Back to Journals » Clinical Ophthalmology » Volume 13
Clinical features of pediatric idiopathic intracranial hypertension
Authors Agraz D, Morgan LA, Fouzdar Jain S , Suh DW
Received 8 August 2018
Accepted for publication 28 February 2019
Published 24 May 2019 Volume 2019:13 Pages 881—886
DOI https://doi.org/10.2147/OPTH.S183087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Daniel Agraz,1 Linda A Morgan,2 Samiksha Fouzdar Jain,1,2 Donny W Suh1,2
1College of Medicine, University of Nebraska Medical Center, Omaha, NE, USA; 2Department of Pediatric Ophthalmology, Children’s Hospital and Medical Center, Omaha, NE, USA
Introduction: Adult idiopathic intracranial hypertension (IIH) is often linked to obesity, however, causes of IIH in children are not well understood. This project identifies potential risk factors and features of pediatric IIH.
Methods: This study consisted of a retrospective chart review of patients ages 5–17 years who were seen at a tertiary care children’s hospital and diagnosed with IIH. Diagnostic criteria included the presence of papilledema, normal neurological exam, normal neuroimaging, normal cerebrospinal fluid composition, and an opening pressure of a lumbar puncture >28 mmHg.
Results: Of the 26 cases of IIH, 19 met all diagnostic criteria for this study, while seven patients were probable IIH, as they lacked papilledema. Intracranial pressure ranged from 28 to 66 mmHg, with a mean of 40.23 mmHg (±10.74). Overall, 50.0% (95% CI: 29.9–70.1%) of IIH patients were obese, with patients 12 years of age and younger exhibiting an overall obesity rate of 30.7% and patients 13 years of age and older having an obesity rate of 69.2%. The overall allergy rate in this IIH patient population was 46.2% (95% CI: 26.6–66.6%).
Conclusion: Obesity appears to have no association with IIH in younger cases, but it is a more common feature in older children. An autoimmune component may play a role in pediatric IIH, given the high rate of atopy observed in this pediatric IIH patient cohort. Because a diagnosis of IIH can have an absence of optic nerve edema, taking a detailed history and performing a thorough examination are keys to diagnosing IIH in the pediatric population.
Keywords: idiopathic intracranial hypertension, papilledema, headache, obesity
Introduction
Idiopathic intracranial hypertension (IIH) is defined as an increase in intracranial pressure (ICP) with no clinical, laboratory, or radiographic evidence of associated infection, vascular abnormality, space-occupying lesion, or hydrocephalus.1 The incidence of IIH in the general population is one case per 100,000 people.2 The typical patient in which adult IIH tends to present is an obese female of reproductive age;3–6 presenting with the classic IIH symptoms of headache, nausea, and vomiting.7,8 The etiology in adults has not been fully elucidated, but hypotheses on its possible causes have been postulated.5,9 Nevertheless, the literature on pediatric IIH is less extensive in comparison to adult IIH. Therefore, the aim of this project is to identify potential risk factors and features associated with the diagnosis of pediatric IIH in patients presenting to Children’s Hospital and Medical Center in Omaha, Nebraska.
Materials and methods
This study consists of a retrospective chart review of patients diagnosed with IIH seen at a tertiary care children’s hospital (Children’s Hospital and Medical Center, Omaha, NE, USA). The protocol was approved by the University of Nebraska Medical Center Institutional Review Board (IRB# 640-15-EP), and the study was carried out in accordance with the principles of the Declaration of Helsinki. As this study was a retrospective chart review, the requirement for consent was waived.
As the aim of the study was to gain insight into pediatric IIH, only patients under the age of 18 were included. During the review, attention was paid to demographic characteristics (eg, age, gender), clinical presentation (eg, headaches, papilledema), imaging, and lumbar puncture results. Lumbar punctures were performed as per hospital protocol in the lateral decubitus position under general anesthesia.
Cases of IIH were further evaluated to ensure they met the diagnostic criteria of IIH based on the Modified Dandy Criteria10 and Revised Diagnostic criteria set forth by Friedman et al11. Diagnostic criteria included the presence of papilledema, normal neurological exam, normal neuroimaging, normal cerebrospinal fluid (CSF) composition, and opening pressure >28 mmHg. This criterion defines definite IIH, while the absence of papilledema resulted in a probable IIH designation. Patients were classified as obese or non-obese based on the 2000 Center for Disease Control and Prevention (CDC) body mass index for age percentile growth charts.12 Cases were divided into pre-pubertal (12 years of age and younger) and pubertal (13 years of age and older) for further analysis.
Microsoft Excel (Microsoft Corp., Redmond, WA, USA) was used to calculate SD, averages and graphs resulting from data analysis. To allow comparison against previously published data, 95% CI for IIH patient data were calculated. Fisher’s exact tests were calculated to determine the strength of any possible associations between continuous variables.
Results
Upon initial chart review, 54 cases of IIH were identified, but only 26 patients had sufficient data in the EMR to fulfill the criteria for this study. Of the 26 patients with sufficient data, 19 had a definitive diagnosis of IIH, meeting all diagnostic criteria, while seven patients were probable IIH as they lacked papilledema. The 26 IIH patients were separated into two groups: patients 12 years of age and younger and patients 13–17 years of age. The total age range was from 5 to 17 years of age, with a mean age of 12.1 (±3.05). Females contributed to 61.5% (n=26) of the overall patient population. Opening ICP ranged from 28 to 66 mmHg, with a mean of 36.63 mmHg (±10.74). This overall data is displayed in Table 1.
 | Table 1 Idiopathic intracranial hypertension patient population information |
The IIH patient population of 12 years of age and below was composed of 13 patients, ranging from 5 to 12 years of age, with a mean of 9.8 years of age (±2.5). This group was composed of 61.5% (n=13) males. Opening ICP ranges were 30–57 mmHg with a mean of 40.7 mm Hg (±7.63). In this group of IIH patients, the overall obesity rate was 30.7% (n=13), all of whom were male.
The IIH patient population of ages 13 and above consisted of 13 patients whose ages ranged from 13 to 17 years of age, with a mean of 14.5 years of age (±1.3). Females composed the majority of this age group at 84.6% (n=11). Opening ICP ranged from 28 to 66 mmHg with a mean of 40.1 mmHg (±12.8). The overall obesity rate in this group was 69.2% (n=9), with the majority of obese IIH patients being female at a ratio of 8:1 female to male.
In this study, 50.0% (95% CI: 29.9–70.1%) of all IIH patients were obese. IIH patients 12 years of age and younger exhibited an overall obesity rate of 30.7%, with only males in this age range being obese. In IIH patients 13 years of age and older, the overall IIH patient obesity rate was 69.2%. Females made up the majority of obese cases in this age range with 72.7% (95% CI: 39.0–94.0%) being obese (Table 2).
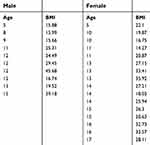 | Table 2 Ages and body mass indices (BMIs) of male and female patients who were diagnosed with pseudotumor |
Presenting symptoms across the overall patient population, along with subgrouping based on opening ICP, were analyzed. Patient data was divided into ICP at or below 40 mmHg and ICP above 40 mmHg. Overall, the most common presenting symptom was headaches found in 80.7% of all pediatric IIH patients (n=26), followed by diplopia (13.3%), blurry vision (13.3%), asymptomatic (13.3%), and emesis (6.67%). Overall trends were consistent within the patient population subgroups. In patients with ICP >40 mmHg (n=11), the most common presenting symptom was headaches at 81.8%, followed by diplopia (27.2%), blurry vision (18.2%), and emesis (9.1%). In patients with ICP at or <40 mmHg (n=15), the most common presenting symptom was headaches at 80%, followed by diplopia (13.3%), blurry vision (13.3%), asymptomatic (13.3%), and emesis (6.7%). Fisher’s exact tests were used to determine the association between each symptom and ICP, with the following results: headaches (P=0.99), diplopia (P=0.62), blurry vision (P=0.99), emesis (P=0.99), and asymptomatic (P=0.49).
Upon funduscopic exam, seven (26.92%) IIH patients exhibited no papilledema. Opening ICP was analyzed for further investigation. IIH patients were separated into the following three groups: ICP range from 28 to 34 mmHg (n=9), ICP range of 35–40 mmHg (n=6), and ICP >40 mmHg (n=11). The group with ICP range of 28–34 mmHg had the largest percent of patients lacking papilledema at 44.4%, followed by ICP >40 mmHg at 18.2%, and ICP between 35 and 40 mmHg at 16.7%. Fisher’s exact test was used to determine that the association between papilledema and ICP was P=0.46.
Upon chart review of the 26 IIH patients in this project, 12 patients (46.2%) were found to have a recorded allergy (95% CI: 26.6–66.6%). Of these 12 patients, 50.0% had more than one item recorded in their chart to which they were allergic. The most common allergies reported were medications (66.7%), environmental sources (41.7%), and food allergies (6.67%), as shown in Table 3. Breaking down these allergy rates by age group, 53.8% of IIH patients 13 years of age and older, and 38.4% of IIH patients 12 years of age and younger, had allergies reported in their charts.
 | Table 3 Patient allergy data related to age |
With regard to medication usage, two female patients were noted to be taking minocycline for acne. The patients, ages 13 and 15, did not have any associated papilledema. Other medications that the remaining patients were taking included albuterol (4 patients), trazodone (1), fluticasone (1), and ondansetron (1).
Discussion
IIH was more common among females with 61.5% of this pediatric IIH patient population. Looking at the population by age group, males composed the majority in the ages 12 and under group at 61.5%, but females made up the majority of the ages 13 and older group at 84.6%. The trends seen in this patient population follow what has been reported in the literature, where males make up a majority of the pre-pubertal IIH cases; however, following puberty, females make up the majority of IIH cases.5,7,14 Current literature suggests that IIH in pediatric pubertal patients tends to follow the risk factor trends seen in adult IIH cases.4,15 In adults, the IIH patient profile most commonly seen is an overweight female of reproductive age.3–6 In this study of patients ages 13 and older, 84.6% were female, and 72.7% of these female patients were obese.
Overall, the obesity rate in this study’s IIH patients (50%) differs from the United States pediatric obesity rate of 18% reported by the CDC.13 Analyzing obesity rate by gender, males had a higher rate of obesity in the 12 and under age group at 50.0% (n=4) compared to the United States age-matched pediatric obesity rate of 17.3%. In males 13–17 years of age, the obesity rate was 50.0% (n=2) compared to the United States age-matched pediatric obesity rate of 19.8% for males.13 While the overall percentage is markedly greater, the United States pediatric obesity rate values lie within the 95% CI of patient data. Female obesity rate noted in IIH patients 12 years of age and younger was 0% (n=0) compared to the United States age-matched obesity rate of 15.6%.13 In the 13–17 age group, 72.7% of females were obese compared to the age-matched obesity rate of 18.3% reported by the CDC, which is a substantial difference.13 As age increases, so does obesity rate. Given the known association of IIH and obesity, this natural history trend of increased obesity with age is also shown to be valid through our patient data. It is not surprising to observe that obesity plays a larger role in the pubertal group, 13 years of age and older, in comparison to IIH patients 12 years of age and younger.
Data from this project mirrors the trends previously reported, where obesity shows an association with IIH children who have reached puberty. Rangwala et al state that there is a well-defined association between IIH and obesity in adults, but a weak association between pediatric IIH and obesity.4 Other studies report that 30% of pre-pubertal children with IIH were overweight.7,16 Balcer et al examined obesity rates in pediatric IIH and found that 43% of those patients aged 3–11 years were obese, while 81% of 12–15 year olds and 91% of 15–17 years of age were obese.17 Furthermore, a larger percentage of IIH cases with advanced age was composed of females.17 In our study, females made up 50% of IIH cases between the ages of 3–11, 88% of IIH cases in 12–14 year age group, and 100% of IIH cases in 15–17 year age group.
Data published by the CDC reports that the most common allergy in the United States pediatric population is respiratory (environmental) allergy at 17%.18 In comparison, our study found the overall allergy rate in this IIH patient population to be 46.2% (95% CI: 26.6–66.6%), which is higher than the most common allergy rate reported by the CDC.
These were interesting observations in this pediatric IIH population, as the etiology of IIH has not been fully elucidated at the time of this writing. A 2007 review article by Rangwala and Liu notes that brain edema, increased cerebral blood volume, and increased CSF secretion could be possible mechanisms by which IIH develops.4,19,20 In addition, Liu states that decreased CSF absorption at the arachnoid villi level has been confirmed by radioisotope cisternography, but it is unclear if that is an inciting event or a consequence of arachnoid villi compression at high ICPs.4 More recently, Margeta et al discusses three general categories for proposed mechanisms contributing to IIH: increased cerebral/water content, CSF overproduction, and decreased CSF resorption at the level of the arachnoid villi or dural venous sinuses.5 Margeta et al also report that current evidence favors the pathologic process behind adult IIH to be decreased CSF outflow and intracranial venous hypertension.5,9 Observing the high rate of atopy in this IIH pediatric patient cohort, an autoimmune component that may be playing a role in pediatric IIH could be contributing to decreased CSF outflow and subsequent elevated ICP, possibly at the arachnoid villi level.
Venous sinus outflow obstruction is a recognized cause of IIH through impaired CSF absorption, as its prominence in the pathophysiology of IIH has been highlighted in several prospective adult studies.28,29 Reports of successful treatment of IIH through venous sinus stent placement, including two pediatric cases, have demonstrated the importance of recognizing venous sinus outflow obstruction in IIH.30,31
A 2010 letter to the editors by Dhungana et al discussed testing for the presence of Aquaporin-4 (AQP-4) antibodies in adult IIH patients.21 Aquaporins are described as playing a role in water homeostasis within the central nervous system and have been suggested to contribute to neuromyelitis optica.21 Dhungana et al hypothesized that AQP-4 could possibly be a factor in adult IIH. That study did not identify the presence of AQP-4 antibodies in their 10 adult female IIH patients (mean age 31.8±7.8 years) when compared to age-matched controls.21 While AQP-4 antibodies were not present, other cellular components could possibly be involved in an autoimmune process. Furthermore, the current consensus is that adult and pediatric IIH may have differing etiologies.4
In our study, specific ICP was not associated with any particular presenting symptoms; however, larger numbers of patients would need to be studied to achieve statistical significance. A headache is the most common complaint in 62–91% of all ages of IIH cases.4 A study by Youroukos et al reported headaches in 26 (72%) of their 36 pediatric patient cases.22 In our study, IIH patients with an ICP >40 mmHg, and those with ICP at or <40 mmHg, had similar rates of headaches as a presenting symptom (81.8% and 80.0%, respectively). Another symptom, diplopia, was more commonly seen in patients with ICPs >40 mmHg (27.2%) compared to ICPs at or below 40 mmHg (13.3%), but it was not significant. In pediatric patients presenting with what they describe as a headache, IIH should be part of the differential diagnosis and worked up once other avenues have been explored, and symptoms persist.
A key component in IIH is examining the optic disk for the presence of papilledema, a swelling of the optic disk in the presence of elevated ICPs.3 Upon reviewing IIH patient charts, it was noted that seven (26.9%) IIH patients lacked papilledema on examination. The absence of papilledema in IIH patients has been reported in the literature previously, ranging from 5.6% to 48% normal optic disks.14,22–27 A 2015 Masri et al article states that a lack of papilledema does not rule out IIH in pediatrics.14 While Digre et al have stated in a previous study that lack of papilledema is most commonly seen in patients with lower ICPs (30.9 cm of water vs 37.3 cm of water, P=0.031),23,27 the data in this study demonstrates the lack of papilledema in various ICP. Furthermore, Faz et al found that, out of 27 of their IIH pediatric patients, 13 (48%) lacked papilledema and therefore suggested revised criteria for pediatric IIH by placing less emphasis on papilledema.26 The current data set shows that lack of papilledema is present to some degree in pediatric IIH. With this project and previous literature presenting instances of IIH in the absence of optic nerve edema, taking a detailed history and performing a thorough examination is key to diagnosing IIH in the pediatric population.
The strengths of this study include the number of pediatric IIH cases as part of the patient cohort, as IIH occurs at a rate of 1:100,000 in the general population. In addition, the study uses the definition of pediatric obesity based on body mass index and uses CDC growth charts to determine obesity status. The limitations of this study include its nature in retroactively reviewing charts of patients seen and referred to Children’s Hospital and Medical Center in Omaha, Nebraska, a tertiary care center. Age was used to define puberty, rather than using physical signs, due to this being a retrospective study and that data concerning signs of puberty were lacking in some patients. No formal testing of our proposed hypothesis, which states pediatric IIH may have an autoimmune component based on high rates of atopy seen in this cohort, was done. For now, this was an observation, and future studies will be needed to test this hypothesis.
Acknowledgment
All authors have indicated that they have no financial relationships relevant to this article to disclose.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495.
2. Gordon K. Pediatric pseudotumor cerebri: descriptive epidemiology. Can J Neurol Sci. 1997;24:219–221.
3. Friedman DI. Papilledema and idiopathic intracranial hypertension. Continuum (Minneap Minn). 2014;20(4):857–876. doi:10.1212/01.CON.0000453314.75261.66
4. Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52(6):597–617. doi:10.1016/j.survophthal.2007.08.018
5. Margeta MA, Buckley EG, El-Dairi MA. Low cerebrospinal fluid protein in prepubertal children with idiopathic intracranial hypertension. J AAPOS. 2015;19(2):135–139. doi:10.1016/j.jaapos.2015.01.006
6. Paley GL, Sheldon CA, Burrows EK, Chilutti MR, Liu GT, McCormack SE. Overweight and obesity in pediatric secondary pseudotumor cerebri syndrome. Am J Ophthalmol. 2015;159(2):344–352. doi:10.1016/j.ajo.2014.11.003
7. Babikian P, Corbett J, Bell W. Idiopathic intracranial hypertension in children: the Iowa experience. J Child Neurol. 1994;9:144–149. doi:10.1177/088307389400900208
8. Phillips PH, Repka MX, Lambert SR. Pseudotumor cerebri in children. J AAPOS. 1998;2:33–38.
9. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83:488–494. doi:10.1136/jnnp-2011-302029
10. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55–56.
11. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. doi:10.1212/WNL.0b013e3182a55f17
12.
13.
14. Masri A, Jaafar A, Noman R, Gharaibeh A, Ababneh OH. Intracranial hypertension in children: etiologies, clinical features, and outcome. J Child Neurol. 2015;30(12):1562–1568. doi:10.1177/0883073815574332
15. Cinciripini GS, Donahue S, Borchert MS. Idiopathic intracranial hypertension in prepubertal pediatric patients: characteristics, treatment, and outcome. Am J Ophthalmol. 1999;127:178–182.
16. Stiebel-Kalish H, Lusky M, Yassur Y, et al. Swedish interactive thresholding algorithm fast for following visual fields in prepubertal idiopathic intracranial hypertension. Ophthalmology. 2004;111:1673–1675. doi:10.1016/j.ophtha.2004.03.031
17. Balcer IJ, Liu GT, Forman S, et al. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52:870–872.
18.
19. Johnston IH, Duff J, Jaconson EE, Fagan E. Asymptomatic intracranial hypertension in disorders of CSF circulation in childhood-treated and untreated. Pediatr Neurosurg. 2001;34:63–72. doi:10.1159/000055997
20. Soler D, Cox T, Bullock P, et al. Diagnosis and management of benign intracranial hypertension. Arch Dis Child. 1998;78:89–94.
21. Dhungana S, Waters P, Ismail A, Woodroofe N, Vincent A, Sharrack B. Absence of aquaporin-4 antibodies in patients with idiopathic intracranial hypertension. J Neurol. 2010;257:1211–1212. doi:10.1007/s00415-010-5499-2
22. Youroukos S, Psychou F, Fryssiras S, et al. Idiopathic intracranial hypertension in children. J Child Neurol. 2000;15:453–457. doi:10.1177/088307380001500706
23. Digre KB, Nakamoto BK, Warner JE, Langeberg WJ, Baggaley SK, Katz BJ. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49:185–193. doi:10.1111/j.1526-4610.2008.01324.x
24. Tibussek D, Schneider DT, Vandemeulebroecke N, et al. Clinical spectrum of the psuedotumor cerebri complex in children. Child Nerv Syst. 2010;26:313–321. doi:10.1007/s00381-009-1018-0
25. Tibussek D, Distelmaier F, von Kries R, Mayatepek E. Pseudotumor cerebri in childhood and adolescence-results of a Germany-wide ESPED-survey. Klin Padiatr. 2013;225:81–85. doi:10.1055/s-0033-1333757
26. Faz G, Butler IJ, Koenig MK. Incidence of papilledema and obesity in children diagnosed with idiopathic “benign” intracranial hypertension: case series and review. J Child Neurol. 2010;25:1389–1392. doi:10.1177/0883073810364853
27. Aylward SC, Aronowitz C, Roach SE. Intracranial hypertension without papilledema in children. J Child Neurol. 2016;31:177–183. doi:10.1177/0883073815587029
28. Farb RI, Vanek I, Scott JN, et al. Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology. 2003;60:1418–1424. doi:10.1212/01.WNL.0000066683.34093.E2
29. Higgins JNP, Tipper G, Varley M, Pickard JD. Transverse sinus stenoses in benign intracranial hypertension demonstrated on CT venography. Br J Neurosurg. 2005;19:137–140. doi:10.1080/02688690500145563
30. Higgins JNP, Cousins C, Owler BK, Sarkies N, Pickard JD. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry. 2003;74:1662–1666.
31. Rajpal S, Niemann DB, Turk AS. Transverse venous sinus stent placement as treatment for benign intracranial hypertension in a young male. Case report and review of the literature. J Neurosurg. 2005;102(3 Suppl):342–346.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
