Back to Journals » Clinical Ophthalmology » Volume 14
Clinical Features of Pseudoexfoliative Glaucoma in Treated Polish Patients
Authors Łukasik U , Kosior-Jarecka E , Wróbel-Dudzińska D , Kustra A , Milanowski P , Żarnowski T
Received 20 November 2019
Accepted for publication 6 April 2020
Published 21 May 2020 Volume 2020:14 Pages 1373—1381
DOI https://doi.org/10.2147/OPTH.S239371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Urszula Łukasik, Ewa Kosior-Jarecka, Dominika Wróbel-Dudzińska, Agnieszka Kustra, Piotr Milanowski, Tomasz Żarnowski
Department of Diagnostics and Microsurgery of Glaucoma, Medical University of Lublin, Lublin, Poland
Correspondence: Urszula Łukasik
Department of Diagnostics and Microsurgery of Glaucoma, 1 Chmielna Street, Lublin 20-079, Poland
Email [email protected]
Purpose: The aim of this study was to describe clinical characteristics of glaucomatous optic neuropathy in treated Polish patients with pseudoexfoliative glaucoma.
Methods: In the course of the study, 348 eyes of 231 patients with pseudoexfoliative glaucoma. The patients involved in the study were treated in the Department of Diagnostic and Microsurgery of Glaucoma in Lublin between 2012 and 2019. The following parameters were assessed in the examination: visual acuity, slit-lamp biomicroscopy with evaluation of anterior segment of the eye, gonioscopic examination, stereoscopic fundus examination of the eye, intraocular pressure, visual field, and pachymetry.
Results: The mean age of all the patients was 73.16 years (SD± 8.03). The mean age of women was 74.06 (SD± 6.97), and the mean age of men was 71.8 (SD± 8.51, p=0.006265). Women represented 37.93% (n=132) of the studied group, while men 62.07% (n=216). In the group of patients younger than 65 years of age, 27.9% were male and 15% female (p=0.0021). In the whole studied group, mean peak IOP was 29.25 mmHg with higher mean values in male patients (M vs F: 33.24 mmHg vs 26.86 mmHg; p=0.000). Peak values exceeding 30 mmHg were significantly more frequent in males (M vs F: 56.5% vs 31.9%; p=0.0000). Peak IOP never exceeding 21 mmHg was observed in 18.6% of the patients. The mean value of MD (Mean Deviation) was − 12.85 dB in the whole group. The men were more likely to have more advanced glaucoma, according to MD (M vs F: − 16.35 dB vs − 11.13 dB; p=0.0000).
Conclusion: Pseudoexfoliative glaucoma was more frequently observed in men with younger age, higher IOP, and more advanced glaucoma. Normotensive glaucoma was observed in 18.6% of the patients with pseudoexfoliative glaucoma.
Keywords: pseudoexfoliation syndrome, pseudoexfoliative glaucoma, glaucoma, epidemiology
Introduction
Pseudoexfoliation syndrome (XFS) was initially reported by the Finnish ophthalmologist John Lindberg in 1971.1 It affects between 60 and 70 million people worldwide2 and between 0.3% and 30% of people aged 60 or more.3 XFS is an age-related systemic disease characterised by production and deposition of extracellular fibrillar material in several ocular and extraocular tissues.4 In the eye, XFS appears as fine “dandruff-like” material typically localised on the anterior lens capsule, but its deposits can also be found on the pupillary margin, lens zonules, trabecular meshwork, the face of the ciliary body, and on the corneal endothelium.5 The presence of exfoliation material in the eye affects the prevalence of some intraocular diseases, such as glaucoma, cataract, lens subluxation, iris atrophy, or keratopathy similar to Fuchs keratopathy.6
The etiopathogenesis of XFS involves both genetic and non-genetic factors. Development of XFS is strongly associated with variants of the lysyl oxidase-like 1 (LOXL1) gene, particularly, three single-nucleotide polymorphisms (SNPs) increase the risk of XFS7 The lysyl oxidase-like (LOXL) gene is relevant to XFS pathogenesis in that it codes for a family of enzymes that catalyzes the covalent cross-linking of collagen and elastin in extracellular matrix.8 CACNA1A was discovered as the second locus associated with susceptibility to XFS.9 Numerous environmental factors such as solar irradiation and climatic variables are hypothesised to be responsible for the latitude effect.10 Additionally, dietary factors are mentioned: low folate intake is related to elevated homocysteine levels, which is in turn associated with increased risk of XFS.11
Elevated intraocular pressure, with or without glaucomatous neuropathy, occurs in approximately 25% XFS eyes.12 Pseudoexfoliative glaucoma (XFG) is the most common type of secondary open-angle glaucoma.13 XFS is confirmed as a significant risk factor for glaucoma; glaucoma occurs 6 to 10 times more often in eyes with pseudoexfoliation syndrome compared to eyes without XFS.14 Pathogenesis of glaucoma during the course of XFS still remains unclear and is attributed to various reasons, such as the mechanical blockage of the trabecular meshwork (TM) caused by exfoliation material and ischemic or molecular insults which cause irreversible damage to the tissue.15
There are numerous publications on the epidemiology of XFS, but it is difficult to find epidemiological data regarding pseudoexfoliative glaucoma. Therefore, the aim of the present study was to describe clinical characteristics of glaucomatous optic neuropathy in diagnosed and treated Polish patients with pseudoexfoliative glaucoma.
Materials and Methods
The research project was designed as cross-sectional one-center study carried out in the Department of Diagnostic and Microsurgery of Glaucoma of the Medical University of Lublin in the years 2012 to 2019. The study was approved by local Ethics Committee (approval number 127/12) and tenets to the Declaration of Helsinki. The studied group consisted of 348 eyes of 231 Caucasian patients with pseudoexfoliative glaucoma. The study involved all the patients with glaucomatous neuropathy in the course of pseudoexfoliation syndrome in at least one eye who had provided written the informed consent. The diagnosis of XFS was based on presence dandruff-like exfoliative material on the anterior lens capsule in a central disc and peripheral band (double concentric ring) pattern and/or in the anterior segment of the eye. In the case of pseudophacic eyes without detected exfoliation material on slit-lamp examination, the diagnosis was based on medical records.
Glaucoma was diagnosed in cases of optic nerve neuropathy with a characteristic optic disc damage pattern detected during stereoscopic examination, with characteristic visual field loss or changes in RNFL typical for glaucomatous neuropathy observed in OCT. Patients with pseudoexfoliation syndrome without glaucoma or elevated IOP, nor with ocular hypertension in course of XFS but without glaucomatous neuropathy were not included to the study. The patients with peak IOP above 21 mmHg were designated as high-tension cases and those under 21 mmHg, as normal-tension cases.
Before being included in the study, in order to confirm the diagnosis, the following parameters were assessed: best-corrected visual acuity (BCVA), using Snellen charts with decimal scale; slit-lamp biomicroscopy with the evaluation of the anterior segment of the eye; gonioscopy, using Zeiss four-mirror gonioscope (grading width of the angle according to Schaffer’s classification); as well as the stereoscopic fundus examination of the eye with a detailed assessment of the optic disc morphology. Intraocular pressure was measured by Goldman applanation tonometry (3 measurements were performed for each eye, the mean value was calculated, IOP was accedes during office hours). Measurements of the central corneal thickness (CCT) were performed using an ultrasonic pachymeter (10 measurements were performed for each eye and in case of SD lower than the mean value was calculated, PIROP, EHOSON S.A), and IOP measurements were corrected according to the results. Visual field test was performed with the use of Humphrey automated perimeter using 30–2 SITA Fast strategy when BCVA was above 0.1 and semikinetic mode with BCVA under 0.1. The staging of visual field defects was assessed using the Hodapp-Parish-Anderson classification:
Early glaucomatous loss MD<-6 dB, moderate glaucomatous loss: MD <-12 dB, advanced glaucomatous loss MD >-12 dB.16
The evaluation of the retinal nerve fiber layer was performed with OCT (Optical Coherence Tomography, Cirrus™ HD-OCT, Model 4000). The history of glaucoma (duration, family history) and chronic general disorders were obtained using a questionnaire.
Statistic evaluation of the data was performed using Statistica 13.1 (Polish version, Statsoft Poland). The results were reported mainly as mean±SD or percentage values. A p-value lower than 0.05 was considered statistically significant. Normal distribution was assessed with the Shapiro–Wilk test. The Mann–Whitney test was used for non-normally distributed data. Proportions were analysed by means of the chi-square test with Yates correction when needed.
Results
The study involved 348 eyes of 231 patients with pseudoexfoliative glaucoma. Demographic characteristic of studied population is put in Table 1. Women represented 37.93% (n=132) of the studied group, and men 62.07% (n=216) (p=0.006).
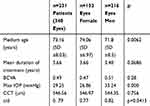 |
Table 1 Characteristics of the Study Group |
The mean age of all the patients was 73.16 years (SD ±8.03). The men were significantly younger than the women (M vs F: 71.8±8.51 vs 74.06±6.97; p=0.006265). In the group of patients younger than 65 years, 27.9% were male participants and 15% were women (p=0.0021). In the whole group, the mean duration of antiglaucoma treatment was 3.66 years and there was the tendency for male to be treated over a shorter period of time (M vs F: 3.40 vs 3.66y; p=0.0686).
The exfoliation syndrome was clinically unilateral in 40.25% patients (n=93 patients), bilateral in 59.75% (n=138 patients) in the whole studied group. In women, XFS was clinically unilateral in 35.25% of the cases, and in men in 47.82%. The bilateral involvement was more frequent in female patients (F vs M: 64.7% vs 52.1%; p=0.056). Bilateral involvement was significantly more frequent in patients younger than 65 years of age (younger vs older: 78.32% vs 42.85%; p=0.000). In patients with higher peak IOP (IOP ≥ 30 mmHg), bilateral involvement was less frequent (64.18% vs 76.68%; p=0.0137) in comparison to the group with peak IOP lower than 30 mmHg.
Best-corrected visual acuity was (BCVA) 0.49 for the studied group and it did not differ between the sexes (F vs M: 0.47 vs 0.51; p=0,08). Central corneal thickness (CCT) was 546.56 µm, similar in females and males (p=0.756).
In the whole studied group, the mean peak IOP was 29.25 mmHg, with higher mean values in male patients (M vs F: 33.24 mmHg vs 26.86 mmHg; p=0.000) [Figure 1]. Peak values exceeding 30 mmHg were also significantly more frequent in male patients (M vs F: 56.5% vs 31.9%; p=0.0000). Peak IOP never exceeding 21 mmHg was observed in 18.6% patients (n=43; F vs M: 79% vs 21%, p<0.05).
 |
Figure 1 Relationship between IOP and gender. 1, Female. 2, Male. |
Iridocorneal angle closure (Grade 0) was observed in 2.71% eyes of the whole studied group, Grade 1 in 6.79%, Grade 2 in 15.84%, Grade 3 in 34.84%, and Grade 4 in 39.82%. Closed and narrow-angle was described in 25.34% eyes. In the group of women, angle closure (Grade 0) was found in 3.62%, Grade 1 in 5.80%, Grade 2 in 14.5%, Grade 3 in 39.13%, and Grade 4 in 36.95%. In the group of men, angle closure (Grade 0) was found in 1.2%, Grade 1 in 8.43%, Grade 2 in 18.08%, Grade 3 in 27.71%, and Grade 4 in 44.58% (p=0.31). In the study, narrow or closed-angle was found to be more frequent in women (F vs M 14.93% vs 10.4%, p=0.62). The cup to disc ratio was 0.79 in the whole group (F vs M: 0.77 vs 0.82; p=0.0415). The mean value of MD (Mean Deviation) was −12.85 dB in the whole group.
According to MD (M vs F: −16.35 dB vs −11.13 dB; p=0.0000), men were likely to have more advanced glaucoma [Figure 2]. Pattern standard deviation was 8.1866 dB in the group of men and 6.6901 dB in the group of women (p=0.007204). MD > −20 dB was assessed in 36.26% of men and 18.24% of women (p=0.0015). Early visual field defects (0–6 dB) were detected in 26.83% of women, moderate visual field defects (6–12 dB) in 25.61% of women, while advanced (>12 dB) in 47.56% of women. Early visual field defects (0–6 dB) were detected in 13.3% of men, moderate (6–12 dB) in 20.%, while advanced (>12 dB) in 66.7% (p= 0.056994). The correlations between IOP, CCT and the demographic and clinical features are put in Table 2.
 |
Table 2 Correlations Between IOP, CCT and Demographic Variables |
 |
Figure 2 Relationship between MD and gender. 1, Female. 2, Male. |
The assessment of the scotoma pattern resulted in the following findings in the group of women: normal VF in 8.47%, paracentral scotoma in 14.84%, arcuate scotoma in 27.55%, nasal step in 10.17%, altitudinal visual field defect in 13.56%, and the advanced VF defect in 25.42%; corresponding results in the group of men were as follows: normal VF in 4.0%, paracentral scotoma in 14.67%, arcuate scotoma in 16.0%, nasal step in 4.0%, altitudinal visual field defect in 13.33%, advanced VF defect in 48.0%. Their morphology of VF scotoma differed significantly between the sexes (p=0.016633).
When assessing the mean defect in VF in the group of patients with early changes, XFS was clinically bilateral in 70.0%, in moderate changes group, XFS was bilateral in 63.63% and in the advanced changes group, XFS was bilateral in 68.35%. In the group of patients with severe damage in VF (MD > −20 dB), XFS was clinically bilateral in 72.34%., whereas in less severe cases group (MD < −20 dB), XFS was clinically bilateral in 56.45%. In patients with more advanced glaucoma, bilateral involvement was more frequent (p=0.0196). Family history of glaucomatous neuropathy was reported by 6.32% patients.
When assessing general chronic diseases, 15.47% of the studied patients suffered from diabetes with no difference in its prevalence between the sexes (p=0.617), 16.05% from hypertension with significant female preponderance (F vs M: 28.22% vs 10.0%; p=0.005) and 5.19% from the ischaemic heart disease.
Discussion
The pseudoexfoliation syndrome is an age-related disease characterised by production of abnormal fibrillar-granular protein in the eye and the body. The exfoliation material plays an important role in the development of decreased aqueous humour outflow and elevation of IOP, which may lead to the development of glaucoma. In our study, the medium age of patients with pseudoexfoliative glaucoma was 73.16, which is similar to the mean age of our primary open-angle glaucoma (POAG) patients.17 Arnarsson et al found that prevalence of XFS increases significantly with age: 2.5% of people aged 50–59 years had XFS; whereas 40.6% of those aged 80 years or more were affected.18 Numerous studies confirm greater rates of prevalence with increasing age19–21 but when adjusting for age and gender, the life span was not statistically different in persons with and without XFS.22 However, it is interesting that, in our study, male patients who had more severe course of pseudoexfoliative glaucoma were significantly younger than the women. Additionally, IOP had weak negative correlation with age which may show that in PEX patients IOP tends to decrease with age. This observation needs further studies.
Studies have reported varying prevalence of XFS as far as gender is concerned. Kounsar et al revealed that 62% of the cases were males,23 similarly to our results for pseudoexfoliative glaucoma; however, other researchers also reported female24–26 or no gender preponderance. Some studies suggest that male individuals with XFS may suffer more commonly from glaucoma,27,28 as is the case in our study. A Greek study29 established a male to female ratio of 2.8:1 in patients undergoing filtration surgery. Similarly, in a Hungarian PEXG cohort, the male to female ratio was 1.67:1,30 comparably to our results (M to F: 1.63:1). They reported that 14.8% of the male individuals in their studied population and 18.2% of females had XFS, whereas XFG was present in 35.9% of the affected male individuals, and in 25.7% of females.31 When we additionally extracted younger patients with pseudoexfoliative glaucoma, even higher male preponderance (M to F: 1.81:1) was observed, which indicates that, in our group, pseudoexfoliative glaucoma was more frequently observed among male participants, especially in the younger group.
XFS often appears as unilateral in clinical examination. In a series of 418 Caucasians patients, XFS was unilateral in 48% of cases;32 the Blue Mountains Study indicated that XFS was unilateral in 52% of the cases,33 but in a Japanese group, 85% of patients had unilateral involvement.34 Immunohistochemical studies of autopsy specimens have shown similarly labeled deposits around blood vessels of the iris in every involved and fellow eye, which indicates that unilateral XFS is probably never truly unilateral, but rather asymmetric.35 Unilateral XFS is often a precursor of bilateral involvement. Aasved et al observed that 43% of Norwegian patients developed binocular involvement after 6 to 7 years.36 In our study concerning XFG, unilateral involvement was much less frequent than in the studies cited above, which indicates that bilateral involvement may be a risk factor for conversion of XFS to glaucoma, perhaps due to more XFS material in the eyes. On the other hand, in this study, unilateral involvement was more frequent in male patients who generally tend to have a more severe course of glaucoma.
Intraocular pressure in XFG is known to be higher than in POAG and, according to CIGS, it is about 31.9 mmHg,37 similarly to our results. Patients with XFS also show a greater 24-hr pressure fluctuation.38 Elevation of IOP is suspected to be caused by blockage of aqueous humor outflow by pigment deposits and/or exfoliation material as well as increased outflow resistance, primarily in the trabecular meshwork.39 Furthermore, IOP has been reported to be significantly higher in male patients with XFG, which is similar to our results.
Pseudoexfoliative glaucoma is typically described as a hypertensive form. However, normotensive variants of XFG have been reported. In the study by Koz et al, normal-tension glaucoma was diagnosed in 24% of eyes with the pseudoexfoliation syndrome.40 We also observed a group of patients with XFS, glaucomatous damage of the optic nerve, and normal IOP. In our study, normotensive type of glaucoma occurred in 18.6% of eyes with pseudoexfoliative glaucoma, which is a percentage similar to the one found for NTG in the POAG group. Pressure independent mechanisms for the XFG are complex and not well known. Abnormal ocular and retrobulbar perfusion and abnormality of the elastic tissue of the lamina cribrosa increase the risk of glaucomatous damage.41 Reduced blood flow values in the optic nerve head and the peripapillary retina, measured by Heidelberg retinal flowmeter, have been observed in eyes with clinically detected exfoliation.42 The cytotoxic properties of exfoliation deposits may be involved here.43 On the other hand, it may be a form of typical normal-tension glaucoma, with exfoliative deposits as an accidental finding. The description of the risk factors and course of normal-tension glaucoma with PEX require further evaluation.
Pseudoexfoliative glaucoma is a subtype of secondary open-angle glaucoma, but narrow angles are relatively common. A considerable part (9% to 18%) of patients with XFS have occludable angles,44,45 which seems to be even more significant in our XFG group, where narrow angles were observed in 25.34% of eyes. Furthermore, narrow angles were more frequent in women, as well as the risk for primary narrow angle in Caucasian patients. Studies estimating the global prevalence of the disease have shown that females represent 70% of all the cases of primary angle-closure glaucoma.46 To our knowledge, there is no data in literature about the width of the angle in relation to gender in pseudoexfoliative glaucoma patients.
The course of pseudoexfoliative glaucoma is often asymptomatic until advanced visual field loss occurs. Frequently, in the eyes with XFG, visual field deterioration is, at the time of the first visit, already severe, which was observed in our study; in the whole group, the average MD was −12.85 dB and moderate and advanced neuropathy was frequently described. It may be related to the fact that our Ward is a reference center for glaucoma treatment in Poland. On the other hand, the course of pseudoexfoliative glaucoma is known to be more aggressive and with poorer prognosis when compared to POAG.47
In our study, male participants were likely to have more advanced glaucoma changes with higher peak IOP values with unilateral eye involvement. However, the mechanisms of male preponderance and more severe course of glaucoma are unknown. Studies estimating global disease prevalence have shown that females represent 59% of all glaucoma cases,48 because of their greater prevalence of angle-closure glaucoma, as well as their relatively greater longevity. Women are estimated to have twice as much visual impairment and blindness overall.49 There are numerous relevant studies about the role of female hormones in glaucoma. There are data suggesting that estrogen levels in women may influence POAG,50,51 but very little is known about the role of male sex hormones in glaucoma. Two datasets, one from the United States and another from Australia, evaluated gene variants related to testosterone metabolism collectively and POAG risk, demonstrating that testosterone metabolism pathway SNPs were consistently associated with the high-tension subtype of POAG.52 Moreover, there are no data about the role of hormones in PEXG. Possible influence of testosterone metabolism on pseudoexfoliative glaucoma patients, as typical high-tension glaucoma, requires further evaluation.
There are some data on the difference between genders in course of pseudoexfoliative glaucoma on molecular basis. PEX is thought to represent aberrant extracellular matrix synthesis; matrix metalloproteinases (MMPs) and their inhibitors (TIMPs) are the enzymes participating in ECM remodeling. Imbalance in the levels of MMPs and TIMPs may be an important factor in the maintenance and regulation of ECM in the TM.53,54 The study also shows elevated concentrations of TIMP1, TIMP2, TIMP4 in aqueous humor samples from POAG. This may lead to changed ECM composition in the TM and, thereby, result in increased outflow resistance.55 Significantly higher activity of aqueous humor TIPM-1 in male patients with XFX was shown, with a significantly higher activity of aqueous humor TIMP-4 in older male patients in the group with PEX glaucoma.56
Pseudoexfoliation syndrome and pseudoexfoliative glaucoma are frequently associated with systemic vascular diseases. Several studies have reported an association between cardiovascular and cerebrovascular morbidity, Alzheimer’s disease, aorta aneurysm, and elevated plasma homocysteine level.57–60 In our study, 16.05% of the patients suffered from hypertension, and 5.19% from the ischaemic heart disease. Furthermore, some studies suggest that diabetic patients are at increased risk for the development of glaucoma.61 In our study, 15.47% of the patients suffered from diabetes. It is a higher frequency in comparison to the prevalence of diabetes in the overall population (8.8% among adults of the global population).62
Our study has some limitations. It describes patients treated in a clinic specializing in treatment of glaucoma so they may include only the patients with most severe forms and stages of the disease. Additionally, given the asymptomatic nature of the condition, referral bias could have occurred with females seeking ophthalmic examination less frequently in the community as one possible confounding variable. However, in the general medical practice in Poland, women significantly more frequently seek medical examination than men.63 Similar reporting may be observed for ophthalmic examination; however, there is no data.
To sum up, in the Polish population, pseudoexfoliative glaucoma is more frequently observed among males who are younger, have higher IOP values, and more advanced glaucoma neuropathy. Normotensive glaucoma was observed in 18.6% of the patients with pseudoexfoliative glaucoma.
Acknowledgments
No funds were obtained for the study. All data and materials are available for request from the corresponding author. All authors declare no financial interests.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Lindberg JG Kliniska Undersökningar över depigmenteringen av pupillarranden och genomlysbarheten av iris vid fall av åldersstarr samt i normala ögen hos gamla personer. Thesis. Helsingfors Univesitat. Helsingfors;1917.
2. Belovay GW, Varma DK, Ahmed IIK. Cataract surgery in pseudoexfoliation syndrome. Curr Opin Ophthalmol. 2010;21:25–34. doi:10.1097/ICU.0b013e328332f814
3. You QS, Xu L, Wang YX, et al. Pseudoexfoliation: normative data and associations: the Beijing eye study 2011. Ophthalmology. 2013;120(8):1551–1558. doi:10.1016/j.ophtha.2013.01.020
4. Elhawy E, Kamthan G, Dong CQ, et al. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Hum Genomics. 2012;6:22. doi:10.1186/1479-7364-6-22
5. Zenkel M, Schlötzer-Schrehardt U. The composition of exfoliation material and the cells involved in its production. J Glaucoma. 2014;23:S12–4. doi:10.1097/IJG.0000000000000123
6. Hassanee K, Ahmed I. Pseudoexfoliation syndrome in cataract surgery ophthalmol. Clin N Am Am. 2006;19(4):507–519.
7. Thorleifsson G, Magnusson KP, Sulem P, et al. Common sequence variants in the loxl1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317(5843):1397–1400. doi:10.1126/science.1146554
8. Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19–20):2304–2316. doi:10.1007/s00018-006-6149-9
9. Aung T, Ozaki M, Mizoguchi T, et al. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nat Genet. 2015;47:387–392. doi:10.1038/ng.3226
10. Pasquale LR, Jiwani AZ, Zehavi-Dorin T, et al. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. JAMA Ophthalmol. 2014;132:1439–1445. doi:10.1001/jamaophthalmol.2014.3326
11. Xu F, Zhang L, Li M. Plasma homocysteine, serum folic acid, serum vitamin B12, serum vitamin B6, MTHFR and risk of pseudoexfoliation glaucoma: a meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1067–1074. doi:10.1007/s00417-011-1877-4
12. Ritcg R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22(3):253–275. doi:10.1016/S1350-9462(02)00014-9
13. Ritch R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3(2):176–177. doi:10.1097/00061198-199400320-00018
14. Kanthan GL, Mitchell P, Burlutsky G, et al. Pseudoexfoliation syndrome and the long-term incidence of cataract and cataract surgery: the Blue Mountains Eye Study. Am J Ophthalmol. 2013;155:83–88. doi:10.1016/j.ajo.2012.07.002
15. Aboobakar IF, Johnson WM, Stamer WD, Hauser MA, Allingham RR. Major review: exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp Eye Res. 2016;11(154):88–103.
16. Hodapp E, Parrish RK
17. Kosior-Jarecka E, Łukasik U, Wróbel-Dudzińska D, et al. Risk factors for normal and high-tension glaucoma in Poland in connection with polymorphisms of the endothelial nitric oxide synthase gene. PLoS One. 2016;11(1):e0147540. doi:10.1371/journal.pone.0147540
18. Arnarsson A, Damji KF, Sverrisson T, Sasaki H, Jonasson F. Pseudoexfoliation in the Reykjavik Eye Study: prevalence and related ophthalmological variables. Acta Ophthalmol Scand. 2007;85:822–827.
19. Miyazaki M, Kubota T, Kubo M, et al. The prevalence of pseudoexfoliation syndrome in a Japanese population the Hisayama study. J Glaucoma. 2005;14:482–484. doi:10.1097/01.ijg.0000185436.15675.b3
20. Arvind H, Raju P, Paul PG, et al. Pseudoexfoliation in south India. Br J Ophthalmol. 2003;87:1321–1323. doi:10.1136/bjo.87.11.1321
21. Astrom S, Stenlund H, Linden C. Incidence and prevalence of pseudoexfoliation syndrome and open-angle glaucoma in northern Sweden. Part I and II. Acta Ophthalmol Scand. 2007;85:828–837. doi:10.1111/j.1600-0420.2007.00992.x
22. Slettedal JK, Sandvik L. Ringvold A ocular pseudoexfoliation syndrome and life span. EBioMedicine. 2015;2:765–769. doi:10.1016/j.ebiom.2015.05.024
23. Kounsar H, Shaheen N, Rather SR. Epidemiology of pseudoexfoliation syndrome: a hospital based comparative study. Int J Res Med Sci. 2018;6(4):1314–1321. doi:10.18203/2320-6012.ijrms20181289
24. Hiller R, Sperduto RD, Krueger DE. Pseudo-exfoliation, intraocular pressure, and senile lens changes in a population-based survey. Arch Ophthalmol. 1982;100:1080–1082. doi:10.1001/archopht.1982.01030040058007
25. McCarty CA, Taylor HR. Pseudoexfoliation syndrome in Australian adults. Am J Ophthalmol. 2000;129(5):629–633. doi:10.1016/S0002-9394(99)00466-3
26. Karger RA, Jeng SM, Johnson DH, Hodge DO, Good MS. Estimated incidence of pseudoexfoliation syndrome and pseudoexfoliation glaucoma in Olmsted County, Minnesota. J Glaucoma. 2003;12(3):193–197. doi:10.1097/00061198-200306000-00002
27. Ringvold A. Epidemiology of glaucoma in Northern Europe. Eur J Ophthalmol. 1996;6:26–29. doi:10.1177/112067219600600107
28. Ringvold A. Epidemiology of the pseudoexfoliation syndrome. A review. Acta Ophthalmol Scand. 1999;77:371–375. doi:10.1034/j.1600-0420.1999.770401.x
29. Konstas AG, Allan D. Pseudoexfoliation glaucoma in Greece. Eye. 1989;3:747–753. doi:10.1038/eye.1989.116
30. Sziklai P, Suveges I. Glaucoma capsulare in patients with open angle glaucoma in Hungary. Acta Ophthalmol Suppl. 1988;184:90–92. doi:10.1111/j.1755-3768.1988.tb02635.x
31. Ringvold A, Blika S, Elsas T, et al. The Middle-Norway eye-screening study. II. Prevalence of simple and capsular glaucoma. Acta Ophthalmol. 1991;69:273–280.
32. Tarkkanen A. Pseudoexfoliation of the lens capsule. A clinical study of 418 patients with special reference to glaucoma, cataract, and changes of the vitreous. Acta Ophthalmol. 1962;(Suppl):40.
33. Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: the Blue Mountains eye study. Arch Ophthalmol. 1999;117:
34. Shimizu K, Kimura Y, Aoki K. Prevalence of exfoliation syndrome in the Japanese. Acta Ophthalmol. 1988;184:112–115.
35. Kivela¨ T, Hietanen J, Uusitalo M. Autopsy analysis of clinically unilateral exfoliation syndrome. Invest Ophthalmol Vis Sci. 1997;38:2008–2015.
36. Aasved H. Mass screening for fibrillopathia epitheliocapsularis, so-called senile exfoliation or pseudoexfoliation of the anterior lens capsule. Acta Ophthalmol. 1971;49:334–343. doi:10.1111/j.1755-3768.1971.tb00958.x
37. Musch DC, Shimizu T, Niziol LM, Gillespie BW, Cashwell LF, Lichter PR. Clinical characteristics of newly diagnosed primary, pigmentary and pseudoexfoliative open-angle glaucoma in the Collaborative initial glaucoma treatment study. Br J Ophthalmol. 2012;96(9):1180–1184. doi:10.1136/bjophthalmol-2012-301820
38. Altintaş O, Yüksel N, Karabaş VL, Qağlar Y. Diurnal intraocular pressure variation in pseudoexfoliation syndrome. Eur J Ophthalmol. 2004;14(6):495–500. doi:10.1177/112067210401400608
39. Gharagozloo NZ, Baker RH, Brubaker RF. Aqueous dynamics in exfoliation syndrome. Am J Ophthalmol. 1992;114:473–478. doi:10.1016/S0002-9394(14)71860-4
40. Koz OG, Turkcu MF, Yarangumeli A, Koz C, Kural G. Normotensive glaucoma and risk factors in normotensive eyes with pseudoexfoliation syndrome. J Glaucoma. 2009;18(9):684–688. doi:10.1097/IJG.0b013e31819c4311
41. Nucci C, Cerulli L, Osborne NN, et al. Glaucoma: An Open-Window to Neurodegeneration and Neuroprotection; 2008; Newnes.
42. Ocakoglu O, Koyluoglu N, Kayiran A, Tamcelik N, Ozkan S. Microvascular blood flow of the optic nerve head and peripapillary retina in unilateral exfoliation syndrome. Acta Ophthalmol Scand. 2004;82(1):49–53. doi:10.1046/j.1600-0420.2003.00196.x
43. Zenkel M. Extracellular matrix regulation and dysregulation in exfoliation syndrome. J Glaucoma. 2018;27(Suppl 1):S24–S28. doi:10.1097/IJG.0000000000000902
44. Wishart PK, Spaeth GL, Poryzees EM. Anterior chamber angle in the exfoliation syndrome. Br J Ophthalmol. 1985;69:103–107. doi:10.1136/bjo.69.2.103
45. Gross FJ, Tingey D, Epstein DL. Increased prevalence of occludable angles and angle-closure glaucoma in patients with pseudoexfoliation. Am J Ophthalmol. 1994;117:333–336. doi:10.1016/S0002-9394(14)73141-1
46. Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ. 1995;73(1):115–121.
47. Anastasopoulos E, Founti P, Topouzis F. Update on pseudoexfoliation syndrome pathogenesis and associations with intraocular pressure, glaucoma and systemic diseases. Curr Opin Ophthalmol. 2015;26:82–89. doi:10.1097/ICU.0000000000000132
48. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
49. Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295(5557):1077–1079. doi:10.1126/science.1066901
50. Kosior-Jarecka E, Sagan M, Wróbel-Dudzińska D, et al. Estrogen receptor gene polymorphisms and their influence on clinical status of Caucasian patients with primary open angle glaucoma. Ophthalmic Genet. 2019;19:1–6.
51. Pasquale LR, Loomis SJ, Weinreb RN, et al. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Mol Vis. 2013;12(19):1471–1481.
52. Bailey JNC, Gharahkhani P, Kang JH, et al.; Australian and New Zealand Registry of Advanced (ANZRAG) Consortium. Testosterone pathway genetic polymorphisms in relation to primary open-angle glaucoma: an analysis in two large datasets. Invest Ophthalmol Vis Sci. 2018;59(2):629–636.
53. Rönkkö S, Rekonen P, Kaarniranta K, Puustjärvi T, Teräsvirta M, Uusitalo H. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):697–704. doi:10.1007/s00417-006-0440-1
54. Määttä M, Tervahartiala T, Vesti E, Airaksinen J, Sorsa T. Levels and activation of matrix metalloproteinases in aqueous humor are elevated in uveitis-related secondary glaucoma. J Glaucoma. 2006;15(3):229–237. doi:10.1097/01.ijg.0000212229.57922.72
55. Ashworth Briggs EL, Toh T, Eri R, Hewitt AW, Cook AL. TIMP1, TIMP2, and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients Molecular Vision. Mol Vis. 2015;21:1162–1172.
56. Djordjević-Jocić J, Zlatanović G, Veselinović D, Stankovic-Babić G, Cekić S. Gender-related difference in aqueous humor matrix metalloproteinases MMP-2 and tissue inhibitor of matrix metalloproteinases in patients with pseudoexfoliation syndrome/glaucoma. Acta Medica Medianae. 2010;49(1):5–12.
57. Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124(5):685–687. doi:10.1016/S0002-9394(14)70908-0
58. Linnér E, Popovic V, Gottfries CG, Jonsson M, Sjögren M, Wallin A. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer’s type. Acta Ophthalmol Scand. 2001;79(3):283–285. doi:10.1034/j.1600-0420.2001.790314.x
59. Schumacher S, Schlötzer-Schrehardt U, Martus P, Lang W, Naumann GO. Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet. 2001;357(9253):359–360. doi:10.1016/S0140-6736(00)03645-X
60. Puustjärvi T, Blomster H, Kontkanen M, Punnonen K, Teräsvirta M. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242(9):749–754. doi:10.1007/s00417-004-0918-7
61. Song BJ, Aiello LP, Pasquale LR. Presence and risk factors for glaucoma in patients with diabetes. Curr Diab Rep. 2016;16(12):124. doi:10.1007/s11892-016-0815-6
62. International Diabetes Federation. IDF Diabetes Atlas.
63. Bujnowska-Fedak MM, Sapila BJ, Steciwko A. Epidemiologia schorzeń i struktura zachorowań w praktyce lekarza rodzinnego/Epidemiology of diseases and structure of morbidity in family medicine practice. Family Med Prim Care Rev. 2011.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
