Back to Journals » Clinical Ophthalmology » Volume 13
Clinical outcome after air-assisted manual deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment
Authors Uchio E , Saeki Y, Tsukahara-Kawamura T , Kadonosono K, Ozaki H
Received 3 April 2019
Accepted for publication 15 August 2019
Published 26 September 2019 Volume 2019:13 Pages 1913—1919
DOI https://doi.org/10.2147/OPTH.S211099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Eiichi Uchio,1 Yusuke Saeki,1 Tomoko Tsukahara-Kawamura,1 Kazuaki Kadonosono,2 Hiroaki Ozaki1
1Department of Ophthalmology, Fukuoka University School of Medicine, Fukuoka, Japan; 2Department of Ophthalmology, Yokohama City University Medical Center, Yokohama, Japan
Correspondence: Eiichi Uchio
Department of Ophthalmology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
Tel +81 92 801 1011
Fax +81 92 865 4445
Email [email protected]
Purpose: Fungal keratitis remains an important disorder because of difficulty in its diagnosis, and some patients do not respond to medical treatment using antifungal local and systemic agents. This study was carried out to determine the therapeutic value of air-assisted manual therapeutic deep anterior lamellar keratoplasty (TDALK) in the treatment of fungal keratitis not curable by antifungal chemotherapy.
Methods: Seventeen patients (18 eyes) who were referred to Fukuoka University Hospital and treated surgically from January 2006 to April 2018, in whom a diagnosis of fungal keratitis was confirmed by typical clinical findings and microbiological or histological analysis of corneal specimens, and who were poorly responsive to topical and systemic antifungal medication, whereas the lesion had not resulted in corneal perforation, were enrolled in this study and were treated by air-assisted manual TDALK. Clinical outcomes including treatment course, therapeutic success rate, visual acuity outcomes and graft clarity rate were analyzed.
Results: The most common pathogen was Fusarium, followed by Candida and Aspergillus. Beneficial therapeutic results (a clear or translucent graft) were achieved in 15 of 18 eyes (83%). There was no recurrence of infection and resulting visual acuity ≤0.15 logarithm of minimal angle of resolution unit was achieved in 15 eyes (83%). Intraoperative microperforation of Descemet’s membrane (DM) was not observed in any patients.
Conclusion: Air-assisted manual TDALK can be effective for treating severe fungal keratitis. In addition, air-assisted manual TDALK might be an alternative procedure to big-bubble DALK, because it can provide ambulatory vision and can preserve potentiality of vision with less risk of intraoperative perforation of DM.
Keywords: fungal keratitis, lamellar keratoplasty, fusarium, candida, therapeutic keratoplasty
Introduction
Fungal keratitis remains an important disorder in clinical ophthalmology because of difficulty in its diagnosis, and some patients do not respond to medical treatment using antifungal local and systemic agents.1 Therapeutic keratoplasty (TKP) has been reported for sight-threatening situations in cases of suppurative keratitis with impending corneal perforation or intraocular inflammatory invasion with typical symptoms such as hypopyon or corneal hyphae.2–4 Two surgical methods, penetrating keratoplasty (PKP) and lamellar keratoplasty (LKP), have been considered for these disorders, and the surgical outcomes with each procedure have been reported. An excellent anatomical or visual outcome has been reported with PKP in fungal keratitis in several studies;5,6 however, there is a high incidence of postsurgical graft failure, rejection and recurrence of fungal infection.7,8 To reduce the incidence of complications after PKP for fungal keratitis, minimal trephination PKP, equal to the size of the corneal ulcer, has been reported for severe fungal keratitis complicated with hypopyon and showed a very high rate of graft clarity (95%) after surgery; however, intraoperative graft edge irrigation and anterior chamber irrigation with antifungal agents were necessary with this method.9 Deep anterior lamellar keratoplasty (DALK) has recently been carried out for the treatment of refractory fungal keratitis, and a higher cure rate and better visual outcome compared with those with PKP have been reported in several studies of infectious keratitis including fungal keratitis.2,10,11 Since the introduction of the big-bubble DALK method,12 this technique has been introduced especially for the treatment of deep fungal keratitis with favorable clinical outcomes.13 However, there still remains a considerable problem relating to complicated surgical procedures in big-bubble DALK, namely, intraoperative macro-perforation of Descemet’s membrane (DM).13,14 As an alternative therapeutic option to avoid this type of complication, Sabatino et al15 reported that fungal keratitis poorly responsive to medical treatment could be treated by early DALK performed between 15 and 50 days after the onset, and no case showed recurrence or rejection during the follow-up, and they also reported that big-bubble technique was accomplished in 74%, whereas manual dissection was performed in the remaining 26% of the eyes.
To reduce the risk of perforation of scarred corneas, DALK has been performed by more superficial intrastromal air injection, avoiding the formation of a big bubble, and this technique was named “air-assisted manual DALK”.16 A clinical outcome with a safe intraoperative profile and resultant significant visual recovery was reported in corneal opacity after herpes simplex virus (HSV) keratitis.16 Taken together with these studies, we herein report the clinical outcomes after air-assisted manual therapeutic DALK in cases of fungal keratitis poorly responsive to medical treatment.
Materials and methods
Patients
This was a consecutive case series study of fungal keratitis patients who were referred to our hospital and treated surgically from January 2006 to April 2018. The diagnosis of fungal keratitis in these patients was based on clinical examination with a slit lamp microscope, confocal microscopic examination of the corneal lesion and laboratory examination of corneal scrapings with fungal culture positivity or histological evaluation mentioned later. Corneal ulcers were classified as mild, moderate or severe depending upon the degree of corneal involvement as described elsewhere:17 mild ulcers: <1/3 superficial stromal involvement; moderate ulcers: 1/3–2/3 stromal involvement; severe ulcers: >2/3 stromal involvement, ulcer near the limbus, impending perforation or perforated ulcer. Seventeen patients (18 eyes) whose disease was not arrested or cured after 21 or more days of topical and systemic antifungal therapy, but in whom the lesion had not resulted in corneal perforation were included in this study and therapeutic DALK (TDALK) was planned for surgical treatment. Those cases that showed corneal perforation clinically or by anterior segment optical coherence tomography18 before surgery were excluded in the study population and therapeutic PKP was performed. This study was carried out according to the Declaration of Helsinki and approved by the Ethics Review Committee of Fukuoka University (11-9-07) and written informed consent was obtained from all patients.
Antifungal treatment
In all cases, the patients were hospitalized and medical management included topical 5% natamycin eye ointment (Senju Pharmaceutical Co. Ltd., Osaka, Japan) 3 times a day, 1% voriconazole eye drops (In-hospital preparation from infusion), 8 times a day and intravenous infusion of voriconazole (Pfizer Japan Inc., Tokyo, Japan), 200 mg twice a day. The fungal lesions in all patients were scraped and curetted to eliminate superficial necrotic tissue. The efficacy of medical management was assessed biomicroscopically by evaluating the edge of the infiltrate, density of suppuration, cellular infiltrate and edema of surrounding stroma, and hypopyon. Antifungal treatment was carried out for 21 days to 1 month, with an average of 24±16 (mean ± SD) days. However, most cases were treated at the local clinic for several weeks. Thus, the exact interval between initiation of treatment at the local clinic and corneal transplantation will be mentioned later. If the infection was not arrested or cured, surgical treatment was selected. In the postoperative period, all patients were treated with broad-spectrum topical antibiotic eye drops (1.5% levofloxacin (Santen Pharmaceutical Co. Ltd, Osaka, Japan)) 4 times a day for 3 months and oral voriconazole (Pfizer) 150 mg twice a day for 3 months, and these regimens were tapered according to the clinical findings after 3 months observation. Corticosteroid eye drops (0.1% betamethasone (Shionogi Pharma Co. Ltd., Osaka, Japan)) were prescribed in all cases at the initial dose of 4 times a day for at least 4 months, with tapering.
Surgical procedure
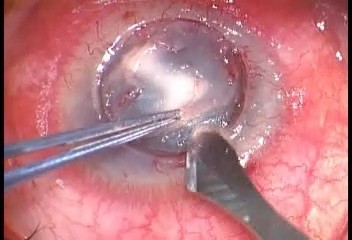
The surgical procedure of air-assisted manual TDALK was performed according to the previous report by Leccisotti et al.16 In brief, in all patients, surgery was started using a Hessburg–Barron trephine (Katena Products, Inc. NJ, USA) with a diameter 0.5 mm larger than the area of fungal infection. Trephination was performed to a depth of 400 μm when the peripheral cornea was thicker than 500 μm. After initial dissection to 2/3 the depth with a surgical knife, a bent 27-gauge needle was inserted to half the depth of the remaining corneal stroma from the edge of the trephination at 10-o’clock to the paracentral area. Air was slowly injected until the stroma was whitened by small intrastromal bubbles but avoiding the formation of a big bubble (Figure 1). Deeper layer-by-layer stromal dissection was then carried out until a thin layer of stroma was left. A donor button 0.5 mm larger than the trephined diameter was cut using a Barron punch (Katena), and the endothelium and DM were peeled off using forceps. The donor button was finally secured with 16-stitch continuous 10-0 nylon suture. The excised corneal specimen was sent for microbiological and histological investigations. An additional therapeutic step of antimicrobial irrigation of the stromal bed before transplantation of donor tissue was not advocated in this study.
Results
Demographics
The age ranged from 18 to 94 and its mean ± SD was 59±21 years. Nine patients were men and 8 were women. The proportion of cases with predisposing ocular trauma, including foreign matter on the cornea or recent refractive surgery, was 56%. Mean ± SD of follow-up time was 57±49 months.
Culture results, treatment course and surgical procedures
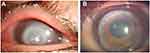
Fourteen eyes (78%) had positive fungal cultures. These included 7 patients (39%) with Fusarium, 3 with Candida (17%), 2 with Aspergillus (11%), 1 with Paecilomyces (Figures 2) and 1 with Cladosporium. One patient (Case 11) showed 18 small subunit ribosomal RNA by PCR from corneal scrapings, and fungal infection was confirmed histologically in 2 cases (Cases 9 and 12) by fungal spore and 1 case (Case 8) by fungal hyphae using surgical specimens, respectively (Table 1). Mean time between the initiation of treatment including that in the local clinic and surgery was 52±20 days (mean ± SD). Mean donor size was 7.5 mm. No patient had a recurrence of infection after surgery.
 |
Table 1 Clinical profiles of cases with TDALK |
Visual acuity outcomes and clinical severity
The Snellen visual acuity was converted to its logarithm of minimal angle of resolution (logMAR) equivalent as previously described; count-fingers was assigned a logMAR value of 1.9 and hand motion 2.3.19 Preoperative best-corrected visual acuity (BCVA): VA of ≤1.0 logMAR unit was observed in 3 eyes (17%); however, all cases maintained light perception before surgery (Table 1). Postoperative BCVA: VA of ≤0.15 logMAR unit was achieved in 15 eyes (83%). The reasons for cases of postoperative BCVA of higher than 0.4 logMAR unit resulted from graft opacity (Cases 10, 12 and 16). Seventeen eyes (94%) had a moderate corneal ulcer and 1 (6%) had a severe corneal ulcer, but no case showed a mild ulcer. The relationship between final visual outcome and ulcer severity is shown in Table 2.
 |
Table 2 Relation between visual outcome and severity of ulcer |
Graft clarity observation
Beneficial therapeutic results (a clear or translucent (partial layer cloudy) graft) were achieved in 15 of 18 eyes (83%), and a clear graft was observed at the final follow-up in 12 eyes (67%). Opaque graft due to whole layer opacity was observed in 3 cases (17%). No case resulted in phthisis bulbi or adherent leucoma, and late endothelial failure was not observed. Analysis of graft clarity was based on patients whose therapy was successful and who underwent one procedure only. Using Kaplan–Meier analysis, the 1-year survival rate was 94% and 5-year survival rate was 85% (Figure 3). Among 18 cases, repeated surgery was carried out in 3 cases at 7, 12 and 12 months, respectively, after the first surgery. In terms of infection control as therapeutic success, all cases achieved success in this case series.
 |
Figure 3 Kaplan–Meier survival curves of graft clarity. Survival indicates graft clarity was maintained and no additional surgery was performed. |
Complications
Allograft rejection and intraoperative DM microperforation did not occur. Postoperative DM detachment was seen in 1e patient (6%); the detachment resolved after intracameral injection of air (Case 3). Delayed corneal endotheliitis due to HSV was observed in 1 case (Case 5) 2 years after TDALK (Table 1); graft rejection was considered at first, but HSV was detected from aqueous humor by PCR and the patient was treated successfully with combined therapy of oral valaciclovir and corticosteroid tablets. In this case series, no patient progressed to develop fulminant endophthalmitis requiring evisceration.
Discussion
Regarding the mean interval between the initiation of treatment and corneal transplantation, a long tendency was observed in our study (52 days). This was presumed to be based on the clinical severity that refractory cases were included but the more threatening clinical status resulted in PKP within short range. A past study of PKP cases in fungal keratitis reported shorter mean duration (16 days) between the first visit and the surgery,7 whereas a similar long mean duration was reported in medically treated fungal keratitis cases (48 days).20 Several recent reports of refractory fungal keratitis noted that the minimum recommended antifungal medical treatment duration should be 14 days.13,21 From these combined reports, 50 days might be proposed as the maximum duration of medical treatment until corneal transplantation in refractory fungal keratitis to achieve a favorable visual prognosis.
A favorable clinical outcome was reported in PKP-treated fungal keratitis in several studies, in which the anatomical success rate was 76–88%.7,22,23 However, higher rates of recurrent infection (14–78%)7,22,23 and graft rejection (18–22%)22,23 were also reported in these studies. Overall, a poor final visual outcome with an average best-corrected logMAR of (mean, 95% CI) 0.7 (0.4–1.0) was noted in fungal keratitis, including patients treated with PKP.24 In contrast, higher anatomical success (93%) and lower recurrence of infection (7%) were obtained in a recent study of the treatment of fungal keratitis by DALK, with good visual acuity ranging from 20/63 to 20/20.10 In DALK, the pathologic corneal stroma is replaced, while the healthy endothelium of the host is preserved. This helps to retain all the advantages of anterior lamellar keratoplasty over full-thickness keratoplasty, providing a clearer interface. In this study, 83% of the patients had ≤0.15 logMAR BCVA after DALK, which is similar to the results of PKP for the treatment of infectious keratitis11,25 and DALK for the treatment of deep fungal keratitis.13 In addition, the DALK procedure avoids most of the complications associated with open-sky surgery and grossly avoids postoperative endothelial rejection. Our results of a high anatomical success rate (100%) and a low rate of recurrence of infection (0%) showed a similar tendency to those of DALK cases.10 The surgical procedure reported by Xie et al10 was similar to that of our study; however, the recipient LKP bed was washed with 0.2% fluconazole and their procedure was conventional manual DALK without using the big-bubble method or an air-assisted step.10 Anshu et al compared the clinical outcome between TDALK and therapeutic PKP (TKP) in advanced infectious keratitis including fungal keratitis in a retrospective study and reported that the therapeutic success rate was similar in both groups, but the proportion of better visual acuity and the 1-year graft survival rate were significantly better in the TDALK group, with a lower recurrent infection rate than that in the TKP group, and they concluded that for medically unresponsive infectious keratitis, TDALK may be considered instead of TKP, yielding similar graft survival without an increased risk of disease recurrence.11 Considering these results together with ours, TDALK could be a powerful procedure in cases refractory to prolonged medical treatment and with a clinical situation of impending perforation.
Gao et al described the procedure and surgical outcome of DALK using the big-bubble technique (big-bubble DALK) for deep fungal keratitis unresponsive to medication and concluded that big-bubble TDALK was effective and safe.13 Whereas, the incidences of intraoperative perforation of the DM (9%) during stromal dissection and double anterior chamber formation (13%) were higher than those of TDALK cases in our study, 0% and 6%, respectively. It was interesting that fungal recurrence was found in 2 patients (9%) with big-bubble TDALK; in contrast, no case showed fungal recurrence in our case series of air-assisted manual TDALK. From these results, air-assisted manual TDALK might have clinical merit compared with big-bubble TDALK. However, there are few studies on the surgical outcome after air-assisted manual TDALK for severe fungal keratitis. More secure preservation of DM is an important advantage of air-assisted manual TDALK over big-bubble TDALK; however, complete removal of an abscess lesion can be obtained more securely by big-bubble TDALK. There are limitations of our study, the lack of randomized choice of surgical procedure and the small scale of study population. Therefore, further case accumulation, such as a prospective study with randomized selection of the procedure, is necessary to determine the appropriate surgical procedure for deep fungal keratitis resistant to medical treatment.
In summary, air-assisted manual TDALK might be an alternative procedure to big-bubble TDALK, because it provides structural stability and ambulatory vision and can preserve potentiality of vision with less risk of intraoperative perforation of DM. The presence of infection and an inflammatory state of the eye at the time of TKP is still challenging; however, the clinical outcome of air-assisted manual TDALK in our study suggests its potential in the future.
Acknowledgment
This work was supported by a Grant-in-Aid for Encouragement of Scientists (15K10911) from the Ministry of Education, Science, Sports and Culture of Japan. We thank Dr. W. Gray for editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19(3):210–220. doi:10.1111/1469-0691.12126
2. Ti SE, Scott JA, Janardhanan P, Tan DT. Therapeutic keratoplasty for advanced suppurative keratitis. Am J Ophthalmol. 2007;143(5):755–762. doi:10.1016/j.ajo.2007.01.015
3. Xu LJ, Song XS, Zhao J, Sun SY, Xie LX. Hypopyon in patients with fungal keratitis. Chin Med J (Engl). 2012;125(3):470–475.
4. Vasantha Ruban V, Geraldine P, Kaliamurthy J, Jesudasan CA, Thomas PA. Keratitis due to Fusarium langsethiae: clinical profile, molecular identification, and susceptibility to antifungals. Mycopathologia. 2015;179(5–6):453–458. doi:10.1007/s11046-015-9866-5
5. Sukhija J, Jain AK. Outcome of therapeutic penetrating keratoplasty in infectious keratitis. Ophthalmic Surg Lasers Imaging. 2005;36(4):303–309.
6. Yalniz-Akkaya Z, Burcu A, Doğan E, Onat M, Ornek F. Therapeutic penetrating keratoplasty for infectious and non-infectious corneal ulcers. Int Ophthalmol. 2015;35(2):193–200. doi:10.1007/s10792-014-9931-y
7. Barut Selver O, Egrilmez S, Palamar M, Arici M, Hilmioglu Polat S, Yagci A. Therapeutic corneal transplant for fungal keratitis refractory to medical therapy. Exp Clin Transplant. 2015;13(4):355–359. doi:10.6002/ect.2014.0108
8. Gregory ME, Macdonald EC, Lockington D, Ramaesh K. Recurrent fungal keratitis following penetrating keratoplasty: an unusual source of infection. Arch Ophthalmol. 2010;128(11):1490–1491. doi:10.1001/archophthalmol.2010.264
9. Liu Y, Jia H, Shi X, et al. Minimal trephination penetrating keratoplasty for severe fungal keratitis complicated with hypopyon. Can J Ophthalmol. 2013;48(6):529–534. doi:10.1016/j.jcjo.2013.05.017
10. Xie L, Shi W, Liu Z, Li S. Lamellar keratoplasty for the treatment of fungal keratitis. Cornea. 2002;21(1):33–37.
11. Anshu A, Parthasarathy A, Mehta JS, Htoon HM, Tan DT. Outcomes of therapeutic deep lamellar keratoplasty and penetrating keratoplasty for advanced infectious keratitis: a comparative study. Ophthalmology. 2009;116(4):615–623. doi:10.1016/j.ophtha.2008.12.043
12. Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117–124. doi:10.1016/j.ajo.2006.09.025
13. Gao H, Song P, Echegaray JJ, et al. Big bubble deep anterior lamellar keratoplasty for management of deep fungal keratitis. J Ophthalmol. 2014;2014:209759.
14. Ziaei M, Ormonde S. Descemet’s membrane macroperforation during interface irrigation in big bubble deep anterior lamellar keratoplasty. Oman J Ophthalmol. 2017;10(3):241–243. doi:10.4103/ojo.OJO_66_2016
15. Sabatino F, Sarnicola E, Sarnicola C, Tosi GM, Perri P, Sarnicola V. Early deep anterior lamellar keratoplasty for fungal keratitis poorly responsive to medical treatment. Eye (Lond). 2017;31(12):1639–1646. doi:10.1038/eye.2017.228
16. Leccisotti A. Air-assisted manual deep anterior lamellar keratoplasty for treatment of herpetic corneal scars. Cornea. 2009;28(7):728–731. doi:10.1097/ICO.0b013e3181930a7e
17. Mann SS, Singh J, Kalra D, Parihar J, Gupta N, Kumar P. Medical and surgical management of keratomycosis. Med J Armed Forces India. 2008;64(1):40–42. doi:10.1016/S0377-1237(08)80144-X
18. Ono T, Mori Y, Nejima R, Iwasaki T, Amano S, Miyata K. Optical coherence tomography examination of the anterior segment in a case of corneal perforation and lens trauma by chestnut burr. Case Rep Ophthalmol. 2018;9(1):154–159. doi:10.1159/000487076
19. Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch Clin Exp Ophthalmol. 2009;247(1):137–142. doi:10.1007/s00417-008-0926-0
20. Sharma N, Agarwal P, Sinha R, Titiyal JS, Velpandian T, Vajpayee RB. Evaluation of intrastromal voriconazole injection in recalcitrant deep fungal keratitis: case series. Br J Ophthalmol. 2011;95(12):1735–1737. doi:10.1136/bjo.2010.192815
21. Lin TY, Yeh LK, Ma DH, et al. Risk factors and microbiological features of patients hospitalized for microbial keratitis: a 10-year study in a referral center in Taiwan. Medicine (Baltimore). 2015;94(43):e1905. doi:10.1097/MD.0000000000000874
22. Bajracharya L, Gurung R. Outcome of therapeutic penetrating keratoplasty in a tertiary eye care center in Nepal. Clin Ophthalmol. 2015;9:2299–2304. doi:10.2147/OPTH.S92176
23. Chen WL, Wu CY, Hu FR, Wang IJ. Therapeutic penetrating keratoplasty for microbial keratitis in Taiwan from 1987 to 2001. Am J Ophthalmol. 2004;137(4):736–743. doi:10.1016/j.ajo.2003.11.010
24. Nielsen SE, Nielsen E, Julian HO, et al. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015;93(1):54–58. doi:10.1111/aos.12440
25. Mandell KJ, Colby KA. Penetrating keratoplasty for invasive fungal keratitis resulting from a thorn injury involving phomopsis species. Cornea. 2009;28(10):1167–1169. doi:10.1097/ICO.0b013e3181a2ad81
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.