Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Features of Pulmonary Nocardiosis in Patients with Different Underlying Diseases: A Case Series Study
Authors Zhong C , Huang P, Zhan Y, Yao Y, Ye J, Zhou H
Received 23 January 2022
Accepted for publication 11 March 2022
Published 21 March 2022 Volume 2022:15 Pages 1167—1174
DOI https://doi.org/10.2147/IDR.S359596
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Cheng Zhong,1,2 Pingping Huang,1,3 Yasheng Zhan,1,4 Yake Yao,1 Junhui Ye,3 Hua Zhou1
1Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310003, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Affiliated Hangzhou Chest Hospital, Hangzhou, 310003, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Sanmen Bay Branch of the First Affiliated Hospital, Zhejiang University School of Medicine, Taizhou, 317200, People’s Republic of China; 4Department of Critical Care Medicine, Jinhua People’s Hospital, Jinhua, 321000, People’s Republic of China
Correspondence: Hua Zhou; Junhui Ye, Email [email protected]; [email protected]
Objective: To investigate the clinical features of pulmonary nocardiosis (PN) in patients with different underlying diseases.
Methods: Clinical, imaging, treatment and prognosis data from patients diagnosed with PN from July 2011 to June 2021 at the First Affiliated Hospital, Zhejiang University School of Medicine were collected and analyzed. According to different underlying diseases, patients were grouped into immunocompromised host (ICH) group and immunocompetent host (ICO) group, and clinical characteristics were compared between the two groups.
Results: There were 64 patients with PN, including 42 males, aged from 21 to 86 (57.1 ± 15.7) years. The most common clinical manifestations were cough, expectoration, fever. There were 41 cases in the ICH group and 23 cases in the ICO group. There were 11 cases with underlying pulmonary diseases in the ICH group, including 2 cases of bronchiectasis, 4 cases of chronic obstructive pulmonary disease (COPD), etc. There were 11 cases of underlying pulmonary basic diseases in ICO group, including 7 cases of bronchiectasis and COPD, 1 case of bronchiectasis, 1 case of COPD, etc. The proportion of patients with bronchiectasis and COPD in the ICO group was significantly higher (P < 0.05). Extrapulmonary nocardiosis infection occurred in 6 patients of the ICH group. During the period of hospitalization, 87.0% patients in ICO group received SMZ/TMP therapy, 73.2% of patients in ICH group received two drug combination therapy. In the ICH group, mortality at 28 days was 14.6% and 8.7% in the ICO group.
Conclusion: PN mainly occurred in ICH patients, but also occurred in the ICO cases to a lesser extent, especially in patients with bronchiectasis and/or COPD. Complicated with extrapulmonary infections mainly occurred in ICH population and combination of two antibiotics was often used in ICH group. The case fatality rates were 14.6% in ICH and 8.7% in ICO cases, respectively.
Keywords: pulmonary nocardiosis, PN, immunocompromised host (ICH) group, immunocompetent host (ICO) group, bronchiectasis, COPD
Introduction
Nocardia is a genus of aerobic gram-positive bacteria in the phylum Actinobacteria. It widely exists in natural environments, such as soil, water, dust and corrupt plants.1 The common pathogenic species in clinical practice are named Nocardia asteroides complex (including Nocardia nova, Nocardia Gelsenkirchen, Nocardia Pythium and Nocardia abscess), Nocardia brasiliensis and Nocardia guinea pig otitis.2 Nocardia can cause infections in humans through inhalation or skin wound contact. Common infection sites include skin, lung, brain and other organs. It is an opportunistic pathogen, mainly infecting immunocompromised hosts (ICH).3 Several Nocardia infections in immunocompetent hosts (ICO) have also been reported.4 Patients with bronchiectasis and chronic obstructive pulmonary disease (COPD) may be more susceptible to Nocardia lung disease. However, the clinical symptoms of pulmonary nocardiosis (PN) are atypical, image specificity is low, and diagnosis depends on pathogenic evidence.5 What are the differences in clinical manifestations and prognosis of PN patients with ICH or ICO? Why do ICO patients suffer from PN? Is bronchiectasis or COPD the main underlying disease in ICH patients with pulmonary nocardiosis? In this study, 64 patients with PN at the First Affiliated Hospital Zhejiang University School of Medicine were collected, and patients were divided into the ICH or ICO group. We then summarized and compared the clinical characteristics.6
Patients and Methods
Inclusion Criteria and Participants
From July 2011 to June 2021, 74 patients from the First Affiliated Hospital, Zhejiang University School of Medicine diagnosed as PN according to clinical manifestations, lung imaging and microbiological examination. Two patients with incomplete clinical data and 8 patients with pulmonary infection complicated with other pathogens (Pseudomonas aeruginosa, Mycobacterium tuberculosis, non Mycobacterium tuberculosis, Aspergillus spp, etc) were excluded. Two physicians and one radiologist discussed and voted on cases with differences to determine whether to be included. A total of 64 patients with PN were assessed in this study.
Definitions
PN is a pulmonary infectious disease caused by the bacterium Nocardia. The diagnostic criteria include symptoms such as fever, cough, hemoptysis, chest pain and dyspnea and pulmonary imaging shows infectious lesions.7 Nocardia was isolated from clinical specimens, including sputum, bronchoscopy brushings, alveolar lavage fluid, pleural effusion, etc. Pathogen detection methods include weak acid-fast staining and bacterial culture. If the smear or culture of Nocardia was positive, but the clinical symptoms and imaging manifestations were not consistent with PN, it was judged as colonization. The ICH group were patients with at least one of the following conditions: solid organ or hematopoietic stem cell transplantation; neutropenia; solid or hematological malignancies under chemotherapy (within one month of the last chemotherapy); any type of known immunodeficiency disease; receiving systemic corticosteroids (prednisone equivalent dose of >0.3 mg/Kg) or any type of immunosuppressive drugs (such as methotrexate, azathioprine, cyclosporine or cyclophosphamide) for more than 3 weeks within 90 days.8 The ICO group consisted of patients who did not have any of the above risk factors. The chronic lung diseases mentioned in this paper include COPD, bronchiectasis, interstitial lung disease, and asthma.
Data Collection
Demographic information, coexisting conditions, immunosuppressive drugs used within 3 weeks of symptom onset, clinical symptoms, laboratory examination, chest imaging data, treatment and prognosis were collected from 64 patients.
Data Statistics
The original data were collected and analyzed by SPSS 23.0 software (IBM, Chicago, Illinois, USA). Mean and standard deviation was calculated for continuous variables that were normally distributed, and the statistical analysis was performed using Student’s t-test. Median and interquartile range [described as (Q25, Q75)] were calculated for non-normally distributed data, and the statistical analysis was performed using ManneWhitney test. The independent binomial variables were described as Number and percent, and the chi-square test or Fisher’s exact test was used for inter-group comparisons based on the number of observations. P < 0.05 denotes statistical significance.
Ethics and Informed Consent
This retrospective study was approved by the medical ethics committee of the First Affiliated Hospital Zhejiang University School of Medicine and exempt from informed consent. Ethics approval No. IIT20210577A. The requirement for informed consent was waived by the Ethics Commission due to the retrospective and anonymous characters of the study. We confirmed that the data was anonymized and maintained with confidentiality, compliance with the Declaration of Helsinki.
Results
Basic Patient Information
Among the 64 patients, 42 were male (65.6%), 41 were ICH (64.1%), and 23 were ICO (35.9%). The age of the ICH group was 23~79 (56.2 ± 13.4) years, and the ICO group was 21~86 (58.6 ± 19.2) years. There was no significant difference in age and gender between the two groups (P > 0.05). At the time of diagnosis, there were 59 cases in the general ward and 5 cases in the intensive care unit. The most common ward was respiratory and critical medicine, which had 28 cases (43.7%), followed by infectious diseases with 10 cases (15.6%), nephrology with 7 cases (10.9%), then hematology, geriatrics, etc. (Table 1).
 |
Table 1 Basic Clinical Features of 64 Patients with Pulmonary Nocardiosis |
Coexisting Diseases and Previous Medication
41 ICH patients were complicated with systemic basic diseases, including hematological diseases (leukemia, hemolytic anemia, thrombocytopenic purpura, myelodysplastic syndrome, etc.), autoimmune diseases (systemic lupus erythematosus, dermatomyositis, ANCA associated vasculitis, multiple myositis, psoriasis, rheumatic polymyalgia, pemphigus, etc.), kidney disease (nephrotic syndrome, chronic glomerulonephritis, lupus nephritis, etc.). In the ICH group, 21 patients used glucocorticoids within 3 weeks before onset, 6 patients used glucocorticoids and other immunosuppressants at the same time, and 1 patient used immunosuppressants alone. Patients in the ICO group complicated with systemic basic diseases mainly had hypertension, chronic hepatitis B, and so on.
Among 64 patients, 22 cases (34.4%) were complicated with chronic lung disease. Of these, 11 were in the ICH group, including 2 cases of bronchiectasis, 4 cases of COPD, 5 cases of asthma, 2 cases of allergic bronchopulmonary aspergillosis, and 2 cases of interstitial lung disease. There were also 11 cases in the ICO group, including 8 cases of bronchiectasis complicated with COPD, 1 case of bronchiectasis, 1 case of chronic obstructive pulmonary disease, and 1 case of asthma. The proportion of patients with bronchiectasis and COPD in the ICO group was significantly higher than that in the ICH group (P < 0.05).
Clinical Manifestation
The clinical manifestations of the 64 patients with PN included fever, cough, dyspnea, hemoptysis, fatigue, expectoration, and so on. Clinical symptoms of PN patients in the ICH and ICO groups were similar (Table 2).
 |
Table 2 Clinical Symptoms, Imaging, and Clinical Laboratory Features in 64 Patients with PN |
Findings of Lung CT
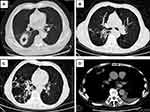
The main manifestations seen on pulmonary CT were consolidation, nodules, cavities, ground glass nodules, masses, mediastinal lymph nodes, bronchiectasis, air bronchial sign, pleural effusion, pericardial effusion, etc (Figure 1). The proportion of bronchiectasis in ICO patients was significantly higher than that in ICH patients (P < 0.05).
Laboratory Examinations
In the ICH group, the average level of C-reactive protein (CRP) was 69.7 (11.4, 131.3) mg/L, erythrocyte sedimentation rate (ESR) was 51.0 (22.0, 90.0) mm/H; In ICO group, CRP was 37.0 (6.11, 24.1) mg/L and ESR was 47.0 (7.0, 72.8) mm/H. There was no significant difference in CRP and ESR between the two groups (P > 0.05).
Microbiological Detection
Nocardia was cultured from clinical samples from each patient. The positive samples included 43 sputum, 16 bronchoalveolar lavage fluid, 4 puncture tissues and 1 pleural effusion. 64 Nocardia strains included 23 cases of N. cyriacigeorgica, 6 cases of N. farcinica, 1 case of N. asteroids, 34 cases of Nocardia spp. There was no significant difference in infection strains between the ICH and ICO groups (P>0.05).
Complicated with Extrapulmonary Infections
Out of 41 patients, 6 in the ICH group were complicated with extrapulmonary infection, including 2 cases with empyema, 2 cases with brain abscess, 1 case with skin and soft tissue infection, and 1 case with skin and soft tissue infection and brain abscess. There were no patients with extrapulmonary infection in the ICO group.
Medical and Treatment
In the entire 64 patient cohort with PN, the time from symptom onset to visit was 15.0 (7.0, 30.0) days, and the time from visit to definitive diagnosis was 3.5 (1.25, 11.0) days. The time from symptom onset to visit was 15.0 (7.0, 30.0) days in the ICH group and 14.0 (7.0, 30.0) days in the ICO group. The time from admission to definitive diagnosis was 6.0 (2.0, 13.5) days in the ICH group and 3.0 (1.0, 7.0) days in the ICO group. The duration of hospitalization was 22.0 (14.5, 35.5) days in the ICH group and 11.0 (7.0, 25.0) days in the ICO group. There was no significant difference between the two groups in treatment time and diagnosis time (P > 0.05), but there was significant difference in length of hospitalization (P < 0.05). Common therapeutic drugs included SMZ/TMP, imipenem, linezolid and amikacin. During the period of hospitalization, 87.0% (20/23) patients in ICO group received SMZ/TMP therapy, 73.2% (30/41) of patients in ICH group received two drug combination therapy. The combination therapy mainly includes SMZ/TMP + imipenem, SMZ/TMP + linezolid, imipenem + amikacin. The rate of receiving combined treatment in ICH group was significantly higher than that in ICO group (P < 0.001).
Outcome
Among the 64 patients with PN, 55 cases (85.9%) improved, 8 cases (12.5%) ended in mortality and 1 case (1.6%) was discharged and the prognosis was unknown. Out of the 41 ICH group cases, 34 cases improved, 6 cases ended in mortality and 1 case was lost to follow-up. Three of the six ICH patients with disseminated nocardiosis died. Out of the 23 ICO group cases, 21 cases improved and 2 cases ended in mortality. The mortality rate of PN of ICH and ICO patients were 14.6% and 8.7%, respectively. There was no significant difference in prognosis between the two groups (P > 0.05).
Discussion
Nocardia bacteria exist widely in the natural environment and is a pathogen of opportunistic infection.9 The clinical symptoms of PN are atypical and the course of disease is diverse (acute, subacute, and chronic clinical). Clinical diagnosis is often delayed and treatment is not timely. PN needs to be differentiated from pulmonary tuberculosis, non-tuberculous Mycobacterium infection, pulmonary fungal infection, pulmonary malignancy and other diseases.10 Patients with PN have a large age span and the incidence rate of males is higher than that in females in this study, which is consistent with the literature.11 PN mainly occurred in ICH patients. Among the 64 patients diagnosed over 11 years in this study, the proportion of ICO was also high, accounting for 35.9%.
Risk factors for PN included systemic underlying diseases and underlying lung diseases. The former mainly includes neutropenia, malignant tumors, solid organ transplantation, acquired immunodeficiency syndrome, diabetes and autoimmune diseases. The latter consisted mainly of chronic lung disease (including COPD, bronchiectasis, etc.). PN occurred in COPD and bronchiectasis patients without any other immunosuppressive factors.12 PN in bronchiectasis or COPD might be related to the decrease of airway clearance and the local pulmonary immune function which leads to an increased chance of Nocardia colonization in the lower respiratory tract.13 Patients with COPD and asthma need to inhale glucocorticoids frequently, and even use systemic glucocorticoids for a short time during acute attacks, which might increase the risk of PN. This study confirmed that bronchiectasis and COPD were the most important risk factors for PN in ICO patients. Patients with ICH had a higher risk of nocardiosis due to severe underlying diseases and use of glucocorticoids and/or immunosuppressants.14
PN could be acute, subacute, or chronic, and its symptoms are diverse and nonspecific.15 Patients with subacute or chronic disease often postponed seeing a doctor because of the slow development of symptoms, and diagnosis was often delayed because of atypical symptoms. At the time of onset, CRP and ESR were significantly increased. Blood leukocytes and neutrophils were not included in this study, mainly considering the effect of coexisting diseases and drugs in the ICH cases. The most common imaging manifestations of PN were nodules, consolidation, and mediastinal lymph node enlargement, with or without cavity.16 This study also found that the tree-in-bud pattern on the basis of bronchiectasis can be a manifestation of PN. However, lung CT manifestations of pulmonary nocardiosis patients were not specific, and were difficult to differentiate from other infectious diseases.
The cases enrolled in this study mainly relied on culture results to diagnose PN. However, the positive rate of nocardiosis smears was low, the culture time was long, which could lead to the possibility of delaying diagnosis. Molecular diagnoses for nocardiosis are more sensitive. Data show that the specificity and sensitivity of using PCR in the diagnosis of nocardiosis were about 74% and 88%, respectively.17 MALDI-TOF MS can quickly and accurately identify strains, and high-throughput sequencing technology also has unique advantages in etiological diagnosis.18 Drug sensitivity test was important in antibiotic selection. This was an important defect of this study. We did not carry out drug sensitivity experiments on cultured positive Nocardia, so there was a lack of drug sensitivity data.
The prognosis of pulmonary nocardiosis depends on timely diagnosis and treatment, and the cure rate of PN under timely and reasonable treatment could be 90%.19 If the patient’s immunosuppressive state was difficult to improve, the diagnosis was not timely and the treatment was not in place, the mortality rate could be 56.7~ 100%.20 In this study, the overall cure rate of PN was 85.9%, patients in ICH group consisted of multiple comorbidities and extrapulmonary infections, the mortality was not higher than that of ICO group. The possible reason was that the time from onset to diagnosis in ICH group was 6 days, and 73.2% of patients received combined treatment. Early diagnosis and combined treatment might be part of the reason for the low mortality. However, the retrospective data of single center were flawed and insufficient. More research is needed, especially prospective research.
There were some deficiencies in this study. Mainly, this was a single center retrospective study that included a small cohort of patients. There is also no drug sensitivity data for Nocardia, making it impossible to verify whether there were drug-resistant strains. The lack of molecular diagnostic technology might have led to missed diagnosis or delayed diagnosis in some patients. The follow-up data after discharge was not obtained, the complete treatment process was not described in the study.
Conclusion
PN mainly occurred in ICH patients, but also occurred in the ICO cases to a lesser extent, especially in patients with bronchiectasis and/or COPD. Complicated with extrapulmonary infections only occurred in ICH population. The combination of two drugs was often used in PN patients in ICH group. The case fatality rates were 14.6% in ICH and 8.7% in ICO cases, respectively.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi:10.1016/j.mayocp.2011.11.016
2. Yu S, Wang J, Fang Q, et al. Specific clinical manifestations of Nocardia: a case report and literature review. Exp Ther Med. 2016;12(4):2021–2026. doi:10.3892/etm.2016.3571
3. Margalit I, Lebeaux D, Tishler O, et al. How do I manage nocardiosis? Clin Microbiol Infect. 2021;27(4):550–558. doi:10.1016/j.cmi.2020.12.019
4. Abe S, Tanabe Y, Ota T, et al. Case report: pulmonary nocardiosis caused by Nocardia exalbida in an immunocompetent patient. BMC Infect Dis. 2021;21(1):776. doi:10.1186/s12879-021-06416-w
5. Liu B, Zhang Y, Gong J, et al. CT findings of pulmonary nocardiosis: a report of 9 cases. J Thorac Dis. 2017;9(11):4785–4790. doi:10.21037/jtd.2017.09.122
6. Igbaseimokumo U, El Shafie S, Al Khal AL. First Human Infection of Nocardia Crassostreae in an Immunocompetent Patient. Chin Med J. 2016;129(1):114–115. doi:10.4103/0366-6999.172609
7. Ott SR, Meier N, Kolditz M, et al. Pulmonary nocardiosis in Western Europe-Clinical evaluation of 43 patients and population-based estimates of hospitalization rates. Int J Infect Dis. 2019;81(4):140–148. doi:10.1016/j.ijid.2018.12.010
8. Zhan Y, Xu T, He F, et al. Clinical Evaluation of a Metagenomics-Based Assay for Pneumonia Management. Front Microbiol. 2021;12:751073. doi:10.3389/fmicb.2021.751073
9. Steinbrink J, Leavens J, Kauffman CA, et al. Manifestations and outcomes of nocardia infections: comparison of immunocompromised and nonimmunocompromised adult patients. Medicine. 2018;97(40):e12436. doi:10.1097/MD.0000000000012436
10. Kandi V. Human Nocardia Infections: a Review of Pulmonary Nocardiosis. Cureus. 2015;7(8):e304. doi:10.7759/cureus.304
11. Singh A, Chhina D, Soni RK, et al. Clinical spectrum and outcome of pulmonary nocardiosis: 5-year experience. Lung India. 2016;33(4):398–403. doi:10.4103/0970-2113.184873
12. Tamakoshi J, Kimura R, Takahashi K, et al. Pulmonary Reinfection by Nocardia in an Immunocompetent Patient with Bronchiectasis. Intern Med. 2018;57(17):2581–2584. doi:10.2169/internalmedicine.0531-17
13. Kuchibiro T, Ikeda T, Nakanishi H, et al. First case report of pulmonary nocardiosis caused by Nocardia mexicana. JMM Case Rep. 2016;3(4):e005054. doi:10.1099/jmmcr.0.005054
14. Margalit I, Goldberg E, Ben Ari Y, et al. Clinical correlates of nocardiosis. Sci Rep. 2020;10(1):14272. doi:10.1038/s41598-020-71214-4
15. Li Y, Tang T, Xiao J, et al. Clinical analysis of 11 cases of nocardiosis. Open Med. 2021;16(1):610–617. doi:10.1515/med-2020-0196
16. Yadav P, Kumar D, Meena DS, et al. Clinical Features, Radiological Findings, and Treatment Outcomes in Patients with Pulmonary Nocardiosis: a Retrospective Analysis. Cureus. 2021;13(8):e17250. doi:10.7759/cureus.17250
17. Rouzaud C, Rodriguez-Nava V, Catherinot E, et al. Clinical Assessment of a Nocardia PCR-Based Assay for Diagnosis of Nocardiosis. J Clin Microbiol. 2018;56(6):74. doi:10.1128/JCM.00002-18
18. Zhou H, Larkin P, Zhao D, et al. Clinical Impact of Metagenomic Next-Generation Sequencing of Bronchoalveolar Lavage in the Diagnosis and Management of Pneumonia: a Multicenter Prospective Observational Study. J Mol Diagn. 2021;23(10):1259–1268. doi:10.1016/j.jmoldx.2021.06.007
19. Yang HQ, Shi HZ, Tong ZH. [Retrospective analysis of 13 cases of nocardiosis]. Zhonghua Jie He He Hu Xi Za Zhi. 2017;40(8):588–591. doi:10.3760/cma.j.issn.1001-0939.2017.08.009. Chinese.
20. Takiguchi Y, Ishizaki S, Kobayashi T, et al. Pulmonary Nocardiosis: a Clinical Analysis of 30 Cases. Intern Med. 2017;56(12):1485–1490. doi:10.2169/internalmedicine.56.8163
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.