Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 11
Clinical Improvements as Predictors of Improvements in Patient-Reported Outcomes: Post Hoc Analysis of a Randomized, Open-Label Study of Etanercept in Latin American Patients with Rheumatoid Arthritis
Authors Guerra Bautista G , Xavier RM , de la Vega M, Simón-Campos JA, Solano G, Pedersen RD, Vlahos B, Borlenghi C
Received 28 August 2019
Accepted for publication 13 November 2019
Published 12 December 2019 Volume 2019:11 Pages 275—281
DOI https://doi.org/10.2147/OARRR.S228866
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Generoso Guerra Bautista,1 Ricardo Machado Xavier,2 Maria de la Vega,3 J Abraham Simón-Campos,4 Gastón Solano,5 Ronald D Pedersen,6 Bonnie Vlahos,6 Cecilia Borlenghi7
1Centro de Investigación Marbella, Paitilla Panamá, Panamá; 2Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil; 3CEIM Investigaciones Medicas, Buenos Aires, Argentina; 4Köhler & Milstein Research, Mérida, México; 5Pfizer, San José, Costa Rica; 6Pfizer, Collegeville, PA, USA; 7Pfizer, Buenos Aires, Argentina
Correspondence: Generoso Guerra Bautista
Consultorios Royal Center, Calle 53 Marbella, Panamá, República de Panamá
Tel +507-263-3283
Email [email protected]
Background: In rheumatoid arthritis (RA), little is known about clinical responses to treatment as predictors of patient-reported outcome (PRO) changes. In this post hoc analysis, we examined the relationship between clinical outcomes at week 12 and PRO changes at week 24 in patients with RA.
Methods: In an open-label study, Latin American patients with moderate-to-severe RA and an inadequate response to methotrexate (MTX) were randomized to receive etanercept 50 mg/week plus MTX (ETN+MTX; n=281) or an additional conventional disease-modifying anti-rheumatic drug (DMARD) plus MTX (DMARD+MTX; n=142) for 24 weeks. The PROs included Health Assessment Questionnaire Disability Index (HAQ-DI), 36-item Short Form (SF-36), Physician and Patient Global Assessment scores (PGA, PtGA), Physician and Patient Satisfaction, and an activity impairment assessment. PRO changes at week 24 were calculated by week-12 improvements using the American College of Rheumatology criteria (ACR <20, ≥20 to <50, ≥50 to <70, and ≥70) and the 28-joint Disease Activity Scores (DAS28 ≥3.2, ≥2.6 to <3.2, and <2.6). Observed-cases data were analyzed using an ANCOVA model with linear contrast, adjusted for baseline PRO and ACR/DAS28 values.
Results: For both ETN+MTX- and DMARD+MTX-treated patients, there was a significant linear trend between week-12 changes in ACR and DAS28 responses and week-24 changes in HAQ-DI (P<0.001 for all), with numerical improvements generally favoring ETN+MTX. Similar relationships were observed for SF-36, PGA, PtGA, Physician Satisfaction, Patient Satisfaction, and activity impairment.
Conclusions: In patients with RA, clinical response after 12 weeks of treatment with ETN+MTX or DMARD+MTX could be a predictor of week-24 response for several PROs.
Trial registration: ClinicalTrials.gov, NCT00848354.
Keywords: etanercept, rheumatoid arthritis, clinical outcome, patient-reported outcome, predictor
Background
Patient-reported outcomes (PROs) in rheumatoid arthritis (RA) are used both in clinical trials1 and in clinical practice.2 In trials, clinical improvements have been shown to correlate with concurrent improvements in various PROs.3–8 However, less is known about clinical responses as predictors of PRO changes.
Since patients’ subjective assessment is an important factor in RA management, including treatment adherence9 and disease remission,10,11 it is important to understand what kind of PRO response can be expected from a certain level of clinical response. In addition, it is of interest to assess predictive relationship between clinical and PRO improvements within a relatively short time: patients are keen to experience improvements as soon as possible, and 6 months are usually considered the maximum time for changing therapies that patients consider to be ineffective.
In a randomized, open-label trial in Latin American patients with moderate-to-severe RA and an inadequate response to methotrexate (MTX) (NCT00848354), adding etanercept (ETN) to MTX was more effective than adding another conventional disease-modifying anti-rheumatic drug (DMARD), for both clinical outcomes and PROs.12,13 In this post hoc analysis, we used data from that trial to examine whether changes from baseline in clinical outcomes at week 12 were predictive of PRO changes at week 24.
Methods
Study Design
All study participants provided written informed consent. In addition, institutional approval was obtained from each center in which the study was conducted (see Table S1). Details of the 24-week, randomized phase of that study were published previously.13 Briefly, patients from Argentina, Chile, Colombia, Mexico, and Panama with moderate-to-severe RA and an inadequate response to MTX were randomized to receive open-label ETN 50 mg/week plus MTX (n=281) or an additional conventional DMARD (hydroxychloroquine or sulfasalazine) plus MTX (n=142) for 24 weeks. Main inclusion criterion was active RA despite MTX monotherapy (≥7.5 and ≤25 mg/week) for at least 3 months, as indicated by ≥6 swollen joints, ≥8 tender joints, and erythrocyte sedimentation rate of ≥28 mm/hr. Main exclusion criteria were previous treatment with ETN or other biologics, treatment with a concurrent DMARD (other than MTX) within 3 months from baseline, and treatment with >1 NSAID at screening. Clinical endpoints were assessed at baseline and at weeks 2, 4, 8, 12, 16, 20, and 24, and included the proportions of patients who achieved the American College of Rheumatology (ACR) improvement criteria (ACR20, ACR50, and ACR70) and the 28-joint Disease Activity Scores (DAS28) consistent with remission (<2.6) and low disease activity (<3.2). The 24-week PROs included Health Assessment Questionnaire Disability Index (HAQ-DI), 36-item Short Form, Physical Component Summary (SF-36 PCS), Hospital Anxiety and Depression Scale subscales for anxiety (HADS-A) and depression (HADS-D), Physician and Patient Global Assessment scores (PGA, PtGA), Physician and Patient Satisfaction, Work Productivity and Activity Impairment: RA (WPAI:RA), and resource utilization.
Data Analysis
Baseline-to-week-24 PRO changes were calculated by week-12 responses on the ACR (<20, ≥20 to <50, ≥50 to <70, and ≥70) and on the DAS28 (≥3.2, ≥2.6 to <3.2, and <2.6). Observed-cases data for ETN+MTX and DMARD+MTX groups were analyzed using ANCOVA with linear contrast, adjusted for baseline PRO and ACR/DAS28 values.
Results
Baseline Characteristics
Baseline characteristics were published previously.13 Most of the 423 randomized patients were women (376; 89%); the average disease duration was 8.3 years (Table 1).
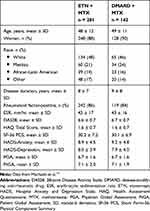 |
Table 1 Baseline Characteristics |
Disability and Physical Component-Related Quality of Life
There was a significant linear trend for improvement in HAQ-DI and SF-36 PCS at week 24 with an increasing level of ACR response or reduction in DAS28 scores at week 12, regardless of the choice of add-on treatment (Figure 1). Consistent with the published, prospectively defined analyses,13 the PRO improvement generally favored ETN over DMARD.
Anxiety and Depression
Overall, patients with a greater ACR or DAS28 response at week 12 also tended to experience a greater reduction in anxiety or depression at week 24, with the linear trends being significant for most depresion-related assessments (Figure 2C-D). There was no significant linear trend in anxiety improvement by DAS28 response for any treatment (Figure 2B).
Global Assessments and Treatment Satisfaction
At week 24, there was an overall significant linear trend for improvement in patients’ global assessment (PGA or PtGA) with an increasing level of week-12 clinical response, regardless of the add-on treatment (ETN or DMARD) (Figure 3). The PRO improvement generally favored ETN over DMARD, consistent with protocol-specified analyses.13 Similar observations were made for patients’ and physicians’ satisfaction with treatment (Figure 4).
WPAI and Resource Utilization
There were significant linear trends for improvement in WPAI:RA subscales by ACR or DAS28 response for ETN treatment, but a low number of patients with available data precluded trend analysis for the DMARD group (Figure S1). The week-24 linear trends were less clear in regard to resource utilization: the trends were significant for ETN-treated patients in terms of reduced visits to rheumatologists, other physicians, or RA-related emergency room visits by ACR, but not by DAS28 response, but there were no significant trends for DMARD treatment (Figure S2).
Discussion
These data suggest that, in patients with moderate-to-severe RA and an inadequate response to MTX, PRO improvement at week 24 was proportional to clinical response at week 12. To the best of our knowledge, this is the first study that evaluated clinical outcomes as predictors of PRO response in RA. Since the treatment groups were similar in terms of baseline characteristics, and since our statistical model was adjusted for baseline PRO and ACR/DAS28 values, the significant linear trends observed are not likely to be baseline-related artifacts.
The fact that significant linear trends in PRO improvement were associated with 2 measures of clinical improvement supports the robustness of our findings. In addition, for certain PROs, significant linear trends were observed in both ETN+MTX- and DMARD+MTX-treated patients, which suggests a predictive relationship independent of the type of treatment or the novelty factor (biologic vs. conventional DMARD). In general, improvements in ETN-treated patients exceeded those in patients receiving DMARD, which is consistent with the prespecified analyses of these data,13 but a trend of greater PRO improvements in patients who had experienced a greater clinical response was observed in both treatment groups.
Limitations of this analysis include its post hoc nature; an open-label design, which typically limits interpretation of PRO data (patients or physicians may be more inclined to be satisfied with a novel treatment); a small number of patients available for certain outcomes (most notably WPAI:RA subscales); and the fact that PtGA is a component of DAS28,14 so the significant linear trend observed for PtGA by DAS28 response was not surprising.
Conclusions
In conclusion, clinical improvements could serve as predictors of certain future PRO improvements, which may be of use in clinical practice, particularly in setting treatment expectations. These findings would need to be corroborated in prospectively designed studies.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation - Good Clinical Practice guidelines, while following all local regulatory requirements, as stated in the primary report on the trial.13
Consent for Publication
This manuscript does not contain any individual person’s data.
Availability of Data and Materials
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices 1) for indications that have been approved in the US and/or EU or 2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Acknowledgment
Medical writing assistance was provided by Vojislav Pejović, PhD, of Engage Medical Affairs, and was sponsored by Pfizer.
Disclosure
GGB: Consultant for Janssen. Speaker for Janssen, Pfizer, and Roche. RMX: Consultant for AbbVie, Janssen, Lilly, and Pfizer. Speaker for AbbVie, BMS, Janssen, Lilly, Novartis, Pfizer, and Roche. RMX also reports personal fees from Abbvie, Pfizer, UCB, Lilly, and Roche, outside the submitted work. MdlV: Consultant and speaker for Pfizer, Sanofi, and Raffo. Speaker for Abbvie, BMS, Janssen, and Lilly. Participant in educational programs supported by Roche. JASC: Received sponsorship from Roche to attend an educational event. GS, CB, and BV are Pfizer employees and may own company stock. RDP was a Pfizer employee during the development of the manuscript and may own company stock. The authors report no other conflicts of interest in this work.
References
1. Kilic L, Erden A, Bingham CO
2. Daul P, Grisanti J. Monitoring response to therapy in rheumatoid arthritis - perspectives from the clinic. Bull NYU Hosp Jt Dis. 2009;67(2):236–242.
3. Baker JF, Conaghan PG, Emery P, Baker DG, Ostergaard M. Relationship of patient-reported outcomes with MRI measures in rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):486–490. doi:10.1136/annrheumdis-2016-209463
4. Cheung PP, Gossec L, Ruyssen-Witrand A, Le Bourlout C, Mezieres M, Dougados M. The relationship of patient-reported joints with active synovitis detected by power Doppler ultrasonography in rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4):490–497.
5. Kekow J, Moots RJ, Emery P, et al. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis. 2010;69(1):222–225. doi:10.1136/ard.2008.102509
6. Li T, Gignac M, Wells G, Shen S, Westhovens R. Decreased external home help use with improved clinical status in rheumatoid arthritis: an exploratory analysis of the Abatacept in Inadequate responders to Methotrexate (AIM) trial. Clin Ther. 2008;30(4):734–748. doi:10.1016/j.clinthera.2008.03.015
7. Strand V, Mease P, Burmester GR, et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther. 2009;11(6):R170. doi:10.1186/ar2859
8. Wiland P, Dudler J, Veale D, et al. The effect of reduced or withdrawn etanercept-methotrexate therapy on patient-reported outcomes in patients with early rheumatoid arthritis. J Rheumatol. 2016;43(7):1268–1277. doi:10.3899/jrheum.151179
9. Hope HF, Bluett J, Barton A, Hyrich KL, Cordingley L, Verstappen SM. Psychological factors predict adherence to methotrexate in rheumatoid arthritis; findings from a systematic review of rates, predictors and associations with patient-reported and clinical outcomes. RMD Open. 2016;2(1):e000171. doi:10.1136/rmdopen-2015-000171
10. Hsiao B, Fraenkel L. Incorporating the patient’s perspective in outcomes research. Curr Opin Rheumatol. 2017;29(2):144–149. doi:10.1097/BOR.0000000000000372
11. van Tuyl LH, Sadlonova M, Davis B, et al. Remission in rheumatoid arthritis: working toward incorporation of the patient perspective at OMERACT 12. J Rheumatol. 2016;43(1):203–207. doi:10.3899/jrheum.141113
12. Machado DA, Guzman R, Xavier RM, et al. Two-year safety and efficacy experience in patients with methotrexate-resistant active rheumatoid arthritis treated with etanercept and conventional disease-modifying anti-rheumatic drugs in the Latin American Region. Open Rheumatol J. 2016;10:13–25. doi:10.2174/1874312901610010013
13. Machado DA, Guzman RM, Xavier RM, et al. Open-label observation of addition of etanercept versus a conventional disease-modifying antirheumatic drug in subjects with active rheumatoid arthritis despite methotrexate therapy in the Latin American region. J Clin Rheumatol. 2014;20(1):25–33. doi:10.1097/RHU.0000000000000055
14. Nikiphorou E, Radner H, Chatzidionysiou K, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016;18(1):251. doi:10.1186/s13075-016-1151-6
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




