Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Clinical Impact of the Capacity-Motivation-Opportunity Pharmacist-Led Intervention in People Living with HIV in Spain, 2019–2020
Authors Morillo-Verdugo R , Robustillo-Cortes MDLA, Navarro-Ruiz A, Sánchez-Rubio Ferrandez J, Fernández Espínola S, Fernández-Pacheco García-Valdecasas M, Vélez-Diaz-Pallares M
Received 6 February 2022
Accepted for publication 27 April 2022
Published 24 May 2022 Volume 2022:15 Pages 1203—1211
DOI https://doi.org/10.2147/JMDH.S361305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ramón Morillo-Verdugo,1 María de las Aguas Robustillo-Cortes,2 Andrés Navarro-Ruiz,3 Javier Sánchez-Rubio Ferrandez,4 Sergio Fernández Espínola,5 María Fernández-Pacheco García-Valdecasas,6 Manuel Vélez-Diaz-Pallares7
1Pharmacy Hospital Service, Hospital Valme, Área de Gestión Sanitaria Sur de Sevilla, Sevilla, Spain; 2Pharmacy Hospital Service, Hospital Juan Ramón Jiménez, Huelva, Spain; 3Pharmacy Hospital Service, Hospital de Elche, Alicante, Spain; 4Pharmacy Hospital Service, Hospital de Getafe, Madrid, Spain; 5Pharmacy Hospital Service, Hospital Serranía de Ronda, Málaga, Spain; 6Pharmacy Hospital Service, Hospital Príncipe de Asturias, Madrid, Spain; 7Pharmacy Hospital Service, Hospital Ramón y Cajal, Madrid, Spain
Correspondence: Ramón Morillo-Verdugo, Pharmacy Hospital Service, Hospital Valme, Área de Gestión Sanitaria Sur de Sevilla, Sevilla, Spain, Email [email protected]
Background: People living with HIV (PLWH) have significantly enhanced their life expectancy. Consequently, age-associated comorbidities and related health conditions are increasingly found in PLWH complicating their clinical management.
Objective: To determine the effect of the capacity-motivation-opportunity (CMO) structured pharmaceutical care intervention for improving clinical health-care results frequently associated to PLWH.
Methods: Multicenter, prospective, pre-post intervention study evaluating the CMO pharmacist-led program in adult PLWH was conducted between September 2019 and September 2020 with six months of follow-up. The primary objective of this study was to determine differences in clinical outcomes (total cholesterol, triglycerides, HDL, blood pressure and glycosylated hemoglobin) and variation in the patient’s activation measure before and after the intervention.
Results: A total of 61 patients were included, 72% were men with a median age of 53 years. After the implementation of the pharmacist-driven program, the percentage of patients with high levels of total cholesterol decreased significantly (18% to 4.9%; p < 0.001). Similarly, the prevalence of patients with high levels of triglycerides, HDL or with hypertension was significantly lower post intervention (13.1% to 6.6%, p < 0.001; 47.5% to 6.6%, p = 0.019 and 24% to 4%, p = 0.009, respectively). The number of patients who achieved the highest activation level increased from 69% to 77.6% (p < 0.001).
Conclusion: The CMO program resulted in significantly better health outcomes during the six months following the pharmacist-led intervention as well as improved activation in PLWH.
Keywords: pharmaceutical care, HIV, clinical outcomes
Plain Language Summary
In recent years, several authors have agreed that the classic definition of pharmaceutical care has already “hit a ceiling” and needs to be re-envisioned rather than changed, marking the way to re-envision the definition of this activity so that it is much more in line with the times and needs of patients. This study was carried out to collect information on the clinical impact of a new pharmaceutical care methodology applied to people living with HIV. To this end, a multicenter prospective study was developed to evaluate this issue. Generating new high-quality evidence is essential to incorporate a new concept and methodology of pharmaceutical care and to become the gold standard in routine practice.
Introduction
Currently, HIV infection is considered a chronic disease. The success of highly active antiretroviral therapy (ART) together with the arrival of new, more powerful, drugs with better dosage guidelines has allowed people living with HIV (PLWH) to substantially reduce the risk of HIV transmission and have a near normal life expectancy. However, increased lifespan has brought a new set of challenges in the management of PLWH, who frequently experience age‐related comorbidities. In fact, analysis of HIV cohorts indicates that medical conditions such as hypertriglyceridemia, hypercholesterolemia, arterial hypertension, or diabetes mellitus are very common among PLWH.1,2 As expected, the appearance of concomitant pathologies leads to an increase in the use of non–antiretroviral drugs and, consequently, the clinical management of these patients is complicated by the greater risk of adverse events and drug interactions, adherence problems, and greater risk of hospitalization.3
Proper management of PLWH requires a multidisciplinary health-care team. The role of specialized HIV clinical pharmacist is especially important in this scenario.4 Traditionally, the pharmaceutical care (PC) model was mostly depending on the medication, not considering the individual characteristics of the patients.5 However, multiple studies have demonstrated that alternatives to the classic medicine-centered design were more effective in increasing patients’ adherence or improving associated health outcomes.6,7 Patient demographic, educational and cognitive factors, as well as the use of health resources should be previously evaluated in order to provide the best care for patients. Moreover, enhancing the empowerment of the patients should be also considered a priority intervention to increase their self-efficacy for medication management.
Considering all the above, five years ago we developed a redefined model of PC based in three differential aspects.8 First, patient stratification. We considered that stratification of the patients is an essential step in order to attend them according to their specific needs, optimizing the use of resources and time. Second, a motivational interview with the objective of setting and defining individualized pharmacotherapy objectives. And third, performing a real-time follow-up of patients using the new technological tools available. The CMO intervention (capacity, motivation, opportunity) intervention has previously been tested in PLWH, showing successful results in improving patient adherence to ART, reducing cardiovascular risk, and increasing patient activation.9–11 However, currently, there are no data on the specific effect of this kind of program in other clinical outcomes of health that are frequently altered in PLWH.
Aim of the Study
The main objective of this study was to evaluate the impact of the CMO pharmaceutical intervention in improving HIV-associated health outcomes such as dyslipidemia, hypertension and diabetes. Also, we analyzed the influence of the CMO-based intervention to improve activation in PLWH.
Ethics Approval
The study was approved by the Ethics Committee “Comité Ético de Investigación del Sur de Sevilla. Hospital de Valme” (Sevilla, Spain), 1841-n-17. Participants were provided with written information regarding the study and its objectives, and a written informed consent form was given to those who took part in the study.
This study has been carried out according to the guidelines of the Declaration of Helsinki for biomedical research.
Materials and Methods
This was a multicentered, prospective cohort study of a structured pharmacist-led health intervention among PLWH patients conducted between September 2019 and September 2020 with six months of follow-up per patient. The study was performed at six tertiary hospitals from four provinces in Spain (Hospital Universitario Virgen de Valme, Sevilla; Hospital Serranía de Ronda, Málaga; Hospital de Elche, Alicante; Hospital Ramón y Cajal, Madrid; Hospital de Getafe, Madrid; Hospital Principe de Asturias, Madrid).
Patients
Participants were included in the study if they met the following criteria: patients with HIV infection ≥18 years of age, receiving active ART during at least one year before the inclusion in the study and under prescription of drugs for the treatment of any concomitant disease at least six months before the start of the study and at the beginning of the investigation period. Patients were excluded if they were pregnant, participating in a clinical trial or did not give their written informed consent.
Interventions
Patients received the pharmacotherapeutic interventions routinely applied to ambulatory care patients according to CMO PC model.8 It consists in an initial stratification of the patients in three levels according to the risk-stratified model for pharmaceutical care in HIV-patients of the Spanish Society of Hospital Pharmacy.12,13 Each patient received intensive PC corresponding to the predetermined interventions for each level of care. During face-to-face visit to the Hospital Pharmacy Service, a motivational interview was performed for each patient. In each interview, pharmacotherapeutic objectives were established or re-evaluated, in consensus with the rest of the medical team taking care of the patient at all times.
Lastly, patients had access to a newly created website (www.proyecto-pricmo.com; not active nowadays) with informative content on the importance of adherence and healthy living habits. It included videos, infographics, diptychs, links to other websites, articles and other relevant information on this matter. This tool was available and updated throughout the follow-up, so that patients could access the uploaded contents at any time in accordance with their digital skills. All patients received permanent contact tools (telephone numbers, institutional email, etc.) with the study pharmacists to resolve any incident or doubt related to their treatment at any time during the study.
Baseline Characteristics
Baseline demographic data (age, gender), HIV infection control variables as viral load (copies/mL) and CD4 count at the time of inclusion (cells/μL) as well as comorbidities and pharmacological therapy were recorded at the initial clinical assessment.
Health Outcomes
The consequences of the CMO pharmaceutical intervention on health outcomes as dyslipidemia, hypertension and diabetes were established by measuring levels of glycosylated hemoglobin (g/dl), total cholesterol (mg/dl), triglycerides (mg/dl), high-density lipoprotein (HDL) (mg/dl) and blood pressure (mm Hg) before and after the introduction of the PC model.
Patient Activation
Patient activation was measured at the initial visit of the study to determine the degree of baseline activation and 6 months after the start of the intervention. Variation of patient activation was measured by patient activation measure (PAM) questionnaire, developed and validated for this purpose and kindly supplied to the Spanish Society of Hospital Pharmacists (SEFH) for its use.14 The PAM is a 10-item survey tool adapted to Spanish and designed to assess a patient’s knowledge, abilities and confidence in managing their own health care. The questionnaire contains 10 items and use a Likert scale with four response options. Points are converted to activation score (range 0 to 100) using a chart provided by the creators of the form. Depending on the results of the questionnaire, patients can be stratified into one of three stages of gradual activation. Level I (PAM score of <52.9) in which patients are not prepared to play an active role in their own health or believe they play an important role but lack assurance and/or knowledge to take action; Level II (PAM score of 53.0–75.4) with patients who are beginning to take action, but may still lack confidence or support to achieve the desired changes; Level III (PAM score of 75.5 or higher) with individuals who have adopted many self-management behaviors, but may not be capable of maintaining actions over time or during stress situations.
Statistical Analysis
Quantitative variables were expressed as means ± standard deviations (SD) or medians and interquartile ranges (IQR) when appropriate. Qualitative variables were presented as counts (percentage). The differences in the variables collected before and after the intervention were assessed using χ2 test in the categories of each of the initial values and after 24 weeks of follow-up. For analytical data that have more than 2 categories, the Bonferroni correction was performed.
Data analysis was performed using the R studio program (v 1.1.456). A p-value of 0.05 or less was considered to be statistically significant.
Results
A total of 66 patients were enrolled (Figure 1). Finally, only 61 patients were included in the study because 5 patients were lost to follow-up. Patients’ baseline characteristics are summarized in Table 1. Seventy-two percent of patients were men, and the median age was 53 (IQR 5). Most of them (59.0%) had sexually acquired HIV and responded well to the antiretroviral treatment, with an undetectable viral load in 93% of patients and a CD4 count higher than 300 cells/u in the 89% of them.
 |
Table 1 Patient Baseline Characteristics |
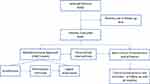 |
Figure 1 Study flow chart. |
The most common retroviral regimens were those including a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) plus an integrase inhibitor (42.6%), followed by two NRTIs plus a non-nucleoside reverse transcriptase inhibitor (16.4%) and two NRTIs plus a protease inhibitor (11.5%). Other combined therapies represented the 29.5% of the patients’ regimens. Regarding prescribed concomitant medications, lipid-lowering drugs were the most frequently used followed by psychotropic drugs (39% and 37% of patients, respectively). The median number of prescribed concomitant drugs per patient was 7 (IQR: 5–8).
Following CMO program indications, patients were stratified in three potential groups (Table 2). Eleven patients (18%) were at intermediate level and the remaining 50 patients (82%) were at level 3 (baseline). Based on these classifications and the conclusions of the motivational interview, specific pharmacist interventions were then applied to each patient.
 |
Table 2 Patient Classification According to CMO Model |
Table 3 shows the effect of applying the CMO model on patients’ pharmacotherapeutic goals regarding cholesterol levels, hypertension, and glycemic control. Total cholesterol levels were significantly reduced after the pharmacist’s intervention, changing from an 18% of patients with high cholesterol levels at the baseline visit to a 4.9% of patients six months after (p < 0.001). Triglycerides and HDL levels were likewise significantly reduced after the intensive pharmacist-led program was implemented (p < 0.001 and p = 0.019, respectively). Reductions in blood pressure were also statistically significant (24% to 4% patients with high blood pressure before and after introduction of specific PC measures; p = 0.009). Statistical differences among glycosylated hemoglobin levels could not be determined because of many patients lost to follow-up.
 |
Table 3 Comparison of HIV-Associated Health Outcomes Before and After Implementation of the CMO Pharmacist-Led Intervention in Spain, 2019–2020 |
With regard to the number of patients who achieved the highest activation level, it increased from 69% to 77.6% yielding statistical significance (p < 0.001) (Table 4).
 |
Table 4 Comparison of Patient Activation Before and After Implementation of the CMO Pharmacist-Led Intervention (N = 58) |
Discussion
Our multicenter study demonstrated that the CMO model of PC, based on patient stratification, motivational interview and the use of new technologies for the follow-up process, has a positive impact on health-care outcomes in PLWH, specifically regarding hypertension and dyslipidemia. Moreover, we found that those personalized pharmacists-led interventions improved patient activation.
Life expectancy for PLWH has increased substantially. Consequently, older PLWH face many health challenges found in other older individuals, although the impact of aging may be greater among PLWH. In fact, cardiovascular events are more frequently observed in patients infected with HIV than in uninfected individuals.15,16 Regarding the classical cardiovascular risk factors, an altered lipid metabolism is commonly observed in HIV-infected patients, while higher prevalence of hypertension in these patients compared to that of the general population is still controversial.17–19 In our study cohort, we found that neither blood pressure nor cholesterol levels or triglycerides were significantly altered in PLWH patients at the initial clinical assessment, with most of the patient having normal or low levels. The heterogenicity in the results found among different studies might be due to residual confounding factors since, in certain settings, PLWH have higher rates of cardiovascular disease risk factors, circumstances such as co-infections (cytomegalovirus, hepatitis B and C viruses), substance abuse (drug use, smoking, alcohol) and also some social characteristics such as homelessness and social isolation.
Previous studies have demonstrated that personalized pharmacist-led interventions based on the CMO model reduce the risk of cardiovascular events.10 In the same line, our results showed that the CMO program reduces health outcomes associated with increased cardiovascular risk, such as total cholesterol, triglycerides or blood pressure. These findings suggest that tailored pharmacist interventions based on the individual needs of the patients successfully improve the pharmacotherapeutic control on HIV infected patients and consequently, improve risk factors for cardiovascular disease. In our study, we found a significant reduction in HDL levels, which seems to contradict the rest of the results regarding dyslipidemia, since prior observational studies have suggested an inverse relationship between HDL cholesterol and both cardiovascular disease and total mortality, so that higher HDL is supposedly better.20–22 However, recent randomization trials and large cohort studies have failed to verify that higher HDL levels are associated with better outcomes.23–25 Indeed, there are some reports of increased cardiovascular events and even increased mortality associated with very high levels of HDL.26,27 On the other hand, pharmaceutical intervention studies designed at increasing HDL levels did not result in improvement of cardiovascular outcomes.28 Thus, there is a discrepancy between recent data and the accepted knowledge regarding the role of higher HDL cholesterol values on cardiovascular outcomes, and reduction of HDL levels might be in fact beneficial in certain settings.
We also found that our pharmacist intervention based positively influenced the activation in HIV-patients, as measured through the PAM questionnaire. Although similar results were found in a previous study,9 this is the first time that we confirmed the impact in activation across multiple hospital centers, suggesting that the results might be representative of the general target population. Furthermore, patient activation has been directly associated with more favorable HIV outcomes in prior research reports,29 in accordance with the results described in our study.
This study presents several strengths, including its prospective and multicentered nature. However, it has also some potential limitations. First, the lack of randomization with a limited sample size could be an important concern. Nonetheless, we considered that there could exist a participant bias when resembling interventions in putative control group, given the expansion and knowledge of the CMO PC model by many national hospital pharmacists and taking into account the results obtained in previous studies. For this reason, we considered that the best design to ponder the influence of our pharmaceutical intervention was using a pre-post design, so that each patient served as his or her own control. Additionally, the follow-up period in the study (6 months) might be considered relatively short within the life of a PLWH receiving a chronic treatment. Yet, methodologically, it is robust enough to determine the impact of a structured health intervention. Longer study periods will be necessary in order to determine whether the clinical effects observed after CMO pharmacist intervention are preserved over time. Despite these limitations, this study has significant implications highlighting the importance of specialized PC in the management of PLWH. Furthermore, this article can be taken as suggestive as a future larger-scale, properly designed, longer trial to validate the results presented in this study.
Conclusion
In conclusion, the CMO PC model, a pharmacist-led intervention based on the stratification of patients according to their specific necessities, in agreement with their pharmacotherapeutic objectives, and reinforced by motivational interviews and tailored follow-up using the new technological tools, might induce an improvement of clinical outcomes frequently associated to HIV disease. Therefore, considering the increased number of age-related comorbidities in PLWH, pharmacists’ role should be given particular attention among the multidisciplinary team taking care of the patients.
Abbreviations
ART, antiretroviral therapy; CMO, capacity, motivation and opportunity; NRTI, nucleoside reverse transcriptase inhibitors; PAM, Patient Activation Measure; PC, pharmaceutical care; PLWHIV, People living with HIV.
Acknowledgments
The authors thank the working groups on Pharmaceutical Care for HIV patients and drug therapy adherence (Adhefar) of the Spanish Society of Hospital Pharmacist (SEFH) for their support in the creation, development and dissemination of the project. Medical writing support was provided by Dr. Vanessa Marfil Vives of Medical Statistics Consulting S.L. (Valencia).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Spanish Society of Hospital Pharmacist (SEFH) grant obtained in 2017.
Disclosure
The authors have no conflicts of interest in relation to this work to report.
References
1. Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622):1417–1426. doi:10.1016/S0140-6736(08)60423-7
2. Palepu A, Sun H, Kuyper L, Schechter MT, O’Shaughnessy MV, Anis AH. Predictors of early hospital readmission in HIV-infected patients with pneumonia. J Gen Intern Med. 2003;18(4):242–247. doi:10.1046/j.1525-1497.2003.20720.x
3. McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E, A Pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37(12):1498–1506. doi:10.1002/phar.2043
4. Schafer JJ, Gill TK, Sherman EM, McNicholl IR, Hawkins B. ASHP guidelines on pharmacist involvement in HIV care. Am J Health Syst Pharm. 2016;73(7):468–494. doi:10.2146/ajhp150623
5. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Health Syst Pharm. 1990;47(3):533–543. doi:10.1093/ajhp/47.3.533
6. Urano K, Ishibashi M, Matsumoto T, et al. Impact of physician-pharmacist collaborative protocol-based pharmacotherapy management for HIV outpatients: a retrospective cohort study. J Pharm Health Care Sci. 2020;6:9. doi:10.1186/s40780-020-00165-9
7. Margulis A, Uhlyar S, Chin-Beckford N, et al. Clinical pharmacist input on HIV management may improve antiretroviral prescribing for psychiatric patients. Am J Health Syst Pharm. 2021;78(Supplement_1):S10–S5. doi:10.1093/ajhp/zxaa310
8. Morillo-Verdugo R, Calleja-Hernández MÁ, de Las Aguas Robustillo-Cortés M. A new pharmaceutical care concept: more capable, motivated, and timely. Hosp Pharm. 2019;54(6):348–350. doi:10.1177/0018578719867657
9. Morillo-Verdugo R, Robustillo-Cortes MA, Manzano Garcia M, Almeida-Gonzalez CV. Influence of pharmacist intervention, based on CMO model, to improve activation in HIV patients. Rev Esp Quimioter. 2019;32(1):40–49.
10. Morillo-Verdugo R, Robustillo-Cortés MA, Martín-Conde MT, et al. Effect of a structured pharmaceutical care intervention versus usual care on cardiovascular risk in HIV patients on antiretroviral therapy: INFAMERICA study. Ann Pharmacother. 2018;52(11):1098–1108. doi:10.1177/1060028018778045
11. Socieda Española de Farmacia Hospitalaria (SEFH). El modelo CMO en consultas externas de Farmacia Hospitalaria [The CMO pharmaceutical care model in hospital pharmacy outpatient consultations]; 2016. Available from: https://www.sefh.es/sefhpdfs/Libro_CMO.pdf.
12. Morillo-Verdugo R, Martinez-Sesmero JM, Lazaro-Lopez A, Sanchez-Rubio J, Navarro-Aznarez H, DeMiguel-Cascon M. Development of a risk stratification model for pharmaceutical care in HIV patients. Farm Hosp. 2017;41(3):346–356. doi:10.7399/fh.2017.41.3.10655
13. Morillo Verdugo R, Villarreal Arevalo AL, Alvarez De Sotomayor M, Robustillo Cortes ML. Development of a taxonomy for pharmaceutical interventions in HIV+ patients based on the CMO model. Farm Hosp. 2016;40(n06):544–568. doi:10.7399/fh.2016.40.6.10567
14. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4 Pt 1):1005–1026. doi:10.1111/j.1475-6773.2004.00269.x
15. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi:10.1001/jamainternmed.2013.3728
16. Drozd DR, Kitahata MM, Althoff KN, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75(5):568–576. doi:10.1097/QAI.0000000000001450
17. Masia M, Perez-Cachafeiro S, Leyes M, et al. Riesgo cardiovascular en pacientes con infección por el virus de la inmunodeficiencia humana en España [Cardiovascular risk in human immunodeficiency virus-infected patients in Spain]. CoRIS cohort, 2011]. Enferm Infecc Microbiol Clin. 2012;30(9):517–527. Spanish. doi:10.1016/j.eimc.2012.02.014
18. Russell E, Albert A, Cote H, et al. Rate of dyslipidemia higher among women living with HIV: a comparison of metabolic and cardiovascular health in a cohort to study aging in HIV. HIV Med. 2020;21(7):418–428. doi:10.1111/hiv.12843
19. Gelpi M, Afzal S, Lundgren J, et al. Higher risk of abdominal obesity, elevated low-density lipoprotein cholesterol, and hypertriglyceridemia, but not of hypertension, in people living with Human Immunodeficiency Virus (HIV): results from the Copenhagen comorbidity in HIV infection study. Clin Infect Dis. 2018;67(4):579–586. doi:10.1093/cid/ciy146
20. Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8(6):737–741. doi:10.1161/01.atv.8.6.737
21. Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. doi:10.1161/01.cir.79.1.8
22. Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi:10.1001/jama.2009.1619
23. Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97(2):E248–56. doi:10.1210/jc.2011-1846
24. Zanoni P, Khetarpal SA, Larach DB, et al. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351(6278):1166–1171. doi:10.1126/science.aad3517
25. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380(9841):572–580. doi:10.1016/S0140-6736(12)60312-2
26. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478–2486. doi:10.1093/eurheartj/ehx163
27. Wilkins JT, Ning H, Stone NJ, et al. Coronary heart disease risks associated with high levels of HDL cholesterol. J Am Heart Assoc. 2014;3(2):e000519. doi:10.1161/JAHA.113.000519
28. Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. doi:10.1136/bmj.g4379
29. Marshall R, Beach MC, Saha S, et al. Patient activation and improved outcomes in HIV-infected patients. J Gen Intern Med. 2013;28(5):668–674. doi:10.1007/s11606-012-2307-y
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
