Back to Journals » International Journal of General Medicine » Volume 14
Clinical Outcome of Acquired Post-Immunosuppressive-Therapy Aplastic Anemia in Pediatric Patients: A 13-Year Experience in Two Southern China Tertiary Care Centers
Authors Huang J, Huang L, Liu S, Lin S, Cheng Y, Jiang X, Xue H, Li C, Chen C
Received 15 April 2021
Accepted for publication 14 June 2021
Published 2 July 2021 Volume 2021:14 Pages 3133—3144
DOI https://doi.org/10.2147/IJGM.S313898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Junbin Huang,1,* Lifen Huang,1,* Su Liu,2 Shaofen Lin,2 Yucai Cheng,1 Xiaoyun Jiang,3 Hongman Xue,1 Chikong Li,4 Chun Chen1
1Division of Hematology/Oncology, Department of Pediatrics, The Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, 518107, People’s Republic of China; 2Department of Pediatrics, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China; 3Department of Pediatrics, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, 518000, People’s Republic of China; 4Division of Haematology/Oncology/BMT, Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong, 999077, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chun Chen
Division of Hematology/Oncology, Department of Pediatrics, The Seventh Affiliated Hospital, Sun Yat-sen University, No. 628 Zhenyuan Road, Guangming District, Shenzhen, 518107, People’s Republic of China
Tel +86 13719003063
Email [email protected]
Chikong Li
Division of Haematology/Oncology/BMT, Department of Paediatrics, The Chinese University of Hong Kong, 30-32 Ngan Shing Street, Shatin, New Territories, Hong Kong, 999077, People’s Republic of China
Tel +86 852-9359 0525
Email [email protected]
Objective: The aim of the present study is to evaluate the efficacy, complications, and contributing factors of immunosuppressive therapy (IST) response in children with acquired aplastic anemia (AA) and to explore optimal therapeutic methods for different clinical AA types.
Methods: A total of 130 children diagnosed with acquired AA underwent IST in the Department of Pediatrics at Sun Yat-sen Memorial Hospital and the Department of Pediatrics at Seventh Affiliated Hospital, Sun Yat-sen University, between January 1, 2006, and July 15, 2020. The overall survival (OS), response rates, complications, and response predictors were analyzed. The response rates were compared according to clinical AA type.
Results: All 130 children with AA were followed up with for a median of 50.6 months. Among the patients, 25 had non-severe AA (NSAA), 64 had severe AA (SAA), and 41 had very severe AA (VSAA). All patients initially received IST. In 13 patients, the IST failed; these patients received an allo-hematopoietic stem cell transplant as a salvage regimen. The OS rate was 90.3% ± 2.8%, and the response rates at 3, 6, 9, and 12 months were 34.19%, 39.32%, 49.57%, and 66.67%, respectively. The prolonged follow-up period might have led to higher response rates, especially in patients with SAA and VSAA. A multivariate logistic regression analysis of prognostic factors was conducted; the results showed that high red blood cell (RBC) and platelet (PLT) counts were associated with a high overall response rate and that the RBC count at diagnosis is a major contributing factor.
Conclusion: With the use of rabbit anti-thymocyte globulin, proper cyclosporine management, and a prolonged IST follow-up period, a higher number of patients with acquired AA than normal achieved response. Proportionally, the number of patients who achieved remission within 12 months was higher in the SAA group (38.18%→ 63.64%) and VSAA group (28.95%→ 65.79%) than in the NSAA group (58.33%→ 75%). Higher RBC and PLT counts at diagnosis can predict a favorable outcome.
Keywords: pediatric, aplastic anemia, immunosuppressive therapy, predicting factor
Introduction
Aplastic anemia (AA) is presented as peripheral blood and hypocellular bone marrow bicytopenia or pancytopenia in the absence of abnormal infiltration. The disease affects approximately 2/1,000,000 population members per year in western countries; in Asia, this rate is two or three times higher.1 Major AA clinical features include persistent anemia and bleeding. Although the precise AA etiology remains unknown, most researchers currently believe that dysfunctional immune regulation might play a central role in the disease’s development.2 Hematopoietic stem cell transplantation (HSCT) with well-matched sibling donors (MSDs) can achieve an 80–90% long-term survival; for this reason, it is considered a first-line treatment in certain countries.3–5 However, challenges have arisen in China regarding this method. These challenges are: (1) a lack of MSDs due to China’s “one-child policy”; (2) a relatively high transplant-related mortality; and (3) high hospital expenses and low insurance coverage. In China, immunosuppressive therapy (IST) based on the use of cyclosporine (CsA) and anti-thymocyte globulin (ATG) is preferable in patients without MSDs or with transfusion-dependent non-severe AA (NSAA), as IST efficacy is comparable with HSCT, is financially affordable, and has a lower mortality rate and good accessibility.2,6–9
In the past, several factors can affect the response rate to IST. The preparation of ATG could significantly affect the IST response rate. Since horse-ATG (hATG) has shown superiority over rabbit-ATG (rATG), ATG preparation could significantly affect the IST response rate.2 Different reports have shown inconsistent, even contradictory, results regarding other factors, such as age, absolute neutrophil count (ANC), absolute reticulocyte count (ARC), and the time needed to receive treatment;10–14 these data are primarily based on reports on adult patients with limited rATG data. However, there are few cases of available data addressing the pediatric population with AA in terms of response rate, response time, and the disease’s predictive factors.
Thus, the present retrospective study analyzes the clinical data of children with acquired AA in two tertiary care centers in order to evaluate IST efficacy, complications, and contributing factors.
Materials and Methods
Patients
A total of 130 pediatric patients with acquired AA who were admitted to the Seventh Affiliated Hospital, Sun Yat-sen University, Shenzhen, and the Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China, between January 1, 2006, and July 15, 2020, were recruited for the present study. All patients received IST with ATG and CsA, and 13 patients received HSCT after IST response failure.
Inclusion Criteria
AA definition: (1) patients with (at least) bicytopenia: (2) patients with a hemoglobin (Hb) level of <10 g/dL, PLT count of <50 × 109/L, and ANC of <1.5 × 109/L; and (3) patients with hypocellular bone marrow determined via bone marrow biopsy.15
The enrolled patients were ineligible for an HLA–identical sibling donor bone marrow transplant, or their guardians chose the IST method due to social or economic factors.
Exclusion Criteria
(1) Patients who had undergone ATG or CsA therapy; (2) patients with a genetic diagnosis of Fanconi anemia, dyskeratosis congenita, or another congenital bone marrow failure syndrome; (3) patients with evidence of myelodysplastic syndrome confirmed by fluorescence in situ hybridization or a bone marrow smear; (4) patients with paroxysmal nocturnal hemoglobinuria (PNH) with evidence of significant hemolysis, a history of PNH-associated thrombosis, or a PNH clone of >50% detected using flow cytometry; (5) patients with a diagnosis or previous history of malignancy; and (6) patients with severe uncontrolled infection, sepsis, or bacteremia.
AA Severity
In the present study, AA (VSAA), severe AA (SAA), and NSAA were defined in accordance with the standard criteria.15
Method
BD FACSCalibur (BD Biosciences, USA) was used for the flow cytometry analysis, and the Sysmex system (Sysmex, Kobe, Japan) was used for the determination of routine hematologic parameters; all procedures were conducted in accordance with their respective user manuals.
Ethics Approval
The present study was approved by the local ethics committees of the Sun-Yat Sen Memorial Hospital and the Seventh Affiliated Hospital, Sun-Yat Sen University. This study was conducted in accordance with the declaration of Helsinki. The parent or legal guardian of the pediatric patients provided informed consent.
Treatment
All 130 patients received ATG and CsA as part of the IST treatment. The rATG (thymoglobulin, Genzyme) was given intravenously through a central venous line at 2.5×3.5 mg/kg·d for at least eight hours for five consecutive days. Meanwhile, CsA was taken orally, first at 3–5 mg/kg/d, then adjusted and maintained at a 100–150 ng/mL trough concentration according to local lab references. The CsA administration was started for at least 6 months directly after the ATG regimen. The CsA trough blood concentration held for 3 months and was followed by slow tapering until discontinuation. Methylprednisolone (Pfizer Manufacturing Belgium NV), along with ATG allergic reaction prevention, was given intravenously at 2 mg/kg for five days. Next, methylprednisolone tablets (Pfizer Italia Srl) were given orally at 1 mg/kg·d for the next nine days and tapered for another week. An Ibuprofen suspension (Shanghai Johnson & Johnson Pharmaceuticals) and loratadine (Shanghai Schering-Plough Pharmaceutical) were administered orally before ATG every day.
Supportive and Alternative Treatments
(1) A compound Zaofan tablet (traditional Chinese medicine, Shaan Xi Haoqijun Pharmaceutical) and Zai Zao Sheng Xue tablet (traditional Chinese medicine, Liaoyuan Yulong Yadong Pharmaceutical Co., Ltd) were administered orally in several patients throughout the IST treatment. (2) The recombinant human granulocyte colony-stimulating factor at 5 ug/kg·d (Qilu Pharmaceutical or Amoytop Biotech) was given to patients with persistent neutropenia or fever. (3) Supportive care: When complete blood count show hemoglobin (Hb) was <60 g/dL during the ATG treatment, Red blood cell (RBC) transfusion was initiated. When the CBC showed a PLT count of <20 × 109/L during the ATG treatment, PLT transfusion was initiated. Antibiotics and anti-fungal treatment were given in the case of infection. Patient liver function was closely monitored every 1–2 weeks, and compound glycyrrhizin, glutathione, or polyene phosphatidylcholine was given as an adjuvant treatment for elevated liver enzymes.
Responsiveness Determination
IST efficacy was evaluated in accordance with Camitta’s criteria:15
- Complete response (CR): patients had a normal Hb for their age, an ANC of >1.5 × 109/L, and PLT count of >150 × 109/L.
- Partial response (PR): patients achieved ANC >0.5×109/L, Hgb >80g/L, platelet count >20 × 109/L and transfusion independent.
- No response: the patient blood count was still severe or had worsened.
- Relapse: patients experienced a reversal to transfusion dependence after PR or CR.
- Response: Both CR and PR were considered as response status.
Adverse Events
Adverse events after IST, especially ATG, included serum sickness, infection tendency, hypertension, elevated liver enzyme, and impaired renal function. Methylprednisolone (1–2 mg/kg·d) was given in prophylactic serum sickness treatment and tapered for at least two weeks.
Follow-Up
All patients were followed up with until July 15, 2020; the median follow-up time was 50.6 months (1–166 months). The peripheral blood cell count, CsA concentration, and infection events were recorded every 2–4 weeks, while the regulatory T cell (Treg) ratio, transfusion dependence, serum sickness, relapse, secondary malignancy, and death were evaluated every 1–2 months.
Statistical Analysis
All results were analyzed using the Statistical Package for Social Sciences (SPSS) 20.0 software (SPSS, version 20.0, Chicago, IL). All statistical tests in the present study were two-sided. A p value of <0.05 was considered statistically significant. The normal distribution measurement data were described as the mean ± standard deviation, and the count data were expressed using percentage (%). The survival analysis was conducted using the Kaplan–Meier curve, the Student’s t-test was used for continuous variables, and the receiver operating characteristic (ROC) curve was performed to determine cutoff points. Furthermore, univariate analysis and multivariate logistic regression analysis were performed via step-down selection to determine which factors were responsible for the IST response rate.
Results
Patient Characteristics and Events
All patients received standard IST with rATG and CsA. Moreover, 13 patients who failed to achieve at least PR underwent HSCT (MSD or well-matched unrelated donor [WMUD]); none of the patients received a second ATG treatment dose. A total of 41 VSAA, 64 SAA, and 25 NSAA cases with transfusion dependency were enrolled in the study. The CBC profiles at diagnosis were as follows: (1) a white blood cell count of 0.47–11.90 × 109/L; (2) neutrophil granulocyte (NEUT) count of 0.01–2.82 × 109/L; (3) lymphocyte (LY) count 0.11–11.34 × 109/L; (4) NEUT% of 0.7%–71.9%; (5) LY% of 9%–99.3%; (6) RBC count of 0.96–4.37 × 1012/L; (7) Hb level of 28–135 g/L; (8) PLT level of 2–303 × 109/L; (9) reticulocyte (RET) level of 0–146.30 × 109/L; (10) RET% of 0%–71.05%; (11) granulocyte and erythrocyte ratios of 0–127; (12) natural killer cell/LY counts of 0.2%–32.6%; (13) B cell/LY counts of 2.8–50.8; (14) T cell/LY counts of 43–93.4; (15) CD8+ T cell/LY counts of 10.1–51.9; (16) CD4+ T cell/LY counts of 7.4–64.4; (17) CD4+/CD8+ of 0.17–5.68; (18) Treg/LY counts of 0.68–5.68; and (19) Tregs/CD4+ T cell counts of 1.6–12.33. A total of 56 patients were eligible for these flow cytometry parameters (Table 1).
 |
Table 1 Characteristics and Events of AA Patients |
Survival Analysis
In all patients, the 5-year overall survival (OS) rate was 85.7% ± 4.0%, and the event-free survival rate was 71.5% ± 5.0% (Figure 1A and B). An event was defined as a transplant, death, relapse, or secondary malignancy. A total of 11 patients relapsed after IST within the 50.6-month median follow-up duration; 13 patients underwent HSCT (MSD or WMUD) at <6 months after IST and were not suitable for responder evaluation; 11 patients died of severe infection; 15 patients underwent blood transfusion therapy without further treatment due to a lack of matched unrelated donors (MUDs), low income, short follow-up, or personal choice; and 1 patient developed secondary acute myeloid leukemia (AML) in the second year after IST and refused chemotherapy.
 |
Figure 1 (A) 5-year OS was 85.7% ± 4.0% in all patients who underwent IST; and (B) the event-free survival rate was 76.0% ± 4.6% in the medium 50.6-month follow up. |
In the subgroup analysis, the 5-year OS rates in the NSAA group, SAA group, and VSAA group were 100%, 90.5% ± 4.1%, and 84.0% ± 6.0%, respectively (Figure 2A). The only significant 5-year OS differences between the groups were between the NSAA group and the VSAA group (p = 0.001) and between the NSAA group and the SAA group (p > 0.05). The difference between the SAA group and the VSAA group was not statistically significant (p > 0.05). Upon exclusion of patients who underwent transplants, the event-free survival rates in the NSAA group, SAA group, and VSAA group were 94.4% ± 5.4%, 71.2% ± 7.1%, and 71.0% ± 9.6%, respectively (Figure 2B). There were significant differences between the NSAA group and the VSAA group (p = 0.035) as well as between the NSAA group and the SAA group (p = 0.026); however, there were no significant differences between the SAA group and the VSAA group (p > 0.05).
Time to IST Response
Excluding 13 patients who received HSCT less than 6 months after IST and were not suitable for responder evaluation, the study included 117 eligible cases for the accumulated response rate comparison at different time points. In all eligible patients, the overall response rates (ORRs) at 3, 6, 9, and 12 months were 34.19%, 39.32%, 49.57%, and 66.67%, respectively (Table 2); significant differences in the NSAA group, SAA group, and VSAA group response rates only showed at the 3-month evaluation. With time, the ORRs continuously improved in all groups; there were no significant differences at the 6-month, 9-month, and 12-month evaluations (Table 2). Moreover, 1 patient in the NSAA group and 1 patient in the SAA group separately achieved an overall response (OR) after 12 months.
 |
Table 2 Different Time Point for Evaluation of IST |
Response Group and Non-Response Group Comparison
The 117 patients who only received ATG and CsA were divided into two groups: the response (OR) group and the non-response group. At diagnosis, clinical parameters and laboratory data, such as age, gender, severity, treatment era, duration from diagnosis to IST, peripheral blood count, bone marrow biopsy, flow cytometry parameters (only 56 eligible patients), and adverse reactions, were collected for further analysis.
The results of the study show that a higher RBC count (p = 0.004), Hb (p = 0.023), PLT count (p = 0.017), and Treg/LY ratio (p = 0.004) had a significant impact on ORR; the factors were capable of improving the ORR. No significant difference was found between the groups regarding other factors (Table 3).
 |
Table 3 Comparison of Clinical Features and Lab Data Between Response and Non-Response Groups |
ROC Curve
The ROC curve was performed to determine cutoff points. The contributing factors of an RBC count of >2.735 × 1012/L (p = 0.007), Hb level of >77.5 g/L (p = 0.024), and a PLT count of >18.5 × 109/L (p = 0.03) were statistically significant (Table 4). The area under the ROC curve for each factor is shown in Figure 3. As only 56 patients had a Treg/LY ratio profile, the ROC curve for this factor was not included.
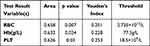 |
Table 4 Receiver Operating Characteristic Curve Analysis, Optimum Cutoff Value and Predict Performance for Factors Affecting Overall Response to IST |
 |
Figure 3 The ROC curve analyzed the correlations of the RBC count, Hb level, and PLT count with the patient IST response. |
Univariate Logistic Regression Analysis
A univariate logistic regression analysis was performed in order to determine which factors were responsible for the IST response rate. The IST response status was considered the dependent variable, and the RBC count, Hb level, and PLT count were considered variables. The logistic regression analysis showed that the RBC count (p = 0.005), Hb level (p = 0.017), and PLT count (p = 0.013) variables were significantly affecting the IST outcome (Table 5.) Since the RBC count and Hb were collinear factors, only the RBC count was used for further analysis.
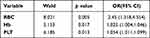 |
Table 5 Contributing Factors for Overall Response to IST: Univariate Analysis |
Multivariate Logistic Regression Analysis
A multivariate logistic regression analysis was performed via step-down selection in order to determine which factors were responsible for the 12-month IST ORR. Validated variables from the univariate logistic regression analysis were chosen for step-down selection. The results showed that higher RBC and PLT counts at diagnosis co-affected the 12-month IST ORR and that the effects of the RBC count were more powerful. The IST OR probability was significantly higher in patients with a higher RBC count ([X1], OR = 2.11, p = 0.025) and PLT count (X2, OR = 1.045, p = 0.05), both calculated at diagnosis (Table 6). The regression equation was established as: logit (p) = −1.674 + 0.749×1 + 0.044×2.
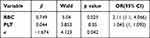 |
Table 6 Contributing Factors for Overall Response to IST: Multivariate Logistic Regression Analysis (Backward, Wald, α = 0.05) |
Discussion
AA is an immune-mediated disorder of bone marrow failure;7 the current cure methods include IST and HSCT. With the in-depth study of the pathogeneses and improvement of supportive care in recent decades, the 5-year IST OS rate in children with AA has reached approximately 90%. Furthermore, approximately 42%–74% of patients obtain complete hematological remission. In Western countries, hATG is widely used in treatment; it seems to be the preferred ATG preparation choice. Due to the limited hATG source, the use of rATG has become the main preparation method in Asian countries, including China. Several studies have shown that the efficacy of the two ATGs is equivalent,2,6 while others have demonstrated that the effect of rATG is relatively poor.2,7–9 However, due to domestic ATG source limitations, rATG combined with CsA was selected for IST in the present study.
The disease-free survival rate of MSD HSCT for pediatric AA, which the first-line AA treatment, is over 90%.16,17 Meanwhile, despite the improvement in HSCT conditioning, along with the continuous enhancement of supportive care and decline of transplant-related mortality, the disease-free survival rate of MUD HSCT is still only 80%.17–19 Although the current HSCT improvements are inspiring, the method is difficult to carry out due to various reasons. For example, China’s long-term implementation of the “one-child policy” has led to a limitation in sibling donors. Additionally, high transplantation costs, the time-consuming process of matching and finding transplant donors, severe infections before transplantation, physical conditions too poor to tolerate transplantation, acute/chronic GVHD, and transplant-related mortality could make patients and their families reconsider treatment. Therefore, IST is still the first choice in most Chinese centers when there are no MSDs available. The therapeutic effects of IST suppress abnormal autoimmunity and restore autologous hematopoiesis from the remaining hematopoietic cells and microenvironment.20 However, the effectiveness of IST treatment is also affected by factors such as the patient’s original physical state, bone marrow hematopoietic microenvironment, and potential malignant clonal transformation. Moreover, there is a lack of reliable indicators for IST with rATG efficacy prediction in pediatric patients with AA. Hence, the present study retrospectively analyzed the efficacy and safety of rATG combined with CsA in the treatment of children with AA and summarized the efficacy of different clinical AA types in children, providing clinical evidence for the IST regimen in the treatment of such patients.
The 12-month IST ORR in the present study was reported at 85.7% ± 4.0%; this is superior to previous studies.10,21–24 The following explanations could be given for these results: the majority of patients included in the present study had NSAA or SAA, leading to a prolonged follow-up period. The results of the present study showed that IST could be an effective therapy for patients with AA. It should be noted that 1 patient developed secondary AML in the second year after IST, and that ATG might contribute to clone evolution.
In the present study, the ORRs at 3 and 6 months were 34.19% and 39.32%, respectively, and the total response rates at 9 and 12 months rose to 49.57% and 66.67%, respectively (Table 2). The efficacy rate was basically the same as the rate stated in previous reports at the 3-month and 6-month time points.15,16 Two versions of the British guidelines15,20 recommend HSCT in patients who fail to respond 3–6 months after IST with hATG. Moreover, the Chinese children’s acquired AA diagnosis and treatment guidelines25 recommend that either HSCT or a second IST should be administered in patients who fail IST treatment after 3–6 months. However, in the present study, a dramatic rise to 66.67% in respondents was observed at the 12-month time point. In other words, an additional 34 patients achieved response after 6 months of follow-up. Moreover, 1 patient in the NSAA group and 1 patient in the SAA group separately achieved response statuses after 12 months. In the subgroup analysis, the response rate of the SAA group and the VSAA group rose from 23.35% to 63.45% and from 28.95% to 65.79%, respectively; the NSAA group had the highest response rate at every time point. The total response rate of all patients with AA, especially in patients with SAA and VSAA, increased along with the follow-up time. Aside from the improved supportive care and antibiotic agents, the rATG pharmacokinetics played an important role in this outcome. Although guideline20 suggests that ATG (mostly hATG) often takes effect at an average of 3–4 months after IST, Xie et al26 showed that rATG can still be detected in peripheral blood 90 days after administration. Feng et al.27,28 found that, compared with hATG, rATG can maintain a higher and more durable drug concentration in plasma, activate a higher number of CD4+, CD3+, CD4–, and CD8– T cells as well as CD19+ B cells, and promote the expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. It has a strong clearance effect on LYs, leading to a lower absolute number of LYs, especially CD4+ T cells. Moreover, rATG cannot compromise the number of Tregs; it can even induce peripheral expansion and new thymic emigration of T cells with a Treg phenotype.29 However, our CsA administration management might have played an important role in allowing Treg development and obtaining a profound immunosuppressive effect. In previous studies,8,9 higher CsA target trough blood (TBC) levels (150–250 ng/mL) did not show significantly superior outcomes to lower TBC levels. Moreover, a relatively lower CsA dose and a lower TBC level (around 87–120 ng/mL) could boost regulatory T cell development, leading to a better response and lower infectious event rate.30–32 In the present study, CsA was initially taken orally at 3–5 mg/kg/d and adjusted to be maintained at 100–150 ng/mL. The CsA administration was started with ATG for at least 6 months, and the trough blood concentration was held for 3 months. After 6 months of IST, CsA was continually administered, and the patients were closely followed up with; each family gave full consent, or there were no other options available. With this CsA administration management, the results of the present study were partially consistent with Zhu’s;33 however, there were no data with porcine anti-LY immunoglobulin included in the present study. Together, the stronger and longer immunosuppressive rATG effect, lower trough level, and prolonged use of CsA within the follow-up period might have contributed to a higher response rate, especially in patients with SAA and VSAA who did not have MSDs.
It was determined that the RBC count (p = 0.004), PLT count (p = 0.017), and Treg/LY ratio (p = 0.004) had a significant impact on the response rate. Emerging evidence regarding Tregs in patients with AA34–37 states that intrinsic impairment of regulatory T cells may contribute to the pediatric AA pathogenesis, as proven in our earlier study.34 However, upon enrolling a higher number of patients (n = 65), it was discovered that a higher Treg/LY ratio (p = 0.004) was significantly associated with a higher response rate. Unfortunately, only 65 patients in the present study had Treg/LY ratio data due to technological and equipment limitations; hence, this parameter was excluded for further ROC curve and logistic regression analyses.
The results showed that higher RBC (>2.735 × 1012/L) and PLT (>18.5 × 109/L) counts at diagnosis co-affected the IST response rate; the RBC count was more powerful. Baseline hematological parameters, such as the RBC count, Hb level, and PLT count could be prognostic factors for IST response statuses; disease severity is the most important predictive factor of IST efficacy. However, many predictive factors confirmed in other studies (even in our previous study), including younger age, higher ARC, higher Treg/LY ratio, higher absolute LY count, higher PLT count, and shorter IST initiation,38–41 were not validated in the present study. As the enrolled patients had different ethnicities, nationalities, disease severity, and ATG dosages, it is not surprising that the studies did not share united conclusions. Thus, such conclusions, including the conclusions of the present study, still require validation in further studies with large cohorts and multi-center groups.
Although the AA pathogenesis remains unknown, the T-LY function imbalance and T cell disease immunodysfuction might be the causes of hematopoietic cell apoptosis.35,42 However, ATG could deplete abnormal T-LYs and restore functional hemopoiesis in AA.26 It is reasonable to hypothesize that the LY change during IST can predict the IST response rate. Gu et al.43 emphasized the role of the decreased LY rate in the first week after IST in IST efficacy prediction. However, the results of the present study found no significant difference in the decreased LY rate in the first week after IST between the response group and the non-response group. These contradictory results may have been caused by the different ratios in the NSAA group, SAA group, and VSAA group.
The limitations of the present study include a relatively small cohort, lack of a Treg flow cytometry profile in all patients, and the study’s retrospective nature. Certain adjuvant Chinese complementary and alternative medications were administered due to personal choice of either the chief physician or patients; these medications might have a confounding role in response achievement. The regression model and Treg parameter changes of our study in multicenter, large-scale, randomized studies should be tested in the future. Furthermore, IST risks include waiting in vain, alloimmunization, invasive fungal infection, iron overload, and clonal evolution.
Overall, the following can be concluded: (1) with rATG, proper CsA management, and a prolonged IST follow-up period, a higher number of patients with acquired AA achieved response. Proportionally, a higher number of patients achieved remission within 12 months in the SAA group (38.18%→63.64%) and the VSAA group (28.95%→65.79%) than in the NSAA group (58.33%→75%); and (2) higher RBC and PLT counts at diagnosis can predict a favorable outcome.
Ethics Approval and Consent to Participate
I confirm that I have read the Editorial Policy pages. This study was conducted with approval from the Ethics Committee of The seventh affiliated hospital, Sun Yat-sen University. This study was conducted in accordance with the declaration of Helsinki. The parent or legal guardian of the pediatric patients provided informed consent.
Acknowledgments
The authors would like to thank Dr. Pan Jingxuan for the assistance and suggestion of this article. We also thank all patients, families, nursing and medical staff for their collaboration. Junbin Huang and Lifen Huang should be considered as co-first authors.
Funding
This research was funded by Sanming Project of Medicine in Shenzhen (SZSM202011004), the National Natural Science Foundation of China (8157010694), Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ20180307150419435), Clinical Founding of The seventh affiliated hospital, Sun Yat-sen University(ZSQYLCKYJJ202024).
Disclosure
The abstract of this paper was presented at the “2019 The American Society of Hematology” Conference name 2019 ASH meeting as a online abstract with interim findings. The poster’s abstract was published in “Poster Abstracts” in Blood Journal name “Clinical Outcome of Pediatric Acquired Aplastic Anemia Underwent Immunosuppressive Therapy and Transplantation: A 13-Year Experience in Two Tertiary Care Centers, Southern China”: https://ashpublications.org/blood/article/134/Supplement_1/5022/424776/Clinical-Outcome-of-Pediatric-Acquired-Aplastic?searchresult=1or https://doi.org/10.1182/blood-2019-128617. The authors report no other conflicts of interest.
References
1. Korthof ET, Bekassy AN, Hussein AA. Management of acquired aplastic anemia in children. Bone Marrow Transplant. 2013;48(2):191–195. doi:10.1038/bmt.2012.235
2. Jeong DC, Chung NG, Cho B, et al. Long-term outcome after immunosuppressive therapy with horse or rabbit antithymocyte globulin and cyclosporine for severe aplastic anemia in children. Haematologica. 2014;99(4):664–671.
3. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica. 2010;95(12):2119–2125. doi:10.3324/haematol.2010.026682
4. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129(11):1428–1436. doi:10.1182/blood-2016-08-693481
5. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK paediatric BMT working party, paediatric diseases working party and severe aplastic anaemia working party of EBMT. Br J Haematol. 2015;171(4):585–594. doi:10.1111/bjh.13614
6. Sakamoto T, Obara N, Kurita N, et al. Effectiveness and safety of rabbit anti-thymocyte globulin in Japanese patients with aplastic anemia. Int J Hematol. 2013;98(3):319–322. doi:10.1007/s12185-013-1418-5
7. Marsh JC, Bacigalupo A, Schrezenmeier H, et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT severe aplastic anaemia working party. Blood. 2012;119(23):5391–5396. doi:10.1182/blood-2012-02-407684
8. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–438. doi:10.1056/NEJMoa1103975
9. Atta EH, Dias DS, Marra VL, et al. Comparison between horse and rabbit antithymocyte globulin as first-line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2010;89(9):851–859. doi:10.1007/s00277-010-0944-y
10. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressivetreatment in the last decade: a report from the European Group for Blood and MarrowTransplantation (EBMT). Haematologica. 2007;92(1):11–18.
11. Chang MH, Kim KH, Kim HS, et al. Predictors of response to immunosuppressive therapy with antithymocyte globulin and cyclosporine and prognostic factors for survival in patients with severe aplastic anemia. Eur J Haematol. 2010;84(2):154–159. doi:10.1111/j.1600-0609.2009.01378.x
12. Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survivalin severe aplastic anaemia. Br J Haematol. 2009;144(2):206–216. doi:10.1111/j.1365-2141.2008.07450.x
13. Boddu P, Garcia-Manero G, Ravandi F, et al. Clinical outcomes in adult patients with aplastic anemia: a single institution experience. Am J Hematol. 2017;92(12):1295–1302. doi:10.1002/ajh.24897
14. Peffault DLR, Tabrizi R, Marcais A, et al. Nationwide survey on the use of horse antithymocyte globulins(ATGAM) in patients with acquired aplastic anemia: a report on behalf of the French reference centerfor aplastic anemia. Am J Hematol. 2018;93(5):635–642. doi:10.1002/ajh.25050
15. Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70. doi:10.1111/j.1365-2141.2009.07842.x
16. Bacigalupo A, Giammarco S, Sica S. Bone marrow transplantation versus immunosuppressive therapy in patients with acquired severe aplastic anemia. Int J Hematol. 2016;104(2):168–174. doi:10.1007/s12185-016-2037-8
17. Bacigalupo A, Socie´ G, Hamladji RM, et al. Aplastic Anemia working party of the european group for blood marrow transplantation. Current outcome of HLA identical sibling versus unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100(5):696–702. doi:10.3324/haematol.2014.115345
18. Marsh JC, Pearce RM, Koh MB, et al. British society for blood and marrow transplantation, clinical trials committee. Retrospective study of alemtuzumab vs ATG-based conditioning without irradiation for unrelated and matched sibling donor transplants in acquired severe aplastic anemia: a study from the British society for blood and marrow transplantation. Bone Marrow Transplant. 2014;49(1):42–48. doi:10.1038/bmt.2013.115
19. Anderlini P, Wu J, Gersten I, et al. Cyclophosphamide conditioning in patients with severe aplastic anaemia given unrelated marrow transplantation: a Phase 1–2 dose de-escalation study. Lancet Haematol. 2015;2(9):e367–e375. doi:10.1016/S2352-3026(15)00147-7
20. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia[J]. Br J Haematol. 2016;172(2):187–207. doi:10.1111/bjh.13853
21. Tichelli A, Schrezenmeier H, Socie G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA working party of the European group for blood and marrow transplantation. Blood. 2011;117(17):4434–4441.
22. Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from a matched family donor versus immunosuppressive therapy. Haematologica. 2014;99(12):1784–1791. doi:10.3324/haematol.2014.109355
23. Nair V, Sondhi V, Sharma A, Das S, Sharma S. Survival after immunosuppressive therapy in children with aplastic anemia. Indian Pediatr. 2012;49(5):371–376. doi:10.1007/s13312-012-0086-5
24. Bacigalupo A. Aplastic anemia: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2007;2007(1):23–28. doi:10.1182/asheducation-2007.1.23
25. Pediatric Hematology Study Group, Chinese Medical Association. Acquired Guidelines for the diagnosis and management of pediatric acquired aplastic anaemia. Zhonghua Er Ke Za Zh. 2014;52(2):103–106. in Chinese
26. Xie X, Zhao H, Qin D, et al. Pharmacokinetics and pharmacodynamics of two antithymocyte globulins in treatment of pediatric aplastic anemia. Int J Clin Exp Med. 2015;8(3):4349–4355.
27. Feng X, Scheinberg P, Biancotto A, et al. In vivo effects of horse and rabbit antithymocyte globulin in patients with severe aplastic anemia. Haematologica. 2014;99(9):1433–1440. doi:10.3324/haematol.2014.106542
28. Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi:10.1182/blood-2008-01-130146
29. Gurkan S, Luan Y, Dhillon N, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–2141. doi:10.1111/j.1600-6143.2010.03210.x
30. Bleyzac N, Philippe M, Bertrand A, et al. Confounding effect of cyclosporine dosing when comparing horse and rabbit antithymocyte globulin in patients with severe aplastic anemia. Haematologica. 2015;100(5):e211–222. doi:10.3324/haematol.2014.122275
31. Brandt C, Pavlovic V, Radbruch A, et al. Low-dose cyclosporine A therapy increases the regulatory T cell population in patients with atopic dermatitis. Allergy. 2009;64(11):1588–1596. doi:10.1111/j.1398-9995.2009.02054.x
32. Kawai M, Kitade H, Mathieu C, et al. Inhibitory and stimulatory effects of cyclosporine A on the development of regulatory T cells in vivo. Transplantation. 2005;79(9):1073–1077. doi:10.1097/01.TP.0000153505.73700.32
33. Zhu Y, Yang Y, Yang W, et al. Efficacy and safety of porcine ALG compared to rabbit ATG as first-line treatment for children with acquired aplastic anemia. Eur J Haematol. 2020;104(6):562–570. doi:10.1111/ejh.13398
34. Lin S, Hou L, Liu S, et al. Roles of regulatory T cells in the pathogenesis of pediatric aplastic anemia. Pediatr Hematol Oncol. 2019;36(4):1–13. doi:10.1080/08880018.2019.1621968
35. Shi J, Ge M, Lu S, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120(8):1624–1632. doi:10.1182/blood-2011-11-390708
36. Kordasti S, Costantini B, Seidl T, et al. Deep phenotyping of Tregs identifies an immune signature for idiopathic aplastic anemia and predicts response to treatment. Blood. 2016;128(9):1193–1205. doi:10.1182/blood-2016-03-703702
37. Lim SP, Costantini B, Mian SA, et al. Treg sensitivity to FasL and relative IL-2 deprivation drive idiopathic aplastic anemia immune dysfunction. Blood. 2020;136(7):885–897. doi:10.1182/blood.2019001347
38. Sasaki N, Shimura K, Yoshida M, et al. Kyoto Clinical Hematology Study Group (KOTOSG) investigators. Immunosuppressive therapy with rabbit antithymocyte globulin therapy for acquired aplastic anemia: a multi-institutional retrospective study in Japanese adult patients. Int J Hematol. 2019;109(3):278–285. doi:10.1007/s12185-018-02583-w
39. Lan Y, Chang L, Yi M, et al. Long-term outcomes of 172 children with severe aplastic anemia treated with rabbit antithymocyte globulin and cyclosporine. Ann Hematol. 2021;100(1):53–61. doi:10.1007/s00277-020-04296-9
40. Li F, He W, Shi W, et al. Efficacy of rabbit antithymocyte globulin as a first-line therapy in children with aplastic anemia. J Pediatr Hematol Oncol. 2020;42(8):e702–e706. doi:10.1097/MPH.0000000000001885
41. Lin SF, Liu S, Xue HM, et al. Comparison of two dosages of rabbit antithymocyte globulin (r-ATG) in treating children with severe aplastic anemia. Pharmazie. 2018;73(5):264–268. doi:10.1691/ph.2018.7353
42. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S119–25. doi:10.1016/j.bbmt.2009.09.013
43. Gu C, Zhu X, Qiao X, et al. Multivariate logistic analysis of predictors of response to immunosuppressive therapy in children with aplastic anemia: a double-center study. Hematology. 2019;24(1):282–289.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

