, for whom average male and female arm length is ~73 cm and ~65 cm,36 and average height is ~163 cm and ~151 cm,36–38 respectively. Furthermore, a viewing distance of ∼50–63 cm (20–25 inches) is recommended while performing tasks using digital screens.15,19,39,40
The current study assessed the effectiveness of TFNT00 in providing this continuous range of vision. In line with previous studies,14,18,24,25,27,28,41 TFNT00 exhibited a high level of distance, intermediate, and near VA. Mean binocular and monocular distance-corrected and uncorrected VAs of 0.1 logMAR or better were achieved at distance (4 m), intermediate (60 cm), and near (40 cm). Overall, ≥70% of patients achieved binocular 0.1 logMAR vision or better across all distances. VA results were supported by the outcome of the binocular defocus curve for TFNT00, which showed that the lens provided consistently excellent vision of approximately 0.1 logMAR (approximately 20/25 Snellen) or better between +0.50 and −2.50 D, or from distance to near. In the range of intermediate vision (between 60 cm and 80 cm), the defocus curve was flat, suggesting stable intermediate vision is provided by TFNT00. Overall, this study shows that the Indian population had similar visual outcomes of ≤0.1 logMAR at all distances to those of patients in other comparative and non-comparative studies who received TFNT00 with a 3-month follow-up period.14,27,41–44 Patient satisfaction with the TFNT00 visual outcomes was reflected in the subjective symptom questionnaire results, with 66 out of 67 patients (98.5%) being “satisfied” or “very satisfied” with their near, intermediate, and distance vision. In this study, TFNT00 recipients could engage in activities that required a range of vision while being spectacle-free.
Previous clinical comparative investigations have demonstrated improved intermediate (60 cm) and near visual outcomes for TFNT00, compared to earlier-generation trifocal IOLs. TFNT00 showed improved VA at 60 cm versus FineVision Micro F (PhysIOL SA) trifocal IOL (p <0.05).18 Furthermore, VA at the preferred reading distance (~42 cm) was 0.07 ± 0.07 and 0.11 ± 0.08 logMAR for TFNT00 and Micro F, respectively (p = 0.04).18 Defocus curve data reported by de Carneros Llorente et al45 for TFNT00, Micro F, and AT LISA 839MP (Carl Zeiss Meditec) trifocal IOLs demonstrated significantly improved VA for patients who received TFNT00 compared with recipients of the other two trifocal IOLs at defocus levels of –1.50 D and –2.00 D (p ≤0.04). In a large multicenter trial, binocular UCIVA and UCNVA were better for TFNT00 recipients compared with 839MP recipients (p = 0.002 and p = 0.003, respectively) 6 months after IOL implantation.23 Additionally, defocus curve VA was improved with TFNT00 versus 839MP at 1.00 D, –1.00 D, –1.50 D, –2.00 D, and –2.50 D.46 In line with these findings, in a non-comparative study, Kohnen et al14 found that TFNT00 provided better VA results between 50 cm and 60 cm than previous outcomes with other trifocal IOLs. However, additional head-to-head studies would be needed to demonstrate such a benefit of TFNT00 in Indian patients.
Previous studies demonstrated a good safety profile for TFNT00, which was further supported by the results of this study.47,48 No patients discontinued the study as a result of an AE, and no SSIs were reported. Furthermore, no eyes underwent Nd:YAG laser posterior capsulotomy during the study; however, three first eyes and one second eye of 3 patients displayed clinically significant PCO by the end of the study, of which two first eyes were assessed to require Nd:YAG laser posterior capsulotomy. Of these four eyes with clinically significant PCO, two eyes were described to have eccentric PCO or capsular plaques within 2 weeks of surgery and the PCO in the other two eyes of the same patient was described as capsular folds. The rates of PCO were higher than those reported in previous comparative 3-month studies of the same lens,42,43 although they were lower than or similar to those reported in comparative and non-comparative 6-month studies.23,28,46,49 Importantly, PCO did not affect patient satisfaction; all but one patient in the study indicated that they were “satisfied” or “very satisfied” with their cataract surgery results (the other patient was “neither satisfied nor dissatisfied”).
Diminished contrast sensitivity has long been a limitation of some multifocal IOL designs, because light from the out-of-focus image may reduce the sharpness of the in-focus image.1,25,50 This effect has been described at multiple spatial frequencies, with and/or withoutglare.1,3,5,50 In this study, mean mesopic binocular contrast sensitivity with and without glare at 3 months was >1.5 log units at spatial frequencies of 1.5 to 6 cpd and within the range set for the measuring device for normal patients of a similar age.35 As expected, only slight clinically insignificant reductions were observed with glare as a function of spatial frequency, representing good quality of vision with TFNT00. These results are in agreement with previous studies that have demonstrated good contrast sensitivity outcomes in TFNT00 recipients.25,51,52 These contrast sensitivity results may be attributable to the TFNT00 IOL design; the higher energy utilization of TFNT00 (up to 88%) than other trifocals (85–86%), and a smaller diffractive zone (4.5 mm and 6.0 mm, respectively), are suggested to result in functional vision that is less dependent on pupil size or lighting conditions and provides better contrast sensitivity.13–15,25,49 These design features could also explain the increased proportion of patients who reported no difficulty driving at night in this study (88.6%).
Increased incidence of visual disturbances, which consist of photic phenomena such as halos, is a common outcome of diffractive multifocalIOLs.2,53 In this study, the majority of TFNT00 recipients (86.6%) experienced halos from “none of the time” to only “some of the time” postoperatively; additionally, only one patient reported their halos as severe. Furthermore, no SSIs were required because of visual disturbances. The low reported frequency and severity of visual disturbances may be due to the design of the TFNT00 lens.18 In the current study, visual disturbances were assessed using a subjective symptom questionnaire, consisting of items derived from the APPLES32 questionnaire. Rasch-based patient-reported outcome measures questionnaires, however, may better capture the subjective patient experience and could facilitate cross-study comparisons.31 Further studies would be needed to assess the perception of additional visual disturbances associated with diffractive multifocal IOLs, such as glare and starbursts, which would help further characterize the quality of vision following TFNT00 implantation.54 Additionally, objective testing methods using straylight meters, halometry, or computer-based simulators could be used to capture supportive quantitative metrics.55
Limitations of the present study include a relatively short follow-up period of 3 months and the lack of a comparator IOL. Future trials should evaluate the long-term outcomes and patient satisfaction with TFNT00 in the Indian population compared with other multifocal lens options for the correction of presbyopia. An additional consideration in this study is that non-toric TFNT00 IOLs were implanted in patients displaying up to 1 D of astigmatism, which may explain the slightly low values for uncorrected VAs, especially for patients with against-the-rule astigmatism. For this patient population, the toric TFNT00 IOL may provide better VA outcomes, although studies need to be conducted to support this hypothesis.
Conclusion
This study has demonstrated that TFNT00 provides excellent vision of 0.1 logMAR (20/25) or better across a range of distances, with a favorable safety profile, and high patient satisfaction. Overall, the diffractive trifocal IOL TFNT00 may provide this population with a continuous range of vision, resulting in spectacle independence over a range of distance to near tasks.
Abbreviations
AE, adverse event; APPLES, Assessment of Photic Phenomena and Lens EffectS; BCDVA, best-corrected distance visual acuity; cpd, cycles per degree; D, diopters; DCIVA, distance-corrected intermediate visual acuity; DCNVA, distance-corrected near visual acuity; IOL, intraocular lens; MedDRA, Medical Dictionary for Regulatory Activities; Nd:YAG, neodymium-doped yttrium aluminum garnet; PCO, posterior capsular opacification; SD, standard deviation; SSI, secondary surgical intervention; SILVER, Spectacle Independence Lens Vision Evaluation and Repurchase; UCDVA, uncorrected distance visual acuity; UCIVA, uncorrected intermediate visual acuity; UCNVA, uncorrected near visual acuity; VA, visual acuity.
Data Sharing Statement
No further data beyond those provided in this manuscript will be shared.
Acknowledgments
The authors thank Aarti Shah for editorial assistance in the preparation of the manuscript, with funding from Alcon Research LLC.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was sponsored by Alcon Research LLC, Fort Worth, Texas. The sponsors participated in the design of the study, data management, data analysis, interpretation of the data, preparation, review, and approval of the manuscript.
Disclosure
Dr Dandapani Ramamurthy reports company-sponsored prospective multicentric study for ALCON, during the conduct of the study; and consultancy for Alcon, outside the submitted work. Dr Abhay Vasavada reports research grant support from Alcon Laboratories, outside the submitted work. Dr Arindam Dey is an employee of Alcon (Alcon Laboratories (India) Private Ltd). The authors report no other conflicts of interest in this work.
References
1. Wang SY, Stem MS, Oren G, Shtein R, Lichter PR. Patient-centered and visual quality outcomes of premium cataract surgery: a systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi:10.5301/ejo.5000978
2. Javitt J, Brauweiler HP, Jacobi KW, et al. Cataract extraction with multifocal intraocular lens implantation: clinical, functional, and quality-of-life outcomes. Multicenter clinical trial in Germany and Austria. J Cataract Refract Surg. 2000;26(9):1356–1366. doi:10.1016/S0886-3350(00)00636-2
3. Zhao G, Zhang J, Zhou Y, Hu L, Che C, Jiang N. Visual function after monocular implantation of apodized diffractive multifocal or single-piece monofocal intraocular lens randomized prospective comparison. J Cataract Refract Surg. 2010;36(2):282–285. doi:10.1016/j.jcrs.2009.08.037
4. de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:CD003169. doi:10.1002/14651858.CD003169.pub4
5. Vingolo EM, Grenga P, Iacobelli L, Grenga R. Visual acuity and contrast sensitivity: AcrySof ReSTOR apodized diffractive versus AcrySof SA60AT monofocal intraocular lenses. J Cataract Refract Surg. 2007;33(7):1244–1247. doi:10.1016/j.jcrs.2007.03.052
6. Kohnen T, Allen D, Boureau C, et al. European multicenter study of the AcrySof ReSTOR apodized diffractive intraocular lens. Ophthalmology. 2006;113(4):578–584. doi:10.1016/j.ophtha.2005.11.020
7. Cillino S, Casuccio A, Di Pace F, et al. One-year outcomes with new-generation multifocal intraocular lenses. Ophthalmology. 2008;115(9):1508–1516. doi:10.1016/j.ophtha.2008.04.017
8. Zeng M, Liu Y, Liu X, et al. Aberration and contrast sensitivity comparison of aspherical and monofocal and multifocal intraocular lens eyes. Clin Exp Ophthalmol. 2007;35(4):355–360. doi:10.1111/j.1442-9071.2007.01452.x
9. Alfonso JF, Fernández-Vega L, Amhaz H, Montes-Mico R, Valcarcel B, Ferrer-Blasco T. Visual function after implantation of an aspheric bifocal intraocular lens. J Cataract Refract Surg. 2009;35(5):885–892. doi:10.1016/j.jcrs.2009.01.014
10. Alfonso JF, Fernández-Vega L, Baamonde MB, Montés-Micó R. Prospective visual evaluation of apodized diffractive intraocular lenses. J Cataract Refract Surg. 2007;33(7):1235–1243. doi:10.1016/j.jcrs.2007.03.034
11. Cochener B, Vryghem J, Rozot P, et al. Clinical outcomes with a trifocal intraocular lens: a multicenter study. J Refract Surg. 2014;30(11):762–768. doi:10.3928/1081597X-20141021-08
12. Mojzis P, Peña-García P, Liehneova I, Ziak P, Alió JL. Outcomes of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2014;40(1):60–69. doi:10.1016/j.jcrs.2013.06.025
13. Kohnen T. First implantation of a diffractive quadrafocal (trifocal) intraocular lens. J Cataract Refract Surg. 2015;41(10):2330–2332. doi:10.1016/j.jcrs.2015.11.012
14. Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62. doi:10.1016/j.ajo.2017.09.016
15. Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySof IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: a clinical overview. Asia Pac J Ophthalmol (Phila). 2019;8(4):335–349. doi:10.1097/APO.0000000000000253
16. Carson D, Xu Z, Alexander E, Choi M, Zhao Z, Hong X. Optical bench performance of 3 trifocal intraocular lenses. J Cataract Refract Surg. 2016;42(9):1361–1367. doi:10.1016/j.jcrs.2016.06.036
17. Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507–514. doi:10.3928/1081597X-20180530-02
18. Gundersen KG, Potvin R. Trifocal intraocular lenses: a comparison of the visual performance and quality of vision provided by two different lens designs. Clin Ophthalmol. 2017;11:1081–1087. doi:10.2147/OPTH.S136164
19. American Optometric Association. Computer vision syndrome; 2015. Available from: https://www.aoa.org/patients-and-public/caring-for-your-vision/protecting-your-vision/computer-vision-syndrome.
20. Aravind S, Haripriya A, Sumara Taranum BS. Cataract surgery and intraocular lens manufacturing in India. Curr Opin Ophthalmol. 2008;19(1):60–65. doi:10.1097/ICU.0b013e3282f2aaed
21. Murthy G, Gupta SK, John N, Vashist P. Current status of cataract blindness and vision 2020: the right to sight initiative in India. Indian J Ophthalmol. 2008;56(6):48994. doi:10.4103/0301-4738.42774
22. Reddy JC, Vaddavalli PK, Sharma N, et al. A new normal with cataract surgery during COVID-19 pandemic. Indian J Ophthalmol. 2020;68(7):1269. doi:10.4103/ijo.IJO_1528_20
23. Lapid-Gortzak R, Bhatt U, Sanchez JG, et al. Multicenter visual outcomes comparison of 2 trifocal presbyopia-correcting IOLs: 6-month postoperative results. J Cataract Refract Surg. 2020;46(11):1534–1542. doi:10.1097/j.jcrs.0000000000000274
24. Lawless M, Hodge C, Reich J, et al. Visual and refractive outcomes following implantation of a new trifocal intraocular lens. Eye Vis (Lond). 2017;4(1):10. doi:10.1186/s40662-017-0076-8
25. García-Pérez JL, Gros-Otero J, Sánchez-Ramos C, Blázquez V, Contreras I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017;17(1):72. doi:10.1186/s12886-017-0462-y
26. Alfonso JF, Fernández-Vega-Cueto L, Fernández-Vega L, Montés-Micó R. Visual function after implantation of a presbyopia-correcting trifocal intraocular lens. Ophthalmic Res. 2020;63(2):152–164. doi:10.1159/000500834
27. Rementería-Capelo LA, Contreras I, García-Pérez JL, Blázquez V, Ruiz-Alcocer J. Visual quality and patient satisfaction with a trifocal intraocular lens and its new toric version. J Cataract Refract Surg. 2019;45(11):1584–1590. doi:10.1016/j.jcrs.2019.06.014
28. Donmez O, Asena BS, Kaskaloglu M, Akova YA. Patients satisfaction and clinical outcomes of binocular implantation of a new trifocal intraocular lens. Int Ophthalmol. 2020;40(5):1069–1075. doi:10.1007/s10792-020-01390-9
29. Barrett GD. An improved universal theoretical formula for intraocular lens power prediction. J Cataract Refract Surg. 1993;19(6):713–720. doi:10.1016/S0886-3350(13)80339-2
30. Alcon Research LLC. Clinical investigation of AcrySof® IQ ReSTOR® +2.5 D multifocal intraocular lens (IOL) Model SN6AD2 [SV25T0]. Available from: https://clinicaltrials.gov/ct2/show/NCT01510717.
31. Grzybowski A, Kanclerz P, Muzyka-Wozniak M. Methods for evaluating quality of life and vision in patients undergoing lens refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2019;257(6):1091–1099. doi:10.1007/s00417-019-04270-w
32. Maxwell A, Holland E, Cibik L, et al. Clinical and patient-reported outcomes of bilateral implantation of a +2.5 diopter multifocal intraocular lens. J Cataract Refract Surg. 2017;43(1):29–41. doi:10.1016/j.jcrs.2016.10.026
33. Alcon Research LLC. Acrysof® ReSTOR® aspheric +3.0 D add power intraocular lens (IOL). Available from: https://clinicaltrials.gov/ct2/show/NCT00684138.
34. Alcon Research LLC. AcrySof® IQ ReSTOR® +2.5 D Multifocal Intraocular Lens (IOL) (Model SV25T0) Product Information. 2015.
35. VectorVision Ocular Health. Normal contrast sensitivity values for CSV-1000; 2020. Available from: http://www.vectorvision.com/csv1000-norms/.
36. Kamal R, Yadav PK. Estimation of stature from different anthropometric measurements in Kori population of North India. Egypt J Forensic Sci. 2016;6(4):468–477. doi:10.1016/j.ejfs.2016.12.001
37. Kim R, Pathak PK, Tripathi N, Subramanian S. Heterogeneity in adult anthropometry by socioeconomic factors: indian national family health survey 2006 and 2016. Eur J Clin Nutr. 2020;74(6):953–960. doi:10.1038/s41430-019-0511-0
38. Goswami AK, Kalaivani M, Gupta SK, Nongkynrih B, Pandav CS. Relationship between height and arm span of elderly persons in an urban colony of New Delhi. Indian J Public Health. 2018;62(2):159–162. doi:10.4103/ijph.IJPH_378_16
39. Charness N, Dijkstra K, Jastrzembski T, Weaver S, Champion M. Monitor viewing distance for younger and older workers.
40. Turgut B. Ocular ergonomics for the computer vision syndrome. J Eye Vis. 2018;1(1):2.
41. Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi:10.1007/s00417-018-4052-3
42. Böhm M, Hemkeppler E, Herzog M, et al. Comparison of a panfocal and trifocal diffractive intraocular lens after femtosecond laser-assisted lens surgery. J Cataract Refract Surg. 2018;44(12):1454–1462. doi:10.1016/j.jcrs.2018.07.060
43. Böhm M, Petermann K, Hemkeppler E, Kohnen T. Defocus curves of 4 presbyopia-correcting IOL designs: diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J Cataract Refract Surg. 2019;45(11):1625–1636. doi:10.1016/j.jcrs.2019.07.014
44. Ribeiro F, Ferreira TB. Comparison of clinical outcomes of 3 trifocal IOLs. J Cataract Refract Surg. 2020;46(9):1247–1252. doi:10.1097/j.jcrs.0000000000000118
45. de Carneros-llorente AM, de Carneros AM, de Carneros-llorente PM, Jiménez-Alfaro I. Comparison of visual quality and subjective outcomes among 3 trifocal intraocular lenses and 1 bifocal intraocular lens. J Cataract Refract Surg. 2019;45(5):587–594. doi:10.1016/j.jcrs.2018.12.005
46. Asena BS. Visual and refractive outcomes, spectacle independence, and visual disturbances after cataract or refractive lens exchange surgery: comparison of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1539–1546. doi:10.1016/j.jcrs.2019.06.005
47. Alcon Research LLC. Investigation of AcrySof® IQ PanOptix™ presbyopia-correcting intraocular lens (IOL) model TFNT00; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT02529488.
48. Alcon Research LLC. AcrySof® IQ PanOptix® Trifocal IOL model TFNT00 directions for use; 2019. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf4/P040020S087D.pdf.
49. Modi S, Lehmann R, Maxwell A, et al. Visual and patient-reported outcomes of a diffractive trifocal intraocular lens compared with those of a monofocal intraocular lens. Ophthalmology. 2020. doi:10.1016/j.ophtha.2020.07.015
50. Cao K, Friedman DS, Jin S, et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Surv Ophthalmol. 2019;64(5):647–658. doi:10.1016/j.survophthal.2019.02.012
51. Vilar C, Hida WT, de Medeiros AL, et al. Comparison between bilateral implantation of a trifocal intraocular lens and blended implantation of two bifocal intraocular lenses. Clin Ophthalmol. 2017;11:1393–1397. doi:10.2147/OPTH.S139909
52. Alió JL, Plaza-Puche AB, Alio Del Barrio JL, et al. Clinical outcomes with a diffractive trifocal intraocular lens. Eur J Ophthalmol. 2018;28(4):419–424. doi:10.1177/1120672118762231
53. Escandón-García S, Ribeiro F, McAlinden C, Queirós A, González-Méijome JM. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. 2018;2018:1–8. doi:10.1155/2018/6165493
54. Almulhim AK, Alarfaj KM, Altaisan AA, Alromaih AZ, Aldawod RA. Visual outcomes and patient satisfaction after bilateral implantation of a new trifocal diffractive intraocular lens. Saudi J Ophthalmol. 2018;32(4):310–317. doi:10.1016/j.sjopt.2018.08.004
55. Kohnen T, Suryakumar R. Measures of visual disturbance in patients receiving extended depth-of-focus or trifocal intraocular lenses. J Cataract Refract Surg. 2020. doi:10.1097/j.jcrs.0000000000000364
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


