Back to Journals » Cancer Management and Research » Volume 12
Clinical Investigation of the Efficacy and Safety of Anlotinib with Immunotherapy in Advanced Non-Small Cell Lung Cancer as Third-Line Therapy: A Retrospective Study
Authors Yang S , Zhang W, Chen Q, Guo Q
Received 8 September 2020
Accepted for publication 2 October 2020
Published 19 October 2020 Volume 2020:12 Pages 10333—10340
DOI https://doi.org/10.2147/CMAR.S280096
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Shengjie Yang,1 Wenjie Zhang,1,2 Qing Chen,1 Qisen Guo1
1Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, People’s Republic of China; 2Department of Oncology, Weifang Medical University, Weifang, Shandong, People’s Republic of China
Correspondence: Qisen Guo
Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, 440 Jiyan Road, Jinan, Shandong 250117, People’s Republic of China
Tel +86 13869199681
Email [email protected]
Purpose: This study was designed to assess the clinical efficacy and safety of anlotinib with immunotherapy in advanced non-small cell lung cancer as third-line therapy.
Patients and Methods: A total of 101 patients with advanced non-small cell lung cancer who were treated with anlotinib combined with immunotherapy were evaluated for progression-free survival, objective response rate, and disease control rate. Univariate and multivariate analyses were performed to determine the prognostic factors. The main adverse events were evaluated as per the Common Terminology Criteria for Adverse Events version 5.0.
Results: Nineteen patients had partial response (18.8%), 61 had stable disease (60.4%), 31 had progressive disease (20.8%), and no patients achieved complete response (0%). The objective response rate was 18.8%, and the disease control rate was 79.2%. In all patients, the median progression-free survival was 6.7 months (95% confidence interval 6.13– 7.24 months). In Cox regression analysis, the Eastern Cooperative Oncology Group performance status score, smoking history and age were predictive indicators for anlotinib treatment efficacy. Treatment-related adverse events were tolerated.
Conclusion: This study demonstrated and confirmed the clinical effectiveness of anlotinib combined with immunotherapy in advanced non-small cell lung cancer as third-line therapy.
Keywords: non-small cell lung cancer, anlotinib, immunotherapy, safety, efficacy
Introduction
Lung cancer is still the most significant worldwide health problem with the highest morbidity and mortality rates in both men and women, and the incidence is still increasing.1 Chemotherapy has always been the most important treatment for advanced non-small cell lung cancer (NSCLC).2 However, there is no standard third-line treatment before the clinical application of anlotinib.3 Anlotinib is a newly developed oral small-molecule receptor tyrosine kinase (RTK) inhibitor that targets vascular endothelial growth factor receptor VEGFR1, VEGFR2/KDR, VEGFR3, c-Kit, PDGFR-α, and fibroblast growth factor receptors (FGFR1, FGFR2, and FGFR3).4 Furthermore, anlotinib can inhibit both tumour angiogenesis and tumour cell proliferation. The ALTER 03035 trial was a multicentre, double-blind, Phase 3 randomized clinical trial designed to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC and to investigate the efficacy of anlotinib in patients with advanced NSCLC that progressed after second- or later-line treatment. The results showed that the median PFS of patients treated with anlotinib could reach 5.4 months, which was better than the results of other third-line treatment schemes. Therefore, on May 8, 2018, the China National Medical Products Administration (NMPA) approved anlotinib for the treatment of patients with advanced NSCLC who have progressed after prior treatment with second- or later-line systemic chemotherapy.6 Moreover, the emergence of immune checkpoint inhibitors in recent years has provided another antitumour treatment method: programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) are the most widely used immune checkpoint targets, together with other immune checkpoints, such as cytotoxic T-lymphocyte-associated protein 4, T cell immunoglobulin and mucin domain-containing protein 3, lymphocyte-activation gene 3, and T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domains. Furthermore, National Comprehensive Cancer Network (NCCN) data have shown that nivolumab can be used as second-line therapy for patients with treatment-resistant mutant NSCLC, while pembrolizumab can also be used as second-line therapy for patients with more than 1% PD-L1-positive NSCLC cells or as first-line treatment for patients with 50% or more PD-L1-positive NSCLC cells.7–12 To date, the results of different clinical trials show that immune checkpoint inhibitors are effective. At the World Conference of Lung Cancer (WCLC) in 2019, Professor Bao-hui Han presented a report13 on Sintilimab combined with Anlotinib first-line therapy for first-line advanced NSCLC13. The results showed that among the 22 patients in the treatment group, 16 cases were effectively treated, the objective response rate (ORR) was as high as 72.7%, close to the results of targeted therapy, and the disease control rate (DCR) was as high as 100%. Such excellent data also provide us with new treatment ideas. Therefore, we conducted a retrospective study to analyse the efficacy and safety of anlotinib combined with immunotherapy in the treatment of third-line non-small cell lung cancer.
Patients and Methods
Research Purpose
By summarizing and analysing the clinical data, we analysed the efficacy and safety of anlotinib combined with immunotherapy in the treatment of third-line NSCLC, in order to guide future clinicians in applying this treatment.
Data and Methods
We collected and followed up the medical records of 101 patients with third-line NSCLC who received anlotinib combined immunotherapy in Shandong Cancer Hospital from October 2018 to November 2019 and analysed these data. All the patients in our study were treated with two-line platinum containing dual drug chemotherapy before, and none of them had targeted mutation.
General Data
The following clinicopathological data were collected from the medical records of the patients: sex; age at diagnosis; tumour-node-metastasis (TNM) stage; pathological type of lung cancer; Eastern Cooperative Oncology Group (ECOG) score; history of smoking tobacco; time until tumour progression; side effects; and laboratory tests, such as routine blood tests, liver and renal function, tumour biomarkers, myocardial zymogram, thyroid function, and imaging examinations (computed tomography, bone scan, skull magnetic resonance imaging, or positron emission tomography-computed tomography).
Treatment Plan
A total of 101 patients received treatment; for these patients, based on the patient’s ECOG PS score, it was up to the physician to decide whether the oral dose of anlotinib was 8 mg, 10 mg or 12 mg, and the drug was used for 14 days and then stopped for 7 days. At the same time, the patients were treated with three-week treatment plan of immune checkpoint inhibitors, and patient tolerance and treatment efficacy were evaluated every 2 cycles. The attending physician recommended the final dose according to the clinical status and treatment tolerance of each patient. The treatment was continued until disease progression, unacceptable serious side effects, death or any other reason was observed.
Evaluation of Efficacy and Adverse Events
The effectiveness of the treatment was evaluated according to the evaluation criteria for solid tumour efficacy (RECIST1.1) in terms of complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The ORR was defined as the total number of CR+PR patients, while the DCR was defined as the total number of CR+PR+SD patients. Progression-free survival (PFS) refers to the duration from the first treatment to PD or death, while overall survival (OS) refers to the duration from the first treatment to death or the last follow-up. Adverse events (AEs) were divided into grades 0–IV according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0; the higher the grade was, the worse the adverse reaction.
Results
Baseline Characteristics
A total of 101 patients with non-small cell lung cancer were treated. The median age of the population was 59 years (28–80 years); 49 patients (48.5%) were older than 60 years old. There were 59 males (58.4%) and 42 females (41.6%) in this study. The pathological types were basically equally distributed, including 47 cases of adenocarcinoma (46.5%) and 54 cases of squamous cell carcinoma (54.5%). We found that 51 patients had a history of smoking (50.5%), and most of the patients were pathologically staged as stage IV (57 cases, 56.4%) during treatment. The baseline characteristics are shown in Table 1.
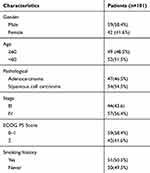 |
Table 1 Baseline Characteristics of the NSCLC Patients (n = 101) |
Initial Dosage and Adjustment of Anlotinib
Table 2 shows an overview of the adjustments to the initial dose and overall dose of anlotinib. A total of 101 patients received different doses of anlotinib. In total, 28 patients initially received 12 mg anlotinib, 5 were adjusted to 10 mg, and 5 were adjusted to 8 mg. Forty-six patients started treatment with 10 mg anlotinib, and 13 were adjusted to 8 mg. The common causes of the dose reductions in these patients included hypertension, gastrointestinal bleeding, haematuria, gingival bleeding, hand-foot syndrome and so on.
 |
Table 2 Treatment Administration and Dose Modification of Anlotinib |
PD1/PD-L1 Inhibitors
In all patients in this study, in addition to the use of anlotinib, they also treated with immunotherapy. Among them, 41 patients were treated with Pembrolizumab, 32 patients were treated with Sintilimab, 15 patients were treated with Nivolumab, and 13 patients were treated with Tislelizumab.
Short-Term Analysis
Of all 101 patients included in this study, 19 had PR (18.8%), 61 had SD (60.4%), 31 had PD (20.8%), and no patients achieved CR (0%). The ORR was 18.8%, and the DCR was 79.2%. Of the 49 patients aged 60 or over, 10 achieved PR, and 27 achieved SD. According to the analysis of the data, the ORR and DCR of older patients were similar to those of younger patients; that is, the benefits to the patients were similar in all age groups. Statistics showed that sex, age, pathological type, pathological stage, ECOG PS score and smoking history were not related to ORR and DCR (Table 3).
 |
Table 3 Association of Clinicopathological Features with the Short-Term Effectiveness of Anlotinib Combined with Immunotherapy in 101 NSCLC Patients |
Long-Term Analysis
All patients received at least one cycle of anlotinib combined with immunotherapy and were followed up. As of November 30, 2019, all patients had progressed, with the longest PFS being 20.5 months. In all patients, the median PFS was 6.7 months (95% confidence interval (CI) 6.13–7.24 months) (Figure 1A), and the median OS was not reached. First, we used the Kaplan-Meier method to analyse the influence of age, pathological type, sex, stage, ECOG PS score and smoking history on PFS. The results showed that the ECOG PS score (Figure 1B) and smoking history (Figure 1C) were significantly correlated with PFS. Among all patients, the median PFS of patients with a low ECOG PS score (0–1) was 7.7 months; in contrast, in patients with an EOCG PS score of 2, the median PFS was 5.8 months. In addition, the median PFS of patients with no history of smoking was 1.4 months longer (7.6 months vs 6.2 months) than that of patients with a history of smoking. There were no significant correlations among age (p=0.102), pathological stage (p= 0.812), pathological type (p=0.361) and sex (p=0.921).
To further eliminate interference from multiple factors, based on univariate analysis and clinical observation, Cox multivariate analysis was used to analyse several important factors (Table 4). The results showed that the p values of the ECOG PS score (p=0.001), smoking history (p=0.000) and age stratification (p=0.002) were all less than 0.05, which was statistically significant. Based on the Cox results, we can conclude that smoking history, age and ECOG PS score were predictive indicators of treatment efficacy. Additionally, we found that sex, pathological type and stage were not risk factors for a shortened PFS.
 |
Table 4 Cox Multivariate Regression Analysis of PFS |
Safety
No grade 4 adverse events were observed in this study. The main treatment-related adverse reactions were hypertension (52.5%), hand-foot syndrome (45.5%), gingival bleeding (25.7%), fatigue (23%), anorexia (19.8%) and bone marrow suppression (9.9%). Table 5 summarizes the main adverse reactions related to treatment.
 |
Table 5 Treatment-Related Adverse Events |
Discussion
NSCLC is the leading cause of cancer-related death worldwide; it has long been the most common cause of cancer and cancer-related death in the world.14 For non-small cell lung cancer patients without gene-driven mutations, if the ECOG PS score is satisfactory, it is recommended that active antineoplastic therapy is continued after second-line chemotherapy. Angiogenesis is closely related to tumor growth, proliferation and metastasis, thus angiogenesis is generally considered an attractive target in NSCLC therapy.15–17 At the same time, because of the benefits of immunotherapy for NSCLC patients, we believe that the combination of amlotinib and immunotherapy should bring good results for patients.
In this study, we evaluated the efficacy of anlotinib combined with immunotherapy in 101 patients with advanced NSCLC. Our short-term efficacy analysis of these data showed that 19 patients had PR (18.8%), 61 patients had SD 60.4%), and 31 patients had PD (20.8%). The ORR of anlotinib combined with immunotherapy was 18.8%, and the DCR was 79.2%. Using these data, our long-term efficacy analysis showed that in these 101 patients, the median PFS was 6.7 months (95% CI 6.13–7.24 months). Univariate analysis showed that ECOG PS score and smoking history were related to PFS. Cox multivariate analysis showed that the regimen of anlotinib combined with immunotherapy had significant advantages in patients who were young (less than 60 years old), had no smoking history and had a low ECOG PS score (0–1), while sex, pathological type, pathological stage and prognosis were not directly related to prognosis.
Although there was no control group in this study, there is still some reference value in comparing the data of this study with the ALERT 03035 data. In the ALERT 03035 study, the median PFS of the group treated with anlotinib was 5.4 months, while in this study, as mentioned above, the median PFS of patients treated with anlotinib combined with immunotherapy reached 6.7 months, and the addition of immunotherapy did not increase the adverse events of the patients. Although the data of these two studies cannot be directly compared, the application of anlotinib combined with immunotherapy does benefit patients. Furthermore, the results of this study can also provide a reference for clinical workers.
The incidence of lung cancer increases with age and peaks in people aged 60 or over.18 Therefore, as the population ages, the incidence of lung cancer may also increase. It is important to explore the survival benefits of immunotherapy for elderly patients because the increase in age is usually accompanied by a decline in the function of the immune system, especially the T cell-mediated immune defence system.19 As a result, immune checkpoint inhibitors and other antitumour methods are less effective in the treatment of elderly patients. In this study, we included 49 elderly patients. Compared with the mPFS of young patients, the mPFS of elderly patients was shorter by 1.2 months (5.9 months vs 7.1 months), which had a p < 0.05 and was considered to be an independent factor for imaging prognosis in the Cox multivariate analysis. As a result, the younger the patient is, the more he or she can benefit from anlotinib combined immunotherapy. Therefore, we infer that in clinical practice, although all patients can benefit from this treatment, young patients should use an anlotinib combined immunotherapy regimen.
In addition, the ECOG PS score was also an important independent prognostic factor in this study. The results showed that when the ECOG PS was 0–1, the mPFS of patients was 7.7 months, which was much longer than that of patients with an ECOG PS score of 2 (5.8 months). These data are easy to understand because when the patient’s ECOG PS score is lower, it means that the patient’s physical condition is better.20 First, good physical condition means that patients have a good tolerance to drugs. In addition, when patients have a good physical condition, they can use a larger dose of anlotinib, which has the best antitumour effect on patients. Therefore, when the patient’s physical condition is good and the ECOG PS score is low, we should recommend anlotinib combined with immunotherapy.
A number of studies have shown that smoking has a certain adverse effect on the development of lung cancer and is a prognostic factor for the treatment of lung cancer.21–23 The data from this study also support this conclusion. The median PFS of patients who never smoked was significantly higher than that of patients with a smoking history (7.6 months vs 6.2 months). According to the statistical analysis, smoking history is an independent factor affecting anlotinib combined with immunotherapy, and this finding also has some reference value for clinical work.
Conclusion
Our current study still has some limitations, such as the lack of a control group, and we did not obtain the median OS of patients. We can only study the PFS of patients but cannot study the effect of anlotinib combined with immunotherapy on the overall survival of patients. We will address these problems in future work. However, it is undeniable that as third-line treatment of advanced NSCLC, anlotinib combined with immunotherapy is a regimen that can benefit patients, and the patients who use this regimen can tolerate the treatment. This study should provide new ideas for clinical anti-tumour therapy, and it is the hope that more patients can benefit from this therapeutic regimen.
Ethics Approval and Informed Consent
This study was approved by the medical ethics committee of Shandong Cancer Hospital and conformed to the provisions of the Declaration of Helsinki. Consent to participate was not applicable due to the retrospective nature of the study, and the data were anonymously analyzed.
Acknowledgments
The authors thank Dr. Jinming Yu of the Shandong Cancer Hospital and Institute (Jinan, China) for his encouragement and inspiration.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Wakelee H, Kelly K, Edelman MJ, 50 years of progress in the systemic therapy of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2014;34:177–189. doi:10.14694/EdBook_AM.2014.34.177
3. Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807–821. doi:10.6004/jnccn.2018.0062
4. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120.
5. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
6. Zhou M, Chen X, Zhang H, et al. China national medical products administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy. Cancer Commun (Lond). 2019;39(1):36. doi:10.1186/s40880-019-0383-7
7. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
8. Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2980–2987. doi:10.1200/JCO.2016.66.9929
9. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
10. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, Phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/S1470-2045(16)30498-3
11. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). 2019;393(10183):1819–1830. doi:10.1016/S0140-6736(18)32409-7
12. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
13. Han B, Chu T, Zhong R, et al. JCSE01.11 efficacy and safety of sintilimab with anlotinib as first-line therapy for advanced Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol. 2019;14(10).S439
14. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
15. Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64(2):327–336. doi:10.1016/0092-8674(91)90642-C
16. Wang W, Zhang L, Xie Y, Zhen T, Su G, Zang Q. Fatal hemoptysis in patients with advanced esophageal cancer treated with apatinib. Onco Targets Ther. 2018;11:2565–2570. doi:10.2147/OTT.S150555
17. Schuch G, Kisker O, Atala A, Soker S. Pancreatic tumor growth is regulated by the balance between positive and negative modulators of angiogenesis. Angiogenesis. 2002;5(3):181–190. doi:10.1023/A:1023893931057
18. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi:10.1016/S1470-2045(12)70211-5
19. Tomihara K, Curiel TJ, Zhang B. Optimization of immunotherapy in elderly cancer patients. Crit Rev Oncog. 2013;18(6):573–583. doi:10.1615/CritRevOncog.2013010591
20. Huemer F, Lang D, Westphal T, et al. Baseline absolute lymphocyte count and ECOG performance score are associated with survival in advanced non-small cell lung cancer undergoing PD-1/PD-L1 blockade. J Clin Med. 2019;8(7):1014. doi:10.3390/jcm8071014
21. Karagueuzian HS, White C, Sayre J, Norman A. Cigarette smoke radioactivity and lung cancer risk. Nicotine Tob Res. 2012;14(1):79–90. doi:10.1093/ntr/ntr145
22. Hang B, Mao JH, Snijders AM. Genetic susceptibility to thirdhand-smoke-induced lung cancer development. Nicotine Tob Res. 2019;21(9):1294–1296. doi:10.1093/ntr/nty127
23. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–9. doi:10.1016/j.lungcan.2004.07.998
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

