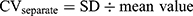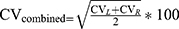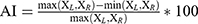Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Clinical Features in Parkinson’s Disease Patients with Hyperechogenicity in Substantia Nigra: A Cross-Sectional Study
Authors Zhu S , Wang Y , Jiang Y , Gu R, Zhong M , Jiang X, Shen B, Zhu J, Yan J, Pan Y, Zhang L
Received 12 May 2022
Accepted for publication 21 July 2022
Published 2 August 2022 Volume 2022:18 Pages 1593—1601
DOI https://doi.org/10.2147/NDT.S374370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Sha Zhu, Yaxi Wang, Yinyin Jiang, Ruxin Gu, Min Zhong, Xu Jiang, Bo Shen, Jun Zhu, Jun Yan, Yang Pan, Li Zhang
Department of Geriatric Neurology, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
Correspondence: Li Zhang, Department of Geriatric Neurology, The Affiliated Brain Hospital of Nanjing Medical University, No. 264 Guangzhou Road, Nanjing, Jiangsu Province, 210029, People’s Republic of China, Email [email protected]
Background: Transcranial ultrasound (TCS) can be used to reveal structural changes in the substantia nigra (SN) and is a potential tool for the early diagnosis of Parkinson’s disease (PD). This study aimed to explore the relationship between substantia nigra hyperechogenicity (SNH) and the clinical features of PD patients.
Methods: A total of 96 PD patients were included in our study. All patients were detected by TCS and divided into two groups: PD patients with SNH (PDSN+) and those with normal SN echogenicity (PDSN-). The Unified Parkinson’s Disease Rating Scale (UPDRS) and the Hoehn & Yahr stage were used to assess the extent of disease-related disability of the PD patients. Non-motor symptoms were evaluated by using several scales. The instrumented stand and walk test was performed on all subjects, and gait data were gathered using a JiBuEn gait analysis system.
Results: Seventy-five PD patients were successfully assessed by TCS. We found that SNH was associated with a higher UPDRS II scores (p = 0.028). In addition, compared with PDSN- group, the PDSN+ group exhibited more severe gait impairment, including increased variability in stride length (p = 0.042), decreased heel strike angle (p = 0.017), decreased range of motion of hip joints (p = 0.031), and a more asymmetrical walking pattern (p = 0.028).
Conclusion: Our study demonstrated that SNH significantly correlated with activities of daily living and gait impairment in Chinese patients with PD, suggesting the formation of SNH might be a dynamic biomarker reflecting disease severity.
Keywords: Parkinson’s disease, transcranial sonography, substantia nigra hyperechogenicity, non-motor symptoms, gait analysis
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra (SN).1 Transcranial sonography (TCS) is a noninvasive and convenient diagnostic technique that can reveal structural changes in the SN and is potentially useful in the diagnosis of PD. SN hyperechogenicity (SNH) was detected by TCS in the SN of PD patients, which could differentiate PD patients from those with other parkinsonian syndromes.2–4 TCS is used extensively in clinical practice. Studies on the use of TCS in PD patients are increasing, but it is still unclear whether the extent of SNH can be a biomarker for monitoring disease severity. Some recent studies have found that PD patients with SNH tend to be older, have more severe non-motor symptoms and are at a more advanced disease stage.5,6 However, other studies have not found similar results.7,8 There is a lack of studies that have explored the relationship between motor symptoms, particularly gait dysfunction, and SNH. Additionally, most previous studies were assessed by scales. Semiquantitative rating scales such as the UPDRS III score are somewhat subjective, and reflect only a portion of motor function in daily life.9 More precise quantification of motor performance is needed to improve in-depth understanding of PD.
Gait impairment in PD patients worsens as the disease progresses, affecting the patient’s quality of life and potentially leading to falls.10,11 Owing to the rapid development of technology, the use of wearable sensors has made it possible to quantify walking patterns. The present study aimed to comprehensively assess the correlation between SNH and the clinical features of PD patients, including gait impairment and non-motor symptoms. We hypothesized that SNH is associated with more severe clinical features in PD patients. Our results may aid the management of PD patients and enhance individualized care for these patients.
Methods
Participants
PD patients were diagnosed according to the Movement Disorder Society criteria.12. Diagnoses were made by at least two experienced neurologists and were based on medical history, neuroimaging studies, and physical examination. None of the PD patients presented atypical features such as cerebellar abnormalities, pyramidal signs, gaze palsy or other secondary parkinsonism. From January 2019 to July 2021, 96 Chinese patients with PD from the Department of Geriatrics, Nanjing Brain Hospital Affiliated to Nanjing Medical University were included in this study. We excluded patients with fractures, cerebrovascular disease, spinal spectrum diseases, and other diseases that may affect gait performance. This study was approved by the Medical Ethics Committee of the Nanjing Brain Hospital Affiliated to Nanjing Medical University. All patients signed a written informed consent form.
Midbrain Transcranial Sonography
The sonographic examinations were performed within 1 week after the clinical scale assessment. Philips EPIQ7 ultrasonic diagnostic apparatus with an emitting frequency of 1.0 MHz ~ 5.0 MHz was used to detect echogenicity in the SN with a dynamic range of 45~55 dB and penetration depth of 14~ 16 cm. TCS was performed through both temporal bone windows in the axial plane by the same experienced ultrasound practitioner who was blinded to the clinical manifestations of the patients. SN echogenic areas were manually encircled with the cursor, and the planimetric area was measured automatically. Patients with at least one SN hyperechogenic size ≥0.20 cm2 were defined as hyperechogenic (SN+),13 whereas normal (SN-) was defined as both left and right SN hyperechogenic areas less than 0.20 cm2. Seventy-five patients (78.125%) had appropriate temporal acoustic bone windows.
Clinical Evaluation
The following demographic data of all participants were collected: age, height, weight, sex, education, shoe size, and disease duration, The Unified Parkinson’s Disease Rating Scale (UPDRS) and the Hoehn & Yahr Stage (H-Y stage) were used to assess the disease disability of the PD patients. The Non-motor Symptoms Scale (NMSS) was used to evaluate non-motor characteristics of PD patients, the Mini-mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were applied to evaluate cognitive function, the Pittsburgh Sleep Quality Index (PSQI) and Parkinson’s Disease Sleep Scale (PDSS) were used to assess patients’ sleep quality, the Parkinson’s Disease Questionnaire-39 (PDQ39) was used for the quality of life, the Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) were used for depression and anxiety, respectively.
Gait Data Collection
We applied a set of gait-analysis system called JiBuEn to collect gait data. A previous study verified the accuracy of this equipment.14 All patients were asked to stop antiparkinsonian medication for 24 h (or 72h for the controlled release anti-PD medications). We collected gait data the next morning while the patient was fully awake. All PD patients completed the instrumented stand and walk test.15 First, the patients stood silently for 30s; when they heard the doctor’s order, they walked 7 meters in a comfortable manner, and then returned to the starting place. The gait data were collected through this process. All patients practiced the test twice before data collection to become acquainted with it.
Statistical Analysis
All analyses were performed with SPSS v.25.0. Qualitative data were summarized as counts of subjects and percentages. Continuous variables are given as the mean ± standard deviation (SD), and statistical significance was set at P < 0.05. A chi-square test was applied for qualitative data. All measurement data were tested for normality by the Kolmogorov–Smirnov test and further data analysis was performed using the two-sample t test and Mann–Whitney U-test. Spearman correlation analysis was performed to examine the associations between SN echogenicity and the factors described above. Formula (1.1) was applied to calculate the variability of gait parameters and then formula (1.2) was used to combine.16,17 We used the asymmetry index (AI) to assess the symmetry of the gait data by using Formula (2).18,19
CV represents the coefficient of variation; the subscripts L and R refer to the left and right sides of subjects, respectively.
where X = [ST, SL, SwPT, StPT, TO, HS, ROM-AJ, ROM-KJ, ROM-HJ], and the subscripts L and R refer to the left and right sides of patients. ST: stride time; SL: stride length; SwPT: swing phase time; StPT: stance phase time; TO: toe-off angle; HS: heel strike angle; ROM: range of motion; AJ: ankle joint; KJ: knee joint; HJ: hip joint.
Results
Of the 96 participants, we failed to acquire appropriate temporal acoustic bone windows in 21 patients; thus, they were excluded from further analysis. The remaining 75 PD patients were divided into two groups according to the TCS examination results. Comparison between PD patients with SN+ (PDSN+) and SN- (PDSN-) was performed and their detailed baseline data are shown in Table 1. There was no statistical difference in any baseline data between the two groups.
 |
Table 1 Clinical Baseline Data of Patients |
Clinical Scale Assessment
The scores of UPDRS Part 2 (UPDRS II) were significantly increased in the PDSN+ group compared with the PDSN- group (15.65±6.68 vs 12.61±7.15, p = 0.028). Spearman correlation analysis also showed that SNH was positively correlated with the UPDRS II scores (r = 0.256, p = 0.026). However, the scores of the NMSS, MoCA, MMSE, HAMD, HAMA, PDSS, PSQI and PDQ-39 were not significantly different between the two groups. All data are presented in Table 2.
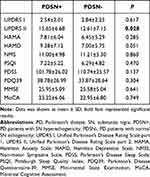 |
Table 2 Clinical Scale Assessment of Patients |
Spatiotemporal Gait Parameters
Spatiotemporal gait parameters including stride length (SL), stride time (ST), gait velocity (GV), cadence (CA), swing phase time (SwPT), stance phase time (StPT) and the variability of ST (CV-ST), SwPT (CV-SwPT) and StPT (CV-StPT) were similar between PDSN+ group and PDSN- group. The only significant difference between the two groups was for the variability of SL (CV-SL) (28.21±6.92 vs 25.23±7.02, p = 0.042). The correlation analysis also showed that SNH was significantly positively correlated with CV-SL (r = 0.237, p = 0.041). The detailed data are presented in Table 3.
 |
Table 3 Spatiotemporal Gait Parameters of Participants |
Kinematic Gait Parameters
The range of motion (ROM) of the hip (ROM-HJ), knee (ROM-KJ), and ankle joints (ROM-AJ) were measured. We acquired the ROM values by calculating the difference between the maximum and minimum angle of the joints in the sagittal plane. The toe-off (TO) and heel strike (HS) angles were also measured. There was no difference in ROM-AJ, ROM-KJ or TO between the PDSN+ group and the PDSN- group. However, we found significant differences in HS (12.10±3.32 vs 13.70±3.35, p = 0.017) and ROM-HJ (31.79±9.29 vs 35.64±7.72, p = 0.031) between the PDSN+ and PDSN- groups, respectively. SNH was significantly and negatively correlated with HS (r = −0.277, p = 0.016) and ROM-HJ (r = −0.251, p = 0.030). All data are shown in Table 4.
 |
Table 4 Kinematic Gait Parameters of Participants |
Symmetry Analysis of Gait Parameters
Spatiotemporal and kinematic gait parameters were included in the symmetry analysis. Compared with the PDSN- group, only the AI-ROM-AJ was significantly increased in the PDSN+ group (15.84±12.51 vs 10.93±10.28, p = 0.028). Spearman correlation analysis also showed that SNH was positively correlated with AI-ROM-AJ (r = 0.256, p = 0.026). All data are provided in Table 5.
 |
Table 5 Symmetry Analysis of Gait Parameters |
Discussion
This study included 96 patients to explore the association between SN echogenicity and non-motor symptom and gait characteristics in Chinese patients with PD. We found that PD patients with SNH showed higher UPDRS II scores and more severe gait impairment than PD patients with normal SN echogenicity.
Several studies have shown that urinary incontinence, depression, UPDRS II scores, cognition, and visual hallucinations are associated with SNH.5,20–23 In our clinical scale assessment, we found that SNH was significantly and positively correlated with the UPDRS II scores, but no correlation was found between SNH and depression. A similar conclusion was drawn in a recent Chinese population-based study.21 We tentatively attribute this result to the following points: First, a recent study demonstrated that the size of the SNH was positively associated with the extent of dopaminergic neuron death in PD animal models.24 Accordingly, we preliminarily inferred that the echogenic pattern of the SN might be associated with disease progression. Moreover, it was also reported that the UPDRS II score increased significantly with the progression and duration of the disease.25 Therefore, UPDRS II scores were higher in the group with a larger echogenic size. Second, PD duration was not significantly different between the groups, but reduced in PDSN- group when considering absolute values. Considering the correlation between UPDRS II scores and disease duration, this may also be one of the potential reasons for the higher UPDRS II score in the PDSN+ group. A recent Chinese study showed that the size of SN echogenicity in PD patients was related to the disease duration,26 but there was only a trend but no significant difference in our study, which may be related to our relatively small sample size. Finally, the UPDRS II focuses on activities of daily living, which is strongly influenced by the patient’s subjective perceptions.27 Because the items of the UPDRS II include falling, freezing and walking, patients with more severe gait disturbance may score higher on the UPDRS II. In our study, gait disturbance was associated with SNH, and this could be one explanation for the higher UPDRS II score. The mechanism underlying the formation of SNH is not fully understood but has been suggested to be related to the iron accumulation and microglia activation.28,29 However, there is also no direct evidence of a correlation between clinical symptoms and microglia activation in the SN in PD.
Gait disturbance is a prototypical feature of PD, and wearable sensors are objective tools that can be used to quantify the walking process. Patients with PD tend to show a slower walking speed and a shorter step length than healthy people.19 However, few studies have investigated the relationship between SNH and gait performance. Among the spatiotemporal gait parameters, we found that the PDSN+ group exhibited an increased CV-SL compared with the PDSN- group. CV refers to the stride-to-stride fluctuation of the gait parameters during walking process. The increased CV-SL is noteworthy because previous studies have shown that CV-SL predicts falls in older people and is associated with disease severity.30,31 Among the kinematic gait parameters, HS and ROM-HJ were decreased in the PDSN+ group compared with the PDSN- group. Spearman correlation analysis demonstrated that SNH was negatively correlated with HS and ROM-HJ. The decreased HS reflects patients’ shuffling gait and manifests the diminished muscle strength of the lower limbs, which may lead to falls.32 In addition, a decreased ROM-HJ value indicates a more rigid gait pattern. From a kinematic point of view, the PDSN+ group had a greater risk of falls than the PDSN- group. Gait asymmetry in PD is related to uncoordinated action in the leg muscles and may predispose the patient to freezing of gait.33,34 Compared with the PDSN- group, the PDSN+ group had increased AI-ROM-AJ values, which manifested in the PDSN+ group walking with a more asymmetrical gait pattern.
In our study, the PD patients with SNH may have suffered more severe impairments in activities of daily living and gait performance. SNH may correlate with disease severity, which is still controversial. A previous study reported that the size of SN echogenicity was unrelated to disease progression and gradual neuron loss in the SN.35 However, 60–70% of dopaminergic neurons in the SN are lost before clinical symptoms appear.36 Studies that have included only patients in advanced stages of PD cannot elucidate how SNH changes over time after the loss of most dopaminergic neurons. Furthermore, there is heterogeneity among the different subsets of PD patients. For example, when patients were classified as tremor dominant (TD) and non-TD subtypes, a correlation between disease duration and SNH was found in the non-TD PD patients.26 More extended studies are required to formalize this result.
In our research, one major concern was the relatively low sensitivity of TCS in this cohort. Only 49.3% of PD patients showed hyperechoic signals in the SN, which was lower than most in previous studies.2,7,37 First, difference in the racial or ethnic makeup of the study population could be one reason for these differences in TCS signal. Our result is in line with other Asian studies that have reported a similar rate (~50%).26,38 Furthermore, previous Chinese studies using single-photon emission computed tomography as the gold standard also showed that the sensitivity of TCS is only approximately 60%.39,40 Second, some previous studies have suggested that echosignal extension is related to disease severity.41,42 Most of the patients in our study were in the early stages of the disease, this may be one of the reasons. Thirdly, although the TCS was performed by the same experienced ultrasound practitioner who was blinded to the clinical symptom of the patients, it is still difficult to avoid subjective errors in identifying SN hyperechoic regions. Finally, considering our relatively small sample size, selection bias cannot be excluded.
Despite these limitations, our research expanded on previous studies and found that the PDSN+ group exhibited higher UPDRS II scores, decreased ROM-HJ and HS, and a more variable and asymmetric gait pattern than the PDSN- group. In our cohort of Chinese patients with PD, SNH was significantly correlated with activities of daily living and gait dysfunction. Our cross-sectional study will help in the management of PD patients with SNH. Based on the results of this study, follow-up studies could explore the development of more individualized treatment or rehabilitation plans for PD patients with SNH. Further studies, especially longitudinal cohort studies, are needed to confirm our findings.
Data Sharing Statement
The data supporting the result of this study are included in the article, and further inquiries can be directed to the corresponding author/s.
Statement of Ethics
The study was approved by the Ethics committee of Nanjing Brain Hospital Affiliated to Nanjing Medical University. All participants signed a written informed consent before the study. All procedures followed the declaration of Helsinki.
Acknowledgments
This research was funded by the National Natural Science Foundation of China [grant numbers 82171249], the Jiangsu Provincial Cadre Health Projects [grant number BJ20005], the Special Funds of the Jiangsu Provincial Key Research and Development Program [grant numbers BE2019612], the Jiangsu Province Elderly Health Project[grant numbers LD2021013], the Nanjng Medical University School Fund Project[grant numbers NMUB20210223], the Nanjing Medical Science and Technology Development Foundation [grant number QRX17026] and the Nanjing Rehabilitation Medicine Center Project [grant number is not available]. We acknowledge all the researchers of JiBuEn gait analysis system for their technical support.
Author Contributions
All authors contributed to the article, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in writing, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors approved the submitted version.
Disclosure
The authors report no conflicts of interest in relation to this work and declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Olson K, Bade A, Schutt C, et al. Manganese-enhanced magnetic resonance imaging for detection of vasoactive intestinal peptide receptor 2 agonist therapy in a model of Parkinson’s disease. Neurotherapeutics. 2016;13(3):635–646. doi:10.1007/s13311-016-0449-z
2. Okawa M, Miwa H, Kajimoto Y, et al. Transcranial sonography of the substantia nigra in Japanese patients with Parkinson’s disease or atypical parkinsonism: clinical potential and limitations. Intern Med. 2007;46(18):1527–1531. doi:10.2169/internalmedicine.46.0271
3. Mei YL, Yang J, Wu ZR, Yang Y, Xu YM. Transcranial sonography of the substantia nigra for the differential diagnosis of Parkinson’s disease and other movement disorders: a meta-analysis. Parkinsons Dis. 2021;2021:8891874. doi:10.1155/2021/8891874
4. Heinzel S, Aho V, Suenkel U, et al. Gut microbiome signatures of risk and prodromal markers of Parkinson disease. Ann Neurol. 2021;90(3):E1–E12. doi:10.1002/ana.26128
5. Yu SY, Cao C, Zuo L, et al. Clinical features and dysfunctions of iron metabolism in Parkinson disease patients with hyper echogenicity in substantia nigra: a cross-sectional study. BMC Neurol. 2018;18(1):9. doi:10.1186/s12883-018-1016-5
6. Toomsoo T, Liepelt-Scarfone I, Berg D, et al. Effect of age on substantia nigra hyper-echogenicity in Parkinson’s disease patients and healthy controls. Ultrasound Med Biol. 2019;45(1):122–128. doi:10.1016/j.ultrasmedbio.2018.09.018
7. Behnke S, Runkel A, Kassar H, et al. Long-term course of substantia nigra hyperechogenicity in Parkinson’s disease. Mov Disord. 2013;28(4):455–459. doi:10.1002/mds.25193
8. Lobsien E, Schreiner S, Plotkin M, Kupsch A, Schreiber S, Doepp F. No correlation of substantia nigra echogenicity and nigrostriatal degradation in Parkinson’s disease. Mov Disord. 2012;27(3):450–453. doi:10.1002/mds.24070
9. Shin C, Hahn S, Park B, et al. Predictors of the placebo response in clinical trials on Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2016;29:83–89. doi:10.1016/j.parkreldis.2016.05.019
10. Lord S, Galna B, Yarnall A, Coleman S, Burn D, Rochester L. Predicting first fall in newly diagnosed Parkinson’s disease: insights from a fall-naïve cohort. Mov Disord. 2016;31(12):1829–1836. doi:10.1002/mds.26742
11. Curtze C, Nutt J, Carlson-Kuhta P, Mancini M, Horak F. Objective gait and balance impairments relate to balance confidence and perceived mobility in people with Parkinson disease. Phys Ther. 2016;96(11):1734–1743. doi:10.2522/ptj.20150662
12. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi:10.1002/mds.26424
13. Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol. 2008;7(11):1044–1055. doi:10.1016/S1474-4422(08)70239-4
14. Tao S, Zhang X, Cai H, Lv Z, Hu C, Xie H. Gait based biometric personal authentication by using MEMS inertial sensors. J Ambient Intell Humaniz Comput. 2018;9(5):1705–1712. doi:10.1007/s12652-018-0880-6
15. Mancini M, King L, Salarian A, et al. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci. 2011;Suppl 1:007. doi:10.4172/2155-9538.S1-007
16. Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture. 2013;37(4):580–585. doi:10.1016/j.gaitpost.2012.09.025
17. Bryant M, Rintala D, Hou J, Collins R, Protas E. Gait variability in Parkinson’s disease: levodopa and walking direction. Acta Neurol Scand. 2016;134(1):83–86. doi:10.1111/ane.12505
18. Patterson K, Gage W, Brooks D, Black S, McIlroy W. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–246. doi:10.1016/j.gaitpost.2009.10.014
19. Zhang M, Artan N, Gu H, et al. Gait study of Parkinson’s disease subjects using haptic cues with a motorized walker. Sensors. 2018;18(10).
20. Walter U, Skoloudík D, Berg D. Transcranial sonography findings related to non-motor features of Parkinson’s disease. J Neurol Sci. 2010;289(1–2):123–127. doi:10.1016/j.jns.2009.08.027
21. Zhou HY, Sun Q, Tan YY, et al. Substantia nigra echogenicity correlated with clinical features of Parkinson’s disease. Parkinsonism Relat Disord. 2016;24:28–33. doi:10.1016/j.parkreldis.2016.01.021
22. Li T, Shi J, Qin B, et al. Increased substantia nigra echogenicity correlated with visual hallucinations in Parkinson’s disease: a Chinese population-based study. Neurol Sci. 2020;41(3):661–667. doi:10.1007/s10072-019-04110-z
23. Del Toro Perez C, Amaya Pascasio L, Arjona Padillo A, et al. Neurosonological findings related to non-motor features of Parkinson’s disease: a systematic review. Brain Sci. 2021;11(6):776. doi:10.3390/brainsci11060776
24. Zhang S, Tao K, Wang J, Duan Y, Wang B, Liu X. Substantia nigra hyperechogenicity reflects the progression of dopaminergic neurodegeneration in 6-OHDA rat model of Parkinson’s disease. Front Cell Neurosci. 2020;14:216. doi:10.3389/fncel.2020.00216
25. Harrison M, Wylie S, Frysinger R, et al. UPDRS activity of daily living score as a marker of Parkinson’s disease progression. Mov Disord. 2009;24(2):224–230. doi:10.1002/mds.22335
26. Zhou HY, Huang P, Sun Q, et al. Substantia nigra echogenicity associated with clinical subtypes of Parkinson’s disease. J Parkinsons Dis. 2018;8(2):333–340. doi:10.3233/JPD-171264
27. Goetz C, Tilley B, Shaftman S, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi:10.1002/mds.22340
28. Ward R, Zucca F, Duyn J, Crichton R, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13(10):1045–1060. doi:10.1016/S1474-4422(14)70117-6
29. Berg D, Godau J, Riederer P, Gerlach M, Arzberger T. Microglia activation is related to substantia nigra echogenicity. J Neural Transm. 2010;117(11):1287–1292. doi:10.1007/s00702-010-0504-6
30. Hausdorff J, Cudkowicz M, Firtion R, Wei J, Goldberger A. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13(3):428–437. doi:10.1002/mds.870130310
31. Callisaya M, Blizzard L, Schmidt M, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40(4):481–487. doi:10.1093/ageing/afr055
32. Schlachetzki JCM, Barth J, Marxreiter F, et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One. 2017;12(10):e0183989. doi:10.1371/journal.pone.0183989
33. Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff J. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exper Brain Res. 2007;177(3):336–346. doi:10.1007/s00221-006-0676-3
34. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff J. Is freezing of gait in Parkinson’s disease related to asymmetric motor function? Ann Neurol. 2005;57(5):656–663. doi:10.1002/ana.20452
35. Berg D, Merz B, Reiners K, Naumann M, Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disorders. 2005;20(3):383–385. doi:10.1002/mds.20311
36. Postuma R, Gagnon J, Montplaisir J. Clinical prediction of Parkinson’s disease: planning for the age of neuroprotection. J Neurol Neurosurg Psychiatry. 2010;81(9):1008–1013. doi:10.1136/jnnp.2009.174748
37. Bártová P, Skoloudík D, Ressner P, Langová K, Herzig R, Kanovsky P. Correlation between substantia nigra features detected by sonography and Parkinson disease symptoms. J Ultrasound Med. 2010;29(1):37–42. doi:10.7863/jum.2010.29.1.37
38. Chen W, Tan Y, Hu Y, et al. Combination of olfactory test and substantia nigra transcranial sonopraphy in the differential diagnosis of Parkinson’s disease: a pilot study from China. Transl Neurodegener. 2012;1(1):25. doi:10.1186/2047-9158-1-25
39. Li D, Zhang L, Hu Y, et al. Transcranial sonography of the substantia nigra and its correlation with DAT-SPECT in the diagnosis of Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(8):923–928.
40. Liu P, Li X, Li F, Ou-Yang Q, Zhang H, Feng T. The predictive value of transcranial sonography in clinically diagnosed patients with early stage Parkinson’s disease: comparison with DAT PET scans. Neurosci Lett. 2014;582:99–103. doi:10.1016/j.neulet.2014.08.053
41. Tsai C, Wu R, Huang Y, Chen L, Yip P, Jeng J. Transcranial color-coded sonography helps differentiation between idiopathic Parkinson’s disease and vascular parkinsonism. J Neurol. 2007;254(4):501–507. doi:10.1007/s00415-006-0403-9
42. Weise D, Lorenz R, Schliesser M, Schirbel A, Reiners K, Classen J. Substantia nigra echogenicity: a structural correlate of functional impairment of the dopaminergic striatal projection in Parkinson’s disease. Mov Disord. 2009;24(11):1669–1675. doi:10.1002/mds.22665
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.